Back to Journals » Cancer Management and Research » Volume 13
LncRNA ELFN1-AS1 Promotes Retinoblastoma Growth and Invasion via Regulating miR-4270/SBK1 Axis
Authors Feng W, Zhu R, Ma J, Song H
Received 11 September 2020
Accepted for publication 6 December 2020
Published 5 February 2021 Volume 2021:13 Pages 1067—1073
DOI https://doi.org/10.2147/CMAR.S281536
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Chien-Feng Li
Wanguo Feng,1 Ruixi Zhu,2 Junlong Ma,3 Han Song2
1Department of Refractive Surgery, Dalian Aier Eye Hospital, Dalian, 116092, People’s Republic of China; 2Department of Ophthalmology, Heilongjiang Provincial Hospital, Harbin Institute of Technology, Harbin, 150036, People’s Republic of China; 3Department of Ophthalmology, Dalian University Affiliated Xinhua Hospital, Dalian, 116021, People’s Republic of China
Correspondence: Han Song
Department of Ophthalmology, Heilongjiang Provincial Hospital, Harbin Institute of Technology, Harbin, 150036, People’s Republic of China
Email [email protected]
Background: Long noncoding RNA (lncRNA) has been reported to play important roles in tumor initiation. However, how lncRNA ELFN1-AS1 affects retinoblastoma development remains unclear. Thus, we sought to elucidate its functions in retinoblastoma progression.
Methods: ELFN1-AS1 expression was measured in retinoblastoma tissues and normal tissues by qRT-PCR. CCK8, colony formation and Transwell assay were carried out to investigate the effects of ELFN1-AS1 knockdown on cell malignant behaviors. Bioinformatics analyses were performed to predict the relationship among ELFN1-AS1, miR-4270 and SBK1.
Results: ELFN1-AS1 was highly expressed in retinoblastoma tissues and cell lines. ELFN1-AS1 was positively correlated with retinoblastoma progression and prognosis. ELFN1-AS1 knockdown curtailed retinoblastoma proliferation, migration and invasion. ELFN1-AS1 was the competing endogenous RNA for miR-4270 and promoted SBK1expression.
Conclusion: Altogether, our findings demonstrated that ELFN1-AS1 promotes retinoblastoma progression through mediating miR-4270/SBK1 axis and might be a promising therapeutic target.
Keywords: ELFN1-AS1, miR-4270, SBK1, retinoblastoma, progression
Introduction
Retinoblastoma (RB) is one of the most common pediatric intraocular cancers.1 Rapid growth and metastasis are the characteristics of RB.2 The current therapeutic strategies include surgery, chemotherapy and radiotherapy.3 However, the prognosis of patients with RB is still rather poor.4 Therefore, it is urgently required to investigate its underlying molecular mechanism and develop novel therapeutic methods.
Long noncoding RNA (lncRNA) is a subgroup of noncoding transcripts, which is characterized by over 200 nucleotides in length and lacking coding ability.5 LncRNA may exert functions through multiple kinds of manners, such as epigenetic or post-transcriptional levels.6 LncRNA participates in the regulation of various biological processes in cancer, including proliferation, invasion, apoptosis and differentiation.7,8 For instance, lncRNA DLX6-AS1 contributes to prostate cancer growth and invasion through enhancing LARGE methylation.9 LncRNA CCAT2 initiates prostate cancer proliferation and metastasis through activation of Wnt signaling.10 LncRNA LINC00491 promotes lung cancer proliferation, migration and invasion by sponging miR-324 to facilitate SP1 expression.11 Thus, it is important to determine the correlation between lncRNA and RB progression.
It is reported that ELFN1-AS1 silencing suppresses colon cancer growth and invasion.12 ELFN1-AS1 also promotes esophageal cancer development.13 A recent work indicates that ELFN1-AS1 accelerates ovarian cancer progression.14 Our study aims to determine the roles of ELFN1-AS1 in RB progression. Our findings illustrated that ELFN1-AS1 promotes RB proliferation, migration and invasion through targeting miR-4270/SBK1 axis.
Materials and Methods
Patient Tissues
Thirty-nine RB tissues and normal adjacent tissues were collected from Heilongjiang Provincial Hospital. These tissues were not treated with radiotherapy or chemotherapy before collection. All tissues were stored in liquid nitrogen until use. Correlation between ELFN1-AS1 expression and clinicopathologic features of RB patients is analyzed in Table 1. Samples stages were analyzed according to the 8th edition AJCC TNM staging. This study was approved by the Ethics Committee of Heilongjiang Provincial Hospital. All patients provided written informed consents.
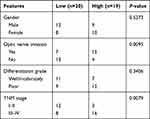 |
Table 1 ELFN1-AS1 Expression and Clinicopathologic Features of Retinoblastoma Patients |
Cell Culture
Normal human retinal epithelial cell line ARPE-19 and RB cell lines were obtained from American Type Culture Collection (Manassas, VA, USA). Cells were cultured using DMEM (Invitrogen) containing 10% fetal bovine serum (FBS; Gibco, MD, USA). siRNAs, miRNA mimics, miRNA inhibitors and negative controls were purchased from GenePharma (Suzhou, China). Plasmids were transfected into cells using Lipofectamine 3000 based on the manufacturer’s instructions.
Real-Time Quantitative PCR
Total RNA was isolated using TRIzol reagent (Invitrogen). cDNA was synthesized using M-MLV Reverse Transcriptase. qPCR was carried out using SYBR green reagents (Invitrogen). Relative expression was normalized to GAPDH or U6 and calculated according to the 2−ΔΔCt approach.
CCK8 Assay
Cell proliferation was assessed through a Cell Counting Kit-8 (CCK-8, Dojindo, Japan). Briefly, cells were seeded into 96-well plates (3000 cells/well) and cultured for indicated time. Then, CCK8 solution was added for 2h and absorbance at 450 nm was measured by a microplate reader (Thermo Fisher Scientific).
Colony Formation Assay
Cells were seeded into 6-well plates and cultured for 14 days. Colonies were fixed with 70% ethanol and incubated with 0.1% crystal violet (Sigma, MO, USA). Colony number was finally determined.
Transwell Assay
Cell migration and invasion were measured using Transwell assay containing chambers (coated with Matrigel for invasion). In brief, cells were seeded into the upper chamber with serum-free medium. Then, the lower chamber was fixed with 600 μL serum-containing medium. After cultured for 48 h, the migratory or invasive cells in the lower chamber were fixed using 4% paraformaldehyde and stained with 0.1% crystal violet. Cell number was quantified with an inverted microscope (Nikon, Japan).
Subcellular Fractionation
Nuclear and cytoplasmic fractions were isolated using A PARIS kit (Thermo Fisher Scientific), followed by RNA isolation and qRT-PCR.
Bioinformatics Analysis
miRDB, StarBase and Targetscan were used to predict the interactions among ELFN1-AS1, miR-4270 and SBK1.
Dual-Luciferase Reporter Assays
Fragments of ELFN1-AS1 or SBK1 containing miR-4270 binding element (wild-type or mutant) were inserted into psiCHECK-2 vector (Promega, Madison, WI, USA) to generate luciferase reporter vectors. Then, luciferase vectors and miR-4270 mimics or negative controls were transfected into cells for 48 h. Finally, the luciferase activity was measured using the Dual-Luciferase Assay System (Promega).
Statistical Analyses
Data were analyzed using SPSS 20.0 statistic software and expressed as mean ± SD. The Student’s t-test or one-way analysis of variance analysis (ANOVA) was utilized to conduct statistical analyses. P<0.05 was considered as statistically different.
Results
ELFN1-AS1 is Highly Expressed in RB Tissues
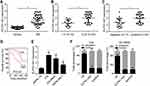
To investigate whether ELFN1-AS1 expression was increased RB tissues, 39 RB tissues were collected and qRT-PCR analysis was performed. ELFN1-AS1 level was raised in RB tissues compared to normal tissues (Figure 1A). Moreover, ELFN1-AS1 expression was higher in advanced RB tissues and metastatic tissues (Figure 1B and C). Notably, ELFN1-AS1 high expression was associated with a low survival rate in RB patients (Figure 1D), indicating that ELFN1-AS1 is a potential prognostic biomarker. In addition, qRT-PCR results showed ELFN1-AS1 level was also upregulated in RB cell lines (Figure 1E). We then analyzed the subcellular distribution of ELFN1-AS1 in RB cells and found that ELFN1-AS1 was mainly expressed in the cytoplasm (Figure 1F).
ELFN1-AS1 Inhibition Suppresses RB Proliferation, Migration and Invasion
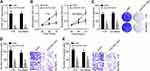
To explore the function of ELFN1-AS1 in RB, specific siRNAs targeting ELFN1-AS1 were designed and transfected. ELFN1-AS1 expression was successfully knocked down by siRNA (Figure 2A). CCK8 and colony formation results showed that ELFN1-AS1 knockdown inhibited the proliferation of RB cells (Figure 2B and C). Moreover, ELFN1-AS1 silencing suppressed the migration and invasion of RB cells (Figure 2D and E). Therefore, ELFN1-AS1 knockdown negatively affects the malignant behaviors of RB cells.
ELFN1-AS1 is the ceRNA for miR-4270 and Facilitates SBK1 Expression
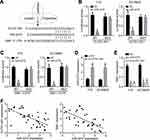
We then performed bioinformatics analysis to search the downstream target of ELFN1-AS1. We identified miR-4270 as the most potential target of ELFN1-AS1 (Figure 3A). Besides, bioinformatics analysis also indicated that SBK1 is the most potential target of miR-4270 (Figure 3A). Through luciferase reporter assays, miR-4270 mimics inhibited the luciferase activities of WT-ELFN1-AS1 and WT-SBK1 (Figure 3B and C), demonstrating their direct interactions. Moreover, ELFN1-AS1 knockdown caused upregulation of miR-4270 level (Figure 3D). However, ELFN1-AS1 knockdown or miR-4270 mimics remarkably suppressed SBK1 expression (Figure 3E). Finally, we noticed that miR-4270 expression was negatively correlated with ELFN1-AS1 or SBK1 in RB tissues (Figure 3F).
ELFN1-AS1 Promotes RB Progression Through Mediating miR-4270/SBK1 Axis
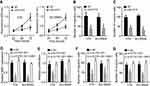
To analyze the function of miR-4270, CCK8 and Transwell assays were carried out. MiR-4270 mimics significantly repressed the proliferation, migration and invasion of RB cells (Figure 4A–C). Then, we rescued SBK1 expression in ELFN1-AS1-silenced RB cells (Figure 4D). We found that SBK1 overexpression rescued the malignant behaviors of RB cells transfected with siRNAs targeting ELFN1-AS1 (Figure 4E–G). Therefore, ELFN1-AS1 contributes to RB development by regulating miR-4270/SBK1 axis.
Discussion
In the past decades, increasing studies have demonstrated that a lot of lncRNAs are involved in human diseases, including cancer.15 Their dysregulation often affects the malignant behaviors of tumor cells.16 Besides, many lncRNAs have been discovered to be potential diagnostic or prognostic biomarkers in several cancers.17 Thus, determining the potential roles of lncRNAs in RB is of significant importance. In this study, we found that ELFN1-AS1 was highly expressed in RB tissues. ELFN1-AS1 high expression is correlated with poor prognosis. Moreover, ELFN1-AS1 knockdown suppressed the proliferation, migration and invasion of RB cells. Therefore, our data uncovered ELFN1-AS1 is a novel oncogene in RB.
Several lncRNAs have been found to regulate RB progression.18 For example, lncRNA TP73-AS1 enhances RB growth and invasiveness by decoying miR-874.18 LncRNA TMPO-AS1 overexpression contributes to RB proliferation, migration and invasion through regulating miR-199a-5p/HIF-1α axis.19 LncRNA PLAC2 dysregulation affects the apoptosis of RB cells.20 ELFN1-AS1 is found to participate in ovarian cancer, esophageal cancer and colon cancer.12–14 Nevertheless, the roles of ELFN1-AS1 in RB is undefined. Our study showed that ELFN1-AS1 expression was upregulated in RB tissues and cell lines. Moreover, ELFN1-AS1 silencing suppressed the malignant behaviors of RB cells.
Evidences have proven that lncRNAs utilize miRNAs to exert functions and miRNAs are important regulators of tumorigenesis and invasion.6,21 For example, lncRNA HOXA-AS2 sponges miR-509-3p to promote prostate cancer development.22 LncRNA DLX6-AS1 is the decoy for miR-195-5p to aggravate ovarian cancer progression.23 Additionally, lncRNA UCA1 sponges miR-138 to upregulate CCR7 expression and enhance tongue squamous cell carcinoma development.24 ELFN1-AS1 was also found to sponge miR-497 and miR-183.13,14 However, we found that ELFN1-AS1 was the sponge for miR-4270 in RB through bioinformatics analysis and luciferase reporter assay validation. Besides, we found that ELFN1-AS1 knockdown promotes miR-4270 expression in RB. MiR-4270 is a poorly researched miRNA. A recent study identified that miR-4270 suppresses liver cancer progression.25 Its role in RB is unclear. In our study, we found that miR-4270 mimics suppressed RB proliferation, migration and invasion. Therefore, miR-4270 is a new tumor suppressor in RB.
Additionally, we identified SBK1 was the downstream target of miR-4270 through bioinformatics analysis. Moreover, we showed that ELFN1-AS1 knockdown or miR-4270 mimics significantly inhibited SBK1 expression. Although two studies imply that SBK1 expression is dysregulated in multiple cancers, its function in RB is still unclear.26,27 Our study revealed that SBK1 expression was negatively correlated with miR-4270 in RB tissues. Moreover, SBK1 overexpression promotes RB cell proliferation, migration and invasion, demonstrating that SBK1 is a key oncogene in RB.
Summarily, our findings demonstrated that ELFN1-AS1 aggravates RB progression through sponging miR-4270 and facilitating SBK1 expression, which may improve the prognosis and treatment for RB.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Yun J, Li Y, Xu CT, Pan BR. Epidemiology and Rb1 gene of retinoblastoma. Int J Ophthalmol. 2011;4(1):103–109. doi:10.3980/j.issn.2222-3959.2011.01.24
2. Narang S, Mashayekhi A, Rudich D, Shields CL. Predictors of long-term visual outcome after chemoreduction for management of intraocular retinoblastoma. Clin Exp Ophthalmol. 2012;40(7):736–742.
3. Wong JR, Tucker MA, Kleinerman RA, Devesa SS. Retinoblastoma incidence patterns in the US surveillance, epidemiology, and end results program. JAMA Ophthalmol. 2014;132(4):478–483.
4. Han S, Song L, Chen Y, Hou M, Wei X, Fan D. The long non-coding RNA ILF3-AS1 increases the proliferation and invasion of retinoblastoma through the miR-132-3p/SMAD2 axis. Exp Cell Res. 2020;393(2):112087.
5. Wang X, Arai S, Song X, et al. Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription. Nature. 2008;454(7200):126–130.
6. Dong Y, Wan G, Yan P, Qian C, Li F, Peng G. Long noncoding RNA LINC00324 promotes retinoblastoma progression by acting as a competing endogenous RNA for microRNA-769-5p, thereby increasing STAT3 expression. Aging (Albany NY). 2020;12(9):7729–7746.
7. Zhao J, Li L, Han ZY, Wang ZX, Qin LX. Long noncoding RNAs, emerging and versatile regulators of tumor-induced angiogenesis. Am J Cancer Res. 2019;9(7):1367–1381.
8. Lecerf C, Le Bourhis X, Adriaenssens E. The long non-coding RNA H19: an active player with multiple facets to sustain the hallmarks of cancer. Cell Mol Life Sci. 2019;76(23):4673–4687.
9. Zhao Z, Liang S, Sun F. LncRNA DLX6-AS1 promotes malignant phenotype and lymph node metastasis in prostate cancer by inducing LARGE methylation. Front Oncol. 2020;10:1172.
10. He P, Xiong G, Guo W, Jiang G, Li Y, Li H. Long non-coding RNA CCAT2 promotes prostate cancer cell proliferation and invasion by regulating the Wnt/beta-catenin signaling pathway. Oncol Lett. 2020;20(4):97. doi:10.3892/ol.2020.11958
11. Zhang X, Zhao X, Wang Y, Xing L. Long Non-Coding RNA LINC00491 contributes to the malignancy of non-small-cell lung cancer via competitively binding to microRNA-324-5p and thereby increasing specificity protein 1 expression. Cancer Manag Res. 2020;12:6779–6793. doi:10.2147/CMAR.S264681
12. Dong L, Ding C, Zheng T, et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells treated with siRNA against ELFN1-AS1 suppress colon adenocarcinoma proliferation and migration. Am J Transl Res. 2019;11(11):6989–6999.
13. Zhang C, Lian H, Xie L, Yin N, Cui Y. LncRNA ELFN1-AS1 promotes esophageal cancer progression by up-regulating GFPT1 via sponging miR-183-3p. Biol Chem. 2020;401(9):1053–1061. doi:10.1515/hsz-2019-0430
14. Jie Y, Ye L, Chen H, et al. ELFN1-AS1 accelerates cell proliferation, invasion and migration via regulating miR-497-3p/CLDN4 axis in ovarian cancer. Bioengineered. 2020;11(1):872–882. doi:10.1080/21655979.2020.1797281
15. Wang M, Zhou L, Yu F, Zhang Y, Li P, Wang K. The functional roles of exosomal long non-coding RNAs in cancer. Cell Mol Life Sci. 2019;76(11):2059–2076. doi:10.1007/s00018-019-03018-3
16. Su S, Gao J, Wang T, Wang J, Li H, Wang Z. Long non-coding RNA BANCR regulates growth and metastasis and is associated with poor prognosis in retinoblastoma. Tumour Biol. 2015;36(9):7205–7211. doi:10.1007/s13277-015-3413-3
17. Alvarez-Dominguez JR, Hu W, Gromatzky AA, Lodish HF. Long noncoding RNAs during normal and malignant hematopoiesis. Int J Hematol. 2014;99(5):531–541. doi:10.1007/s12185-014-1552-8
18. Wang L, Wang C, Wu T, Sun F. Long non-coding RNA TP73-AS1 promotes TFAP2B-mediated proliferation, metastasis and invasion in retinoblastoma via decoying of miRNA-874-3p. J Cell Commun Signal. 2020;Volume 12(2):193–205. doi:10.1007/s12079-020-00550-x
19. Peng X, Yan J, Cheng F. LncRNA TMPO-AS1 up-regulates the expression of HIF-1alpha and promotes the malignant phenotypes of retinoblastoma cells via sponging miR-199a-5p. Pathol Res Pract. 2020;216(4):152853.
20. Song L, Qi Y, Lin M. Long noncoding RNA PLAC2 regulates PTEN in retinoblastoma and participates in the regulation of cancer cell apoptosis. Oncol Lett. 2020;19(3):2489–2494.
21. Dykxhoorn DM. MicroRNAs and metastasis: little RNAs go a long way. Cancer Res. 2010;70(16):6401–6406.
22. Xiao S, Song B. LncRNA HOXA-AS2 promotes the progression of prostate cancer via targeting miR-509-3p/PBX3 axis. Biosci Rep. 2020;40(8).
23. Kong L, Zhang C. LncRNA DLX6-AS1 aggravates the development of ovarian cancer via modulating FHL2 by sponging miR-195-5p. Cancer Cell Int. 2020;20:370.
24. Shi TT, Li R, Zhao L. Long noncoding RNA UCA1 regulates CCR7 expression to promote tongue squamous cell carcinoma progression by sponging miR-138-5p. Neoplasma. 2020.
25. Wang Y, Li CF, Sun LB, Li YC. microRNA-4270-5p inhibits cancer cell proliferation and metastasis in hepatocellular carcinoma by targeting SATB2. Hum Cell. 2020.
26. Wang P, Guo J, Wang F, Shi T, Ma D. Human SBK1 is dysregulated in multiple cancers and promotes survival of ovary cancer SK-OV-3 cells. Mol Biol Rep. 2011;38(5):3551–3559.
27. Li Z, Zhang Y, Meng L, et al. LncRNA-ENST00000501520 promotes the proliferation of malignant-transformed BEAS-2B cells induced with coal tar pitch mediated by target genes. Environ Toxicol. 2019;34(7):869–877.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.