Back to Journals » OncoTargets and Therapy » Volume 12
LncRNA CCAT1 Promotes Colorectal Cancer Tumorigenesis Via A miR-181b-5p/TUSC3 Axis
Authors Chen S, Liu Y, Wang Y, Xue Z
Received 22 May 2019
Accepted for publication 12 September 2019
Published 5 November 2019 Volume 2019:12 Pages 9215—9225
DOI https://doi.org/10.2147/OTT.S216718
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Takuya Aoki
Si Chen,1 Yan Liu,2 Yuanyuan Wang,3 Zhaoping Xue4
1Department of Colorectal and Anal Surgery; 2Department of Spine Surgery; 3Department of Dermatology; 4Anaesthesia Wake Room, First Hospital of Jilin University, Changchun, Jilin 130021, People’s Republic of China
Correspondence: Zhaoping Xue
Anaesthesia Wake Room, First Hospital of Jilin University, 71 Xinmin Street, Changchun, Jilin 130021, People’s Republic of China
Tel +86 15804301580
Email [email protected]
Yan Liu
Department of Spine Surgery, First Hospital of Jilin University, 71 Xinmin Street, Changchun, Jilin 130021, People’s Republic of China
Tel +86 13804331531
Email [email protected]
Aim: The aim was to determine the function and molecular mechanism of long non-coding RNA colon cancer associated transcript-1(lncRNA CCAT1) in the development of colorectal cancer (CRC).
Methods: CCAT1 mRNA expression levels were determined in CRC tissues and cells using reverse transcription-quantitative polymerase chain reaction. Cell Counting Kit-8 and colony formation assays were used to examine the effects of CCAT1 on the proliferation of CRC cells. Luciferase reporter gene analysis was used to confirm the target gene of microRNA-181b-5p (miR-181b-5p) in CRC cells. Tumor xenografts were subsequently used to investigate the role of CCAT1 in CRC growth in vivo.
Results: The relative mRNA expression levels of CCAT1 were significantly higher in CRC tissues and cell lines compared with the normal tissues or cells. CCAT1 knockdown significantly inhibited CRC cell proliferation in vitro and in vivo. Bioinformatics and luciferase reporter assays showed that miR-181b-5p was a direct target of CCAT1, and the expression of miR-181b-5p was negatively correlated with the expression of CCAT1 in CRC tissues. Furthermore, CCAT1 positively regulated the level of tumor suppressor candidate 3 (TUSC3) by competing with miR-181b-5p in CRC cells.
Conclusion: These data suggested that lncRNA CCAT1 promoted colorectal cancer tumorigenesis via a miR-181b-5p/TUSC3 axis.
Keywords: LncRNA, CCAT1, CRC, miR-181b-5p, TUSC3
Introduction
Colorectal cancer (CRC) is the third most common cancer worldwide, and results in > 1 million deaths annually.1,2 Despite numerous advances in therapy for treating CRC, including surgery, chemotherapy, irradiation or combined therapy,3–6 clinical data studies have shown that CRC prognosis remains poor.7,8 Therefore, an improved understanding of the molecular mechanisms underlying CRC tumorigenesis may provide novel insights into the pathogenesis of CRC and thus improve the therapeutic options.
Long non-coding RNAs (lncRNAs) are a family of RNAs >200 nucleotides in length which do not code for proteins.9,10 LncRNAs regulate many hallmarks of cancer, such as proliferation, migration and apoptosis.11–15 Aberrant expression of lncRNAs has been demonstrated in numerous human diseases including many different types of cancer.16,17 Colon cancer associated transcript-1 (CCAT1) is consistently upregulated in and is associated with pathogenesis of a number of malignancies, including gastric carcinoma, colon cancer, gallbladder cancer and hepatocellular carcinoma.18–21 Recently, lncRNAs have been demonstrated to function as competing endogenous RNAs (ceRNA) by competitively binding common microRNAs (miRNAs).22–24 However, the exact molecular mechanisms underlying the involvement of CCAT1 in the development of CRC remains unknown.
Tumor suppressor candidate 3 (TUSC3) is located on the chromosomal band 8p22 and was originally identified as a potential tumor suppressor in prostate cancer.25–27 Recent studies reported that the mRNA and protein expression levels of TUSC3 were significantly upregulated in CRC tissues.28,29 Tang et al28 found that knockdown of TUSC3 inhibited the cell viability, migration and invasion of CRC cells, and overexpression of TUSC3 had the promotion effects on CRC cells. However, the precise upstream regulation mechanism of TUSC3 in carcinogenesis requires further investigation.
The results of the present study demonstrated that knockdown of CCAT1 significantly decreased cell proliferation and growth of CRC. Furthermore, miR-181b-5p directly binds to the 3ʹ untranslated regions (UTRs) of both CCAT1 and TUSC3 in CRC cells. The novel regulatory function of CCAT1/miR-181b-5p/TUSC3 axis in CRC may provide a potential target for treatment of CRC.
Materials And Methods
Clinical Samples
Human CRC tissues and adjacent healthy tissues were obtained from the First Hospital of Jilin University (Changchun, China) between March 2014 and December 2016, and CRC samples were pathologically confirmed. The written informed consent was obtained from each patient, and that this was conducted in accordance with the Declaration of Helsinki. A total of 27 pairs of primary CRC tissues and matching normal tissues were obtained. The samples were stored at −80°C immediately after surgical resections. The present study was approved by The Ethics Committee of the First Hospital of Jilin University. All experimental animals received care in compliance with the Principles of Laboratory Animal Care.
Cell Culture
Human colon immortalized cell line FHC, and four colorectal cancer cell lines, HCT116, HT29, SW480, LoVo, were purchased from The Cell Bank of Type Culture Collection of the Chinese Academy of Sciences. The FHC cells were cultured in Dulbecco’s modified Eagle’s medium (45%); Ham’s F12 medium (45%); 25 mM HEPES; 10 ng/mL cholera toxin; 0.005 mg/mL insulin; 0.005 mg/mL transferrin; 100 ng/mL hydrocortisone; 20 ng/mL human recombinant EGF (Thermo Fisher PHG0311); fetal bovine serum (FBS, 10%), LoVo cells were maintained in F-12K medium (Invitrogen; Thermo Fisher Scientific, Inc.), SW480 and HT29 cells were cultured in RPMI-1640 (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS. All cells were maintained at 37°C with 5% CO2 in a humidified incubator.
Cell Transfection
Short hairpin RNA (shRNA) targeting CCAT1 (shCCAT1) was used to silence CCAT1. The shRNA sequence was, 5ʹ-CACCCCATTCCATTCATTTCTCTTTCCTATTCAAGAGATAGGAAAGAGAAATGAATGGAATGGTTTTTTG-3ʹ. Cells were transfected for 48 h with Lipofectamine® 2000 for 48 h according to the manufacturer’s protocol. Transfected cells were used for all subsequent experiments.
RNA extraction and reverse transcription-quantitative (RT-qPCR)
TRIzol Reagent (Invitrogen; Thermo Fisher Scientific, Inc.) was used to extract the total RNA from CRC tissues or cells according to the manufacturer’s protocol. Subsequently, cDNA was synthesized using PrimeScript RT Reagent kit (Takara Bio, Inc.). qPCR was performed using a SYBR Green PCR kit (Takara Bio, Inc.). U6 and GAPDH were used as the internal controls. The sequences of the primers were: CCAT1 forward, 5ʹ-TTTATGCTTGAGCCTTGA-3ʹ and reverse, 5ʹ-CTTGCCTGAAATACTTGC-3ʹ; miR-181b-5p forward, 5ʹ-AACATTCATTGCTGUCGGTGGGT-3ʹ and reverse, 5ʹ-GGCCAACCGCGAGAAGATGTTTTTTTTT-3ʹ; TUSC3 forward, 5ʹ- GATGTGATTTGGACCATGGCA-3ʹ and reverse, 5ʹ-TCCTGTGGCACACACTGAAT-3ʹ; and U6 forward, 5ʹ-AGAGCCTGTGGTGTCCG-3ʹ and reverse, 5ʹ-CATCTTCAAAGCACTTCCCT-3ʹ. The relative expression levels were calculated using the 2−ΔΔCq method.
Cell Proliferation Analysis
Cell Counting Kit-8 analysis (CCK-8; NeoBioscience) was used to detect the proliferative ability of cells. The LoVo cells were seeded at 6×103/well and SW480 cells were seeded at 5×103/well into 96-well plates, and then transfected for 48 h. CCK-8 reagent was added to each well and further incubated for 4 h at 37°C with 5% CO2. The optical density value at 450 nm was detected using an enzyme labeling instrument.
Colony Formation Analysis
LoVo and SW480 cells following transfection were seeded in 6-well plates with 500 cells in each well. The media was replaced every 3 days. Subsequently, the cells were fixed with 4% paraformaldehyde at 4°C for 1 h and stained with 0.1% crystal violet staining solution (Beyotime Institute of Biotechnology) after incubation for 15 days. The number of clones containing >50 cells were counted, and images were taken.
Western Blotting
The CRC tissues and cells were lysed using RIPA Lysis Buffer (Invitrogen; Thermo Fisher Scientific, Inc.), and the protein concentrations were determined using a Bicinchoninic acid assay. Total protein (20 µg) was resolved on a 10% gel using SDS-PAGE and transferred to a PVDF membrane (EMD Millipore). The membranes were blocked with 5% skimmed milk in TBS-Tween (TBST) buffer overnight at 4°C and incubated with primary antibodies against TUSC3 or GAPDH (Abcam) at 4°C overnight. Subsequently, the membranes were washed three times using TBST after which the membranes were incubated with horseradish peroxidase-conjugated secondary antibody for 2 h according to the manufacturer’s protocol. Signals were visualized using enhanced chemiluminescence (PerkinElmer).
Luciferase Reporter Assay
The CCAT1 3ʹ-UTR containing wild-type (CCAT1 wt) or mutant (CCAT1 Mut) of miR-181b-5p seed sequence fragment was cloned into pcDNA3.1/eGFP at the BamHI and EcoRI restriction sites, downstream of the eGFP coding region. LoVo cells were seeded into 24-well plates and were co-transfected with the luciferase reporter plasmids and miR-181b-5p mimics or mimics control using Lipofectamine® 2000 Reagent for 48 h. Subsequently, cells were harvested, and the relative luciferase activity was detected using a F-4500 Fluorescence Spectrophotometer (Hitachi, Ltd.) according to the manufacturer’s protocol. TUSC3 3ʹ-UTR wild-type (TUSC3-Wt) or TUSC3 3ʹ-UTR mutant (TUSC3-Mut) were also co-transfected with miR-181b-5p mimics or mimics control into LoVo cells using Lipofectamine® 2000 Reagent. The cells were harvested, and the luciferase activity was measured after 48 h of culture.
RNA Immunoprecipitation Assay (RIP)
RIP assay was used to prove the sponge function of lncRNA CCAT1 by Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, MA, USA) according to the manufacture’s protocol. Briefly, cells were transfected with miR-181b-5p mimics, Vector-CCAT1 or controls, and then were lysed with lysis buffer. Subsequently, cell lysates were incubated with anti-Ago2 (Abcam) or anti-IgG (Abcam) and protein A/G magnetic beads. Co-precipitated RNAs were detected by RT-qPCR.
Xenograft Tumor Study In Vivo
Six-week-old male BALB/c nude mice were purchased from Beijing Huafu Kang Biological Technology Co., Ltd. A total of 5x106 transfected LoVo cells were subcutaneously injected into the posterior flank of nude mice. The length (L) and width (W) of the tumors were measured every 6 days with calipers, and the tumor volume in nude mice was calculated according to the following formula: tumor volume (mm3) = (LxW2)/2. The mice were sacrificed by cervical dislocation under anesthesia with diethyl ether after 30 days. The tumor tissues were harvested, weighed, and used to detect miR-181b-5p, CCAT1 and TUSC3 expression. The animal experiments were approved by The Ethics Committee of The First Hospital of Jilin University (Changchun, China)
Statistical Analysis
Statistical analyses were performed using SPSS version 20.0 (IBM, Corp.) Data are presented as the mean ± standard deviation. Differences between groups were analyzed using a Student’s t-test or a one-way ANOVA. Correlations between miR-181b-5p and CCAT1 expression were assessed using Pearson’s correlation coefficient. P<0.05 was considered to indicate a statistically significant difference.
Results
CCAT1 Is Upregulated In CRC Samples And Cell Lines
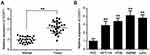
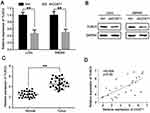
To investigate the potential role of lncRNA CCAT1 in CRC, the relative expression of CCAT1 in 27 pairs of CRC tissues and their respective adjacent normal tissues. The results showed that the expression of CCAT1 in CRC tissues was significantly higher compared with the normal tissues (Figure 1A). Similarly, the expression of CCAT1 in CRC cell lines (HCT116, HT29, SW480 and LoVo) was significantly higher compared with the normal colon normal cell line, FHC (Figure 1B). These data suggest that CCAT1 may be associated with tumorigenesis of CRC.
CCAT1 knockdown suppressed cell proliferation and colony formation of CRC in vitro.
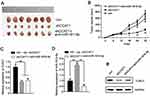
To determine whether knockdown of CCAT1 affected CRC cell proliferation in vitro, shCCAT1 or control shRNA was transfected into LoVo and SW480 cells. RT-qPCR showed that shCCAT1 significantly decreased CCAT1 expression in both the LoVo and SW480 cells (Figure 2A). Subsequently, CCK-8 assay showed that downregulation of CCAT1 protein expression significantly decreased cell proliferation compared with the control LoVo and SW480 cells, respectively (Figure 2B and C). Furthermore, the number of colonies formed by LoVo and SW480 cells was positively associated with the expression level of CCAT1 (Figure 2D). These results suggest that CCAT1 can promote the growth of colorectal cancer cells.
CCAT1 Directly Interacts With miR-181b-5p In CRC
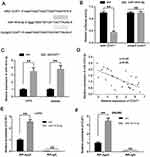
To determine the molecular mechanisms underlying CCAT1 in colorectal cancer cells, online bioinformatics software starBase version 2.0 was used to predict potential lncRNA-miRNA interactions. The results suggested a putative miR-181b-5p binding site located in the 3ʹ-UTR of the CCAT1 mRNA (Figure 3A). A dual luciferase reporter assay was used to confirm binding in LoVo cells. The data showed that miR-181b-5p mimics resulted in significantly lower luciferase activity when transfected with wild-type 3ʹ-UTR of CCAT1; however, there was no significant effect on the luciferase when transfected with the mutated 3ʹ-UTR of CCAT1 in LoVo cells (Figure 3B). The effect of CCAT1 knockdown on the expression levels of miR-181a-5p in LoVo and SW480 cells were determined. The expression levels of miR-181a-5p was significantly increased in the shCCAT1 group compared with the control group (Figure 3C). Pearson’s correlation analysis showed there was a correlation between CCAT1 and miR-181b-5p expression in CRC tissues. As shown in Figure 3D, the expression levels of miR-181b-5p was inversely correlated with the mRNA expression levels of CCAT1 in CRC tissues. Moreover, RIP assay showed that CCAT1 was substantially enriched by miR-181b-5p overexpression with anti-Ago2 in LoVo and SW480 cells. This suggested that there was an endogenous interaction between CCAT1 and miR-181b-5p, and that CCAT1 might work as a miR-181-5p sponge (Figure 3E and F). Together, these data suggest that CCAT1 directly interacts with miR-181b-5p in CRC.
Anti-miR-181b-5p reverses the effect of CCAT1 knockdown on growth of CRC.
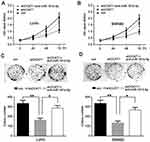
To examine whether anti-miR-181b-5p could rescue CCAT1 knockdown mediated inhibition of tumorigenesis in CRC, anti-miR-181b-5p or negative control were co-transfected with shCCAT1 into LoVo and SW480 cells. A CCK-8 assay was used to determine cell proliferation. As shown in Figure 4A and B, the inhibitory effects of shCCAT1 on cell proliferation were partly rescued by the anti-miR-181b-5p in LoVo and SW480 cells. In addition, a colony formation assay showed similar results (Figure 4C and D). Taken together, these results suggest that anti-miR-181b-5p may rescue CCAT1 knockdown-mediated effects in CRC cells. CCAT1 knockdown suppressed CRC growth partly through binding miR-18b-5p.
TUSC3 Is A Direct Target Of miR-181b-5p
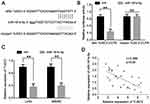
A putative miR-181b-5p binding site located in the 3ʹ-UTR of TUSC3 mRNA which was identified by Pictar and TargetScan (Figure 5A). A dual luciferase reporter assay was used to confirm the binding in LoVo cells. The data showed that miR-181b-5p mimics resulted in a significant decrease in relative luciferase activity of TUSC3-wt but not in TUSC3-mut (Figure 5B). These results suggest that miR-181b-5p may directly bind to the 3ʹ-UTR of TUSC3. The effects of miR-181b-5p on TUSC3 mRNA expression were also determined using RT-qPCR. As shown in Figure 5C, TUSC3 mRNA levels in the cells transfected with miR-181b-5p mimics was significantly lower compared with the control cells. In addition, Pearson’s correlation analysis showed that there was an inverse correlation between TUSC3 and miR-181b-5p expression in CRC tissues (Figure 5D). These data suggest that TUSC3 is a direct target of miR-181b-5p in CRC cells.
CCAT1 Positively Regulates TUSC3 Expression In CRC
To investigate whether CCAT1 regulated TUSC3 expression in CRC, shCCAT1 or control shRNA were transfected into LoVo and SW480 cells. The data shows that the mRNA and protein expression levels of TUSC3 were significantly lower in the shCCAT1 group compared with the control LoVo and SW480 cells (Figure 6A and B). The expression levels of TUSC3 mRNA were measured in CRC tissues and compared with the normal tissues using RT-qPCR. As shown in Figure 6C, TUSC3 mRNA expression levels were significantly increased in CRC tumor tissues compared with the normal tissues. In addition, CCAT1 expression was positively correlated with TUSC3 level in CRC tissues (Figure 6D). Therefore, lncRNA CCAT1 positively regulates TUSC3 expression in CRC.
CCAT1 Promotes CRC Tumor Growth Through miR-181b-5p/TUSC3 In Vivo
The potential involvement of CCAT1 in tumorigenesis of CRC in vivo was determined through injecting LoVo cells into mice. As shown in Figure 7A and B, the tumors of mice injected with shCCAT1 transfected cells were significantly smaller than the tumors observed in mice transfected with the control cells. Co-transfection with anti-miR-181b-5p and shCCAT1 reversed the effects of CCAT1 knockdown effects in vivo. Furthermore, the miR-181b-5p, CCAT1 and TUSC3 expression levels were measured in tumor tissues. As shown in Figure 7C, the expression levels of CCAT1 in the tumor tissues were significantly reduced in the shCCAT1 group and shCCAT1 + anti-miR-181b-5p group compared with the control group. The expression levels of miR-181b-5p was significantly higher in the shCCAT1 group compared with the control group; however, it was significantly lower in the shCCAT1 + anti-miR-181b-5p compared with the shCCAT1 group in tumor tissues (Figure 7D). As shown in Figure 7E, the relative protein expression of TUSC3 was downregulated in the shCCAT1 group. Taken together, these data suggest that CCAT1 promotes CRC tumor growth through a miR-181b-5p/TUSC3 axis in vivo.
Discussion
Colorectal cancer (CRC) is a common malignant tumor of the gastrointestinal tract and is also the third leading cause of cancer-associated death worldwide.30 In recent years, a growing number of studies have shown that lncRNAs are involved in the development of cancer pathogenesis. Therefore, they provide novel avenues for understanding the underlying molecular mechanisms resulting in the development and progression of cancer.31–34 However, the effect of the numerous lncRNAs on the tumorigenesis of CRC is still being elucidated. Understanding the roles of the various lncRNAs in each cancer will potentially improve the therapeutic options available to patients. In the present study, the results demonstrated that CCAT1 promoted CRC cancer cell proliferation partly through a CCAT1/miR-181b-5p/TUSC3 axis.
LncRNA CCAT1 is upregulated in colon cancer. Studies have shown that CCAT1 expression is increased in other types of cancer. However, its role in CRC progression remains unknown. In the present study, CCAT1 knockdown inhibited the growth of CRC cells. Subsequently, it was identified that miR-181b-5p was a potential target of CCAT1 using bioinformatics, and a luciferase reporter assay confirmed the interaction in CRC cells. CCK-8 and colony formation assays showed that anti-miR-181b-5p rescued the suppressive effects of CCAT1 knockdown in CRC cells. To further examine the mechanisms by which CCAT1 altered proliferation of CRC cells, the target genes of miR-181b-5p were predicted using bioinformatics. TUSC3 was determined to be a potential target, and this was confirmed using a luciferase reporter assay. The correlation between CCAT1 and TUSC3 expression was calculated using a Pearson’s correlation analysis. Furthermore, the functional effects of CCAT1 in CRC tumor growth were determined using a subcutaneous xenograft tumor model in vivo. The data demonstrated that CCAT1 promoted CRC tumor growth through miR-181b-5p/TUSC3 in vivo.
In conclusion, the present study identified that knockdown of CCAT1 expression inhibited cell proliferation and colony formation by acting as a ceRNA of miR-181b-5p, and regulated TUSC3 expression in CRC cells. The data suggest that the CCAT1/miR-181b-5p/TUSC3 axis may serve as potential therapeutic targets for treating patients with CRC.
Ethics Approval And Consent To Participate
The present study was approved by The Clinical Research Ethics Committee of Jilin University and the Laboratory Animal Ethics Committee of Jilin University (Changchun, China).
Availability Of Data And Materials
The datasets used and/or analyzed during the present study are available from the author on reasonable request.
Acknowledgments
The present study was supported by a grant from The First Hospital of Jilin University: Skin care measures for patients with cervical and lumbar diseases during perioperative period.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30. doi:10.3322/caac.21442
2. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi:10.3322/caac.21395
3. Capasso A, Pitts TM, Tentler JJ, et al. Rational combination therapy in young vs older patients with advanced colorectal cancer (CRC). J Clin Oncol. 2017;35(4):612. doi:10.1200/JCO.2017.35.4_suppl.612
4. Ng W, Shin J, Yang T, et al. Inhibition of polo-like kinase 1 (PLK1) additively improves outcome of ionising irradiation (IR) in colorectal cancer (CRC). Pathology. 2017;49(1):S137.
5. Lin KY, Denehy L, Frawley HC, Wilson L, Granger CL. Pelvic floor symptoms, physical, and psychological outcomes of patients following surgery for colorectal cancer. Physiother Theory Pract. 2018;34(6):442–452. doi:10.1080/09593985.2017.1422165
6. Golkhalkhali B, Paliany AS, Chin KF, Rajandram R. The roles of adjuvant supplements in colorectal cancer patients on chemotherapy-reaping benefits from metabolic crosstalk. Nutr Cancer. 2018;70(2):184–191. doi:10.1080/01635581.2018.1412470
7. Veettil SK, Lim KG, Chaiyakunapruk N, Ching SM, Abu Hassan MR. Colorectal cancer in Malaysia: its burden and implications for a multiethnic country. Asian J Surg. 2017;40(6):481–489. doi:10.1016/j.asjsur.2016.07.005
8. Ciarloni L, Hosseinian S, Monnier-Benoit S, et al. Discovery of a 29-gene panel in peripheral blood mononuclear cells for the detection of colorectal cancer and adenomas using high throughput real-time PCR. PLoS One. 2015;10(4):e0123904. doi:10.1371/journal.pone.0123904
9. Rao AKDM, Rajkumar T, Mani S. Perspectives of long non-coding RNAs in cancer. Mol Biol Rep. 2017;44(2):203–218. doi:10.1007/s11033-017-4103-6
10. Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet. 2009;10(3):155–159. doi:10.1038/nrg2521
11. Tang Y, He Y, Zhang P, et al. LncRNAs regulate the cytoskeleton and related Rho/ROCK signaling in cancer metastasis. Mol Cancer. 2018;17(1):77. doi:10.1186/s12943-018-0825-x
12. Yang L, Tang Y, Xiong F, et al. LncRNAs regulate cancer metastasis via binding to functional proteins. Oncotarget. 2017;9(1):1426–1443. doi:10.18632/oncotarget.22840
13. Chen S, Wang M, Yang H, et al. LncRNA TUG1 sponges microRNA-9 to promote neurons apoptosis by up-regulated Bcl2l11 under ischemia. Biochem Biophys Res Commu. 2017;485(1):167–173. doi:10.1016/j.bbrc.2017.02.043
14. Zhou X, Ye F, Yin C, Zhuang Y, Yue G, Zhang G. The interaction between miR-141 and lncRNA-H19 in regulating cell proliferation and migration in gastric cancer. Cell Physiol Biochem. 2015;36(4):1440–1452. doi:10.1159/000430309
15. Peng W, Wang Z, Fan H. LncRNA NEAT1 impacts cell proliferation and apoptosis of colorectal cancer via regulation of Akt signaling. Pathol Oncol Res. 2017;23(3):651–656. doi:10.1007/s12253-016-0172-4
16. Roberts TC, Morris KV, Wood MJ. The role of long non-coding RNAs in neurodevelopment, brain function and neurological disease. Philos Trans R Soc Lond B Biol Sci. 2014;369(1652):pii: 20130507. doi:10.1098/rstb.2013.0507
17. Salehi S, Taheri MN, Azarpira N, Zare A, Behzad-Behbahani A. State of the art technologies to explore long non-coding RNAs in cancer. J Cell Mol Med. 2017;21(12):3120–3140. doi:10.1111/jcmm.13238
18. Li Y, Jing F, Ding Y, He Q, Zhong Y, Fan C. Long noncoding RNA CCAT1 polymorphisms are associated the risk of colorectal cancer. Cancer Genet. 2018;222–223:13–19. doi:10.1016/j.cancergen.2018.02.003
19. Ma MZ, Chu BF, Zhang Y, Weng MZ, Qin YY, Gong W. Long non-coding RNA CCAT1 promotes gallbladder cancer development via negative modulation of miRNA-218-5p. Cell Death Dis. 2015;6:e1583. doi:10.1038/cddis.2014.541
20. Deng L, Yang SB, Xu FF, Zhang JH. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge. J Exp Clin Cancer Res. 2015;34(1):18. doi:10.1186/s13046-015-0136-7
21. Yang F, Xue X, Bi J, et al. Long noncoding RNA CCAT1, which could be activated by c-Myc, promotes the progression of gastric carcinoma. J Cancer Res Clin Oncol. 2013;139(3):437–445. doi:10.1007/s00432-012-1324-x
22. Chen L, Hu N, Wang C, Zhao H, Gu Y. Long non-coding RNA CCAT1 promotes multiple myeloma progression by acting as a molecular sponge of miR-181a-5p to modulate HOXA1 expression. Cell Cycle. 2018;17(3):319–329. doi:10.1080/15384101.2017.1407893
23. Liu T, Chi H, Chen J, et al. Curcumin suppresses proliferation and in vitro invasion of human prostate cancer stem cells by ceRNA effect of miR-145 and lncRNA-ROR. Gene. 2017;631:29–38. doi:10.1016/j.gene.2017.08.008
24. Wang W, Zhuang Q, Ji K, et al. Identification of miRNA, lncRNA and mRNA-associated ceRNA networks and potential biomarker for MELAS with mitochondrial DNA A3243G mutation. Sci Rep. 2017;7:41639. doi:10.1038/srep41639
25. Horak P, Reibenwein J, Pils D, et al. N33 (TUSC3) promoter hypermethylation in serum of prostate cancer patients. Cancer Res. 2006;66(8):763–764. doi:10.1158/0008-5472.CAN-05-3771
26. Horak P, Tomasich E. Vaňhara P, Kratochvílová K, Anees M, Marhold M, et al. TUSC3 loss alters the ER stress response and accelerates prostate cancer growth in vivo. Sci Rep. 2014;4:3739. doi:10.1038/srep03739
27. Vašíčková K, Horak P, Vaňhara P. TUSC3: functional duality of a cancer gene. Cell Mol Life Sci. 2018;75(5):849–857. doi:10.1007/s00018-017-2660-4
28. Tang H, Li K, Zheng J, Dou X, Zhao Y, Wang L. microRNA-145 regulates tumor suppressor candidate 3 and mitogen-activated protein kinase pathway to inhibit the progression of colorectal cancer. J Cell Biochem. 2018;28:1–9.
29. Zhu YF, Dong M. Expression of TUSC3 and its prognostic significance in colorectal cancer. Pathol Res Pract. 2018;214(9):1497–1503.
30. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66(4):683–691.
31. Coni P, Madeddu A, Kuqi L, et al. LncRNA colon cancer-associated transcript 1 (CCAT1) in ovarian cancer. Eur Rev Med Pharmacol Sci. 2018;22(6):1525–1527.
32. Xu C, Xiao G, Zhang B, et al. CCAT1 stimulation of the symmetric division of NSCLC stem cells through activation of the Wnt signalling cascade. Gene Ther. 2018;25(1):4–12.
33. Qin N, Wang C, Lu Q, et al. Systematic identification of long non-coding RNAs with cancer-testis expression patterns in 14 cancer types. Oncotarget. 2017;8(55):94769–94779.
34. Zhang Z, Xie H, Liang D, et al. Long non-coding RNA CCAT1 as a diagnostic and prognostic molecular marker in various cancers: a meta-analysis. Oncotarget. 2018;9(34):23695–23703.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.