Back to Journals » Cancer Management and Research » Volume 12
LncRNA DCST1-AS1 Sponges miR-107 to Upregulate CDK6 in Cervical Squamous Cell Carcinoma
Received 27 February 2020
Accepted for publication 10 July 2020
Published 28 August 2020 Volume 2020:12 Pages 7921—7928
DOI https://doi.org/10.2147/CMAR.S251582
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Zhigang Zhou, Na Xia
Department of Gynecological Oncology, Maternal and Child Health Hospital of Hubei Province, Wuhan City, Hubei Province, People’s Republic of China
Correspondence: Na Xia Tel +86 15902772665
Email [email protected]
Introduction: LncRNAs have been reported to play critical roles in liver cancer, while its role in other cancers remains unclear. The aim of this study was to investigate the role of DCST1-AS1 in cervical squamous cell carcinoma (CSCC).
Methods: Expression of DCST1-AS1 in CSCC tissues and non-tumor tissues from 68 CSCC patients was determined by RT-qPCR. A 5-year follow-up study was carried out to explore the prognostic value of DCST1-AS1 for CSCC. Overexpression of DCST1-AS1 and miR-107 was achieved in CSCC tissues to explore the interaction between them. The roles of DCST1-AS1, miR-107 and CDK6 in regulating the proliferation and viability of CSCC cells were assessed by cell proliferation and viability assays, respectively.
Results: We found that DCST1-AS1 was upregulated in CSCC and predicted poor survival. RNA interaction prediction showed potential interaction between DCST1-AS1 and miR-107. However, overexpression experiments revealed no significant interaction between them. Moreover, overexpression of DCST1-AS1 led to upregulate CDK6 and increase cell proliferation rate, while overexpression of miR-107 played an opposite role and attenuate the effects of overexpression of DCST1-AS1.
Conclusion: DCST1-AS1 may sponge miR-107 to upregulate CDK6 in CSCC.
Keywords: DCST1-AS1, miR-107, cervical squamous cell carcinoma, CSCC, CDK6, proliferation
Introduction
Among females, cervical cancer ranks the fourth place for both mortality and incidence among all types of malignancy worldwide.1,2 Human papillomavirus (HPV) infections are the major causes of cervical cancer.3 In effect, HPV infection screening and vaccination are the main approaches to prevent cervical cancer.3 However, HPV vaccination is not helpful for the prevention of cervical cancer among females who have already been infected.4 Moreover, cervical cancer can also affect HPV-negative females,5 indicating the existence of other factors that are involved in the pathogenesis of this malignancy. Compared to HPV-positive cervical cancer, HPV-negative cases are more malignant and the prognosis is even worse.6 Therefore, novel therapeutic and treatment approaches are still needed.
Cyclin-dependent kinase 6 (CDK6) is a type of cyclin-dependent kinase that functions with its partner CDK4 to regulate cell cycle progression, specifically at G1 phase progression and during the transition from G1 to S phase.7 Inhibition of the expression of CDK6 is considered as a promising therapeutic target for cancer treatment.8,9 In effect, certain tumor-suppressive miRNAs, such as miR-107, can target CDK6 to suppress cancer progression.10 It has been reported that long (>200 nt) non-coding RNAs (lncRNAs) can interact with miRNAs to participate in cancer biology. DCST1-AS1 (DCST1 antisense RNA 1) is a recently identified oncogenic lncRNA in liver cancer.12 Our bioinformatics analysis revealed the potential interaction between DCST1-AS1 and miR-107. This study was therefore carried out to investigate the interactions among DCST1-AS1, miR-107 and CDK6 in cervical squamous cell carcinoma (CSCC), a major subtype of cervical cancer.
Materials and Methods
CSCC Patients
A total of 68 CSCC patients (age range from 45 to 67 years old, mean age 55.1 ± 6.0 years old) selected from the 117 CSCC patients admitted to Maternal and Child Health Hospital of Hubei Province between January 2012 and July 2014. This study was approved by the Ethics Committee of aforementioned hospital (No.32876MCHHHP2011). Inclusion criteria: 1) newly diagnosed CSCC patients; 2) therapies were not initiated before admission; 3) after admission, patients completed treatment and a 5-year follow-up. Exclusion criteria: 1) clinical disorders other than CSCC were diagnosed; 2) recurrent CSCC. Based on their medical record, the 68 patients included 18 cases of HPV-11 positive, 16 cases of HPV-16 positive, 25 cases of HPV-18 positive and 9 cases of HPV negative. All patients were informed of the principle of experiments and signed the informed consent.
Biopsy
Before the initiation of therapies, cervical biopsy was performed under the guidance of MRI to collect tumor (CSCC) tissues for diagnosis. For the purpose of this study, adjacent non-tumor tissues were also collected during biopsy. All tissue specimens were confirmed by histopathological exams.
Treatment
The staging of the 68 CSCC patients was performed following the AJCC criteria. The 68 CSCC patients included 12, 18, 17 and 21 cases at clinical stage I, II, III, and IV, respectively. The patients were also staged based on the FIGO system, and 11, 19, 16 and 22 patients were classified into stage I–IV, respectively. The patients were treated according to their clinical stages and health conditions. Surgical resection, chemo- and radio-therapies, or targeted drug therapies were performed.
Follow-Up
From the day of admission, the 68 patients were monitored for 5 years or until their death to record their survival conditions. Follow-up was performed through telephone or outpatient visit. Patients who lost to follow-up and died of causes unrelated to CSCC were excluded from the study.
SiHa Cells and Transfections
Human CSCC cell line SiHa (ATCC, USA) was used. Cell culture conditions were 37°C, 95% humidity and 5% CO2. Cell culture medium was a mixture composed of 90% Eagle’s Minimum Essential Medium and 10% FBS. Following transfections were performed using cells harvested at 75–85% confluence. MiR-107 mimic (5ʹ-AGCAGCAUUGUACAGGGCUAUCA-3ʹ) and miRNA negative control (5ʹ-GUACCGUGGUAUGCUAGGUGCAC-3ʹ) were obtained from Sigma-Aldrich (USA). CDK6 (NCBI Accession: KJ905166.1) and DCST1-AS1 (NCBI Accession: NR_040772.1) expression vectors (pcDNA3.1 as backbone) were obtained from Sangon (Shanghai, China). Lipofectamine 2000 (Sigma-Aldrich) was used to transfect 10 nM vector (empty vector as NC group) or 50 nM miRNA (NC miRNA as NC group) into 3×106 cells. Vector or miRNAs were first mixed with Lipofectamine 2000 to form transfection mixture. Cells were then incubated with the transfection mixture at 37°C for 6 h, followed by washing with fresh cell culture medium to terminate transfections. Following experiments were performed using cells harvested at 24 h post-transfection. Untransfected cells were used as the Control (C).
RNA–RNA Interaction Prediction
The interaction between DCST1-AS1 and miR-107 was predicted using an online RNA–RNA interaction prediction program named IntaRNA 2.0 (http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp). The sequence of DCST1-AS1 was used as long sequence and miR-107 was short sequence. All other parameters were default.
Dual-Luciferase Reporter Assay
Transfections were performed through the same way as mentioned above. It is worth noting that psiCHECK-1 vector (Promega) instead of pcDNA3.1 vector was used in this assay. Two different co-transfections, including DCST1-AS1 + NC miRNA (NC group) and DCST1-AS1 + miR-107 (miR-107 group), were performed. Renilla luminescence was normalized to the expression of firefly luminescence.
RNA Extraction and Digestion
Total RNAs were extracted from SiHa cells (harvested at 24 h post-transfection) and biopsies (ground in liquid nitrogen) using RNAzol reagent (Sigma-Aldrich). All operations were performed in strict accordance with the manufacturer’s instructions. MiRNAs were harvested by precipitation using 85% ethanol. RNA samples were digested with DNase I at 37°C to remove genomic DNAs.
RT-qPCR (Real-Time Polymerase Chain Reaction)
QuantiTect Reverse Transcription Kit (QIAGEN) was used to perform reverse transcriptions (RTs) of total RNA with poly (T) as primer. With cDNA as template, qPCR reactions were prepared using the SYBR Green Master Mix (Bio-Rad, USA). The expression levels of DCST1-AS1 and CDK6 mRNA were measured with GAPDH as the endogenous control. All-in-One™ miRNA qRT-PCR Detection Kit (Genecopoeia) was used to measure the expression levels of mature miR-107. All steps were performed in strict accordance with the manufacturer’s instructions. Three replicates were conducted for each reaction and data were processed following 2−ΔΔCT method.
Western Blot
SiHa cells were harvested at 24 h post-transfection and cells were counted. Cell pellets containing 105 SiHa cells were resuspended in 1 mL RIPA solution (Cell Signaling Technology) to extract total proteins. Concentrations of total proteins were measured using BCA assay (Cell Signaling Technology). In a 95°C incubator, protein samples were denatured for 10 min, followed by electrophoresis using 12% SDS-PAGE gel. After that, proteins were transfected to PVDF membranes and blocking was performed in 5% non-fat milk (PBS) at room temperature for 1 h. GAPDH (1:1800, ab37168, Abcam) and CDK6 (1:1800, ab151247, Abcam) rabbit polyclonal primary antibodies were used to incubate with the membranes at 4°C for 18 h, followed by incubation with HRP (IgG) (1:1800; ab6721; Abcam) goat anti-rabbit secondary antibody at 24°C for 2 h. ECL Western Blotting Substrate (Promega Corporation) was dropped onto the membranes and MYECL™ Imager (Bio-Rad) was used to detect signals. Image J v1.46 software was used for data normalizations.
Cell Proliferation Assay (CCK-8)
SiHa cells were harvested at 24 h post-transfection and cells were counted. Cell pellets containing 5×103 SiHa cells were resuspended in 1 mL medium. The cell suspensions were transferred to a 96-well plate (0.1 mL per well), followed by cell culture under aforementioned conditions. CCK-8 solution (10 uL, Sigma-Aldrich) was added into each well at 4 h before the collection of cells. Cells were collected at 24, 48, 72 and 96 h post-transfection. After cell collection, OD values were measured at 450 nM using Accuris SmartReader 96 Microplate Reader following the manufacturer’s instructions.
MTT Assay
SiHa cells were harvested at 24 h post-transfection and were transferred to a 96-well plate with 0.1 mL medium containing 3000 cells per well. Cells were cultivated for 96 h under aforementioned conditions, followed by the addition of MTT to 10%. OD values at 570 nm were measured 2 h later to represent cell viability.
Statistical Analysis
All experiments were repeated 3 times. Mean values were calculated to be used in statistical analysis. Paired t-test was used to compare differences between non-tumor and CSCC tissues. Differences among multiple groups were explored using ANOVA (one-way) and Tukey’s test. With the median value of DCST1-AS1 expression in CSCC tissue as cutoff value, patients were divided into high and low (n = 34) level groups, followed by plotting survival curves using K-M plotter and comparison of survival curves using Log rank test. P < 0.05 was statistically significant.
Results
Overexpression of DCST1-AS1 in CSCC Tissues Predicted Poor Survival
The differential expression of DCST1-AS1 in CSCC patients was first detected by qPCR, followed by paired t-test analysis to compare non-tumor and CSCC tissues. Compared to non-tumor tissues, the expression levels of DCST1-AS1 were significantly higher in CSCC tissues (Figure 1A, p < 0.0001). Survival curves were plotted and compared. Compared to patients in the low DCST1-AS1 level group, patients in high DCST1-AS1 level group experienced significantly lower overall survival rate (Figure 1B). It is worth noting that no significant differences in treatment approaches, clinical stages, age, BMI and living habits were observed between the high and low DCST1-AS1 level groups.
The Expression of DCST1-AS1 in CSCC Was Not Affected by Clinical Stage and HPV Infections
Comparisons of the expression levels of DCST1-AS1 among patients at 4 clinical stages and patients in 4 HPV infection groups were performed by ANOVA (one-way). As shown in Figure 2, the expression levels of DCST1-AS1 were not significantly different among 4 clinical stage groups (Figure 2A) and 4 HPV infection groups (Figure 2B). It is worth noting that the expression levels of DCST1-AS1 were also not significantly different among 4 FIGO stages (data not shown).
DCST1-AS1 and miR-107 Interact with Each Other but Not Regulate the Expression of Each Other
RNA interaction prediction performed using IntaRNA 2.0 showed that DCST1-AS1 and miR-107 could form strong base pairing (Figure 3A). Dual-luciferase reporter assay was then performed to further explore the interaction between DCST1-AS1 and miR-107. Compared to SiHa cells transfected with DCST1-AS1 + NC miRNA (NC group), cells transfected with DCST1-AS1 + miR-107 (miR-107 group) showed significantly lower relative luciferase activity (Figure 3B, p < 0.05), indicating direct interaction. SiHa cells were transfected with DCST1-AS1 expression vector or miR-107 mimic. Overexpression of DCST1-AS1 and miR-107 was confirmed by qPCR at 24 h post-transfection (Figure 3C, p < 0.05). Compared to C and NC (NC miRNA or pcDNA3.1 vector transfection) groups, overexpression of DCST1-AS1 did not affect the expression of miR-107 (Figure 3D). Moreover, overexpression of miR-107 also did not affect the expression of DCST1-AS1 (Figure 3E).
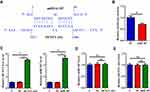 |
Figure 3 DCST1 antisense RNA 1 (DCST1-AS1) and miR-107 interact with each other but not regulate the expression of each other. RNA interaction prediction performed using IntaRNA (http://rna.informatik.uni-freiburg.de/IntaRNA/Input.jsp) showed that DCST1-AS1 and miR-107 can form strong base paring (A). Dual-luciferase reporter assay was the performed to further explore the interaction between DCST1-AS1 and miR-107. Compared to SiHa cells transfected with DCST1-AS1 + NC miRNA (NC group), cells transfected with DCST1-AS1 + miR-107 (miR-107 group) showed significantly lower relative luciferase activity (B). SiHa cells were transfected with DCST1-AS1 expression vector or miR-107 mimic. Overexpression of DCST1-AS1 and miR-107 was confirmed by polymerase chain reaction (QPCR) at 24h post-transfection (C). The effects of the overexpression of DCST1-AS1 on miR-107 (D) and the effects of overexpression of miR-107 on DCST1-AS1 (E) were analyzed by qPCR. Experiments were repeated 3 times and mean values were expressed. *p < 0.05. |
Overexpression of DCST1 -AS1 Resulted in the Upregulation of CDK6
CDK6 is a downstream target of miR-107. qPCR and Western blot were performed to evaluate the effects of overexpression of DCST1-AS1 and miR-107 on the expression of CDK6 at both mRNA (Figure 4A) and protein (Figure 4B) levels. Compared to C and NC (NC miRNA or pcDNA3.1 vector transfection) groups, overexpression of DCST1-AS1 resulted in the upregulation of CDK6, while overexpression of miR-107 played an opposite role and attenuate the effects of overexpression of DCST1-AS1 (p < 0.05).
CST1-AS1 Regulated Cell Proliferation Through the Axis of miR-107/CDK6
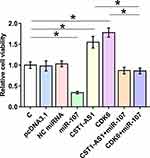
The effects of overexpression of CST1-AS1, miR-107 and CDK6 on the proliferation and viability of SiHa cells were evaluated by CCK-8 assay (Figure 5) and MTT assay (Figure 6), respectively. Compared to C and NC (NC miRNA or pcDNA3.1 vector transfection) groups, overexpression of DCST1-AS1 and CDK6 increased proliferation and viability of SiHa cells, while overexpression of miR-107 played an opposite role and attenuated the effects of overexpression of DCST1-AS1 and CDK6 (p < 0.05).
Discussion
The functions of DCST1-AS1 were investigated in CSCC. To the best of our knowledge, this study is the first to report the upregulation of DCST1-AS1 in CSCC. We also provided evidence that DCST1-AS1 may be an endogenous sponge of miR-107 and can upregulate its downstream target CDK6 to promote cancer cell proliferation.
The functionality of DCST1-AS1 has only been investigated in liver cancer.12 DCST1-AS1 is upregulated in liver cancer and silencing of DCST1-AS1 led to inhibited cancer cell proliferation and promoted apoptosis.12 In this study, we found that DCST1-AS1 was also upregulated in CSCC. In addition, overexpression of DCST1-AS1 led to increased proliferation rate of CSCC cells. Therefore, DCST1-AS1 is also an oncogenic lncRNA in CSCC.
MiR-107 plays oncogenic or tumor-suppressive roles in different types of cancer.13–15 MiR-107 is upregulated in esophageal squamous cell carcinoma and overexpression of miR-107 targets Cdc42 to suppress cancer progression.13 In contrast, miR-107 is upregulated in pancreatic ductal adenocarcinoma and inhibition of miR-107 results in suppressed tumor metastasis through the inactivation of the PI3K/Akt signaling and the activation of PTEN.14 A recent study reported that miR-107 was downregulated in CSCC and inhibited cancer cell invasion by activating the ATR/Chk1 pathway by targeting MCL1,15 which suggested its tumor-suppressive roles in CSCC. Consistent with this study, our study also reported the tumor-suppressive roles of miR-107 in suppressing cancer cell proliferation.
It was reported that miR-107 targeted CDK6 to induce G1 arrest in gastric cancer cells.10 In our study, we also observed the downregulation of CDK6 and decreased proliferation rate of CSCC cells after the overexpression of miR-107. Therefore, miR-107 may also target CDK6 in CSCC cells. We found that DCST1-AS1 can interact with miR-107, while DCST1-AS1 is unlikely a target of miR-107 due to the fact that overexpression of DCST1-AS1 and miR-107 did not affect the expression of each other. It is known that lncRNAs may sponge miRNAs to upregulate their downstream targets.16,17 We observed the upregulation of CDK6 after the overexpression of DCST1-AS1. Therefore, DCST1-AS1 may sponge miR-107 to upregulate CDK6, thereby promoting cancer cell proliferation.
In conclusion, DCST1-AS1 is upregulated in CSCC and may sponge miR-107 to upregulate CDK6.
Data Sharing Statement
The analyzed data sets generated during the study were all presented in this paper.
Disclosure
The authors declare that they have no competing interests.
References
1. Small W
2. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
3. Wright TC, Stoler MH, Behrens CM, et al. Primary cervical cancer screening with human papillomavirus: end of study results from the ATHENA study using HPV as the first-line screening test. Gynecol Oncol. 2015;136(2):189–197. doi:10.1016/j.ygyno.2014.11.076
4. Lowy DR. HPV vaccination to prevent cervical cancer and other HPV-associated disease: from basic science to effective interventions. J Clin Invest. 2016;126(1):5–11. doi:10.1172/JCI85446
5. Rodriguez‐Carunchio L, Soveral I, Steenbergen RDM, et al. HPV‐negative carcinoma of the uterine cervix: a distinct type of cervical cancer with poor prognosis. BJOG. 2015;122(1):119–127. doi:10.1111/1471-0528.13071
6. Nicolás I, Marimon L, Barnadas E, et al. HPV-negative tumors of the uterine cervix. Mod Pathol. 2019;32(8):1189–1196.
7. Malumbres M, Barbacid M. Cell cycle, CDKs and cancer: a changing paradigm. Nat Rev Cancer. 2009;9(3):153–166. doi:10.1038/nrc2602
8. Sherr CJ, Beach D, Shapiro GI. Targeting CDK4 and CDK6: from discovery to therapy. Cancer Discov. 2016;6(4):353–367. doi:10.1158/2159-8290.CD-15-0894
9. Otto T, Sicinski P. Cell cycle proteins as promising targets in cancer therapy. Nat Rev Cancer. 2017;17(2):93–115. doi:10.1038/nrc.2016.138
10. Feng L, Xie Y, Zhang H, et al. miR-107 targets cyclin-dependent kinase 6 expression, induces cell cycle G1 arrest and inhibits invasion in gastric cancer cells. Med Oncol. 2012;29(2):856–863. doi:10.1007/s12032-011-9823-1
11. Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet. 2016;17(5):272–283. doi:10.1038/nrg.2016.20
12. Chen J, Wu D, Zhang Y, et al. LncRNA DCST1-AS1 functions as a competing endogenous RNA to regulate FAIM2 expression by sponging miR-1254 in hepatocellular carcinoma. Clin Sci (Lond). 2019;133(2):367–379. doi:10.1042/CS20180814
13. Sharma P, Saini N, Sharma R. miR-107 functions as a tumor suppressor in human esophageal squamous cell carcinoma and targets Cdc42. Oncol Rep. 2017;37(5):3116–3127. doi:10.3892/or.2017.5546
14. Xiong J, Wang D, Wei A, et al. Deregulated expression of miR-107 inhibits metastasis of PDAC through inhibition PI3K/Akt signaling via caveolin-1 and PTEN. Exp Cell Res. 2017;361(2):316–323. doi:10.1016/j.yexcr.2017.10.033
15. Zhou C, Li G, Zhou J, et al. miR-107 activates ATR/Chk1 pathway and suppress cervical cancer invasion by targeting MCL1. PLoS One. 2014;9(11):e111860. doi:10.1371/journal.pone.0111860
16. Liang WC, Fu WM, Wong CW, et al. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6(26):22513–22525. doi:10.18632/oncotarget.4154
17. D’Angelo D, Mussnich P, Sepe R, et al. RPSAP52 lncRNA is overexpressed in pituitary tumors and promotes cell proliferation by acting as miRNA sponge for HMGA proteins. J Mol Med (Berl). 2019;97(7):1019–1032. doi:10.1007/s00109-019-01789-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.