Back to Journals » International Journal of Women's Health » Volume 14
Liver Angiosarcoma with Poor Prognosis in a 61-Year-Old Woman: A Case Report and Literature Review
Authors Manh Hung T , Tran TPT
Received 3 April 2022
Accepted for publication 20 July 2022
Published 27 July 2022 Volume 2022:14 Pages 957—963
DOI https://doi.org/10.2147/IJWH.S369271
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Everett Magann
Tran Manh Hung,1 Thi Phuong Thao Tran2
1Department of Surgery, Bach Mai Hospital, Hanoi, Vietnam; 2Center for Population Health Sciences, Hanoi University of Public Health, Hanoi, Vietnam
Correspondence: Tran Manh Hung, Department of Surgery, Bach Mai Hospital, Hanoi, Vietnam, Tel +84-912-157-095, Email [email protected]
Purpose: Hepatic angiosarcoma is very rare malignancy and more common in men than in women. To date, only a few female cases of liver angiosarcoma have been reported. Here, we report a female case of liver angiosarcoma, first detected in Vietnam, with a high malignancy stage, rapid progression, and poor prognosis.
Case Presentation: A 61-year-old woman was admitted to the Bach Mai Hospital with fatigue, anorexia, weight loss, and severe pain in the right upper quadrant for 2 weeks prior. Clinical examination detected a firm 4-cm hepatomegaly below the right costal margin and grade I splenomegaly. Abdominal ultrasonography and CT revealed diffuse lesions in the entire liver parenchyma, spreading to the spleen, while MRI showed signs of bone metastasis. Blood tests showed elevated transaminase enzymes, especially Gamma Glutamyl Transferase 501 U/L; thrombocytopenia; no anemia; and other tumor markers such as AFP, CEA, and CA19-9 were within normal limits. On CT images, the dots and nodules in the liver and spleen appeared hyperenhanced in the arterial phase and washout in the venous phase. The results of both histopathology and immunohistochemistry showed liver angiosarcoma. Surgery and radiation were not indicated due to the suspicion of bone metastasis. Chemotherapy with doxorubicin at a dose of 60 mg/m2 and intravenous infusion once every 21 days was administered. Unfortunately, during the first dose of chemotherapy with doxorubicin, side effects appeared. Since the disease developed continuously and uncontrollably, the patient was subsequently exhausted, anemic, presented peritoneal fluid, and eventually died of intra-abdominal bleeding.
Conclusion: For the diagnosis of liver angiosarcoma, ultrasound-guided liver biopsy could be applied for safe and effective histopathology, and selective embolization of the hepatic artery is necessary to prevent bleeding complications. The disease has a very poor prognosis, and if chemotherapy does not respond, the patient can die within six months of diagnosis.
Keywords: liver angiosarcoma, primary tumor, hepatectomy, case report
Introduction
Angiosarcoma is rare malignancy that develops in the endothelial cells of blood vessels. It can occur anywhere in the body but is most common in the skin on the head and neck and rarely seen in deeper tissues, such as the liver, heart, or lungs. Hepatic angiosarcoma is very rare, approximately 200 cases reported every year worldwide, and more common in men than in women, with a ratio of 4:1.1,2 Its clinical symptoms are poor, and its diagnosis is mainly based on histopathology. Treatment is usually a combination of surgery, radiotherapy, and chemotherapy, depending on the tumor location, size, invasion, and metastasis. Among such treatment approaches, extensive resection surgery is the most effective, but radiotherapy and chemotherapy are options in cases wherein surgery cannot be performed, the tumor has metastasized or for adjuvant therapy after surgery.1–4 To date, only a few female cases of liver angiosarcoma have been reported. Here, we report a case of liver angiosarcoma in a 61-year-old woman, first detected in Vietnam, with a high malignancy stage, rapid progression, and poor prognosis.
Case Presentation
A 61-year-old woman with no history of the disease was admitted to the Bach Mai Hospital (Hanoi, Vietnam) with fatigue, anorexia, weight loss, and severe pain in the right upper quadrant for 2 weeks prior, in which the patient did not take any medications. The patient had no family history of cancer.
Clinical examination detected a firm 4-cm hepatomegaly below the right costal margin and grade I splenomegaly. Abdominal ultrasonography revealed hepatomegaly with rough heterogeneous parenchyma, several hypoechoic and hyperechoic nodules with diameters of 20 mm, and splenomegaly with rough heterogeneous parenchyma. Blood tests showed red blood cells 4.12 T/L, platelets 63G/L, white blood cells 7.8 G/L, prothrombin 91%, urea 5.1 mmol/L, creatinine 81μmol/L, glucose 7.3 mmol/L, total protein 65.1 g/L, blood albumin 36.5 g/L, total bilirubin 27.4 μmol/L, GOT 63 U/L, GPT 65, Gamma Glutamyl Transferase (GGT) 501, AFP 2.52 ng/mL, CEA 1.82 ng/mL, and CA 19–9 29.3 U/mL. Computed tomography (CT) revealed hepatomegaly and splenomegaly, 203mm along the right liver, 103mm along the left liver, 160mm along the spleen; and liver parenchyma appeared heterogeneously hypodense before injection. The parenchyma of the bilateral liver and spleen had several pre-contrast hypodense nodules with diameters of 10–20 mm that were not clear and diffused in the parenchyma of the bilateral liver and spleen. The nodules appeared hyperenhanced in the arterial phase with the spreading property from centripetal to the peripheral areas and washout in the venous phase. The remaining liver parenchyma has hypo-enhanced areas (Figure 1). There were several sclerotic dots in the spine and pelvis. Chest CT tomography showed no masses or nodules. Pelvic magnetic resonance imaging (MRI) identified multifocal lesions in the pelvis and femur (Figure 2). A computed tomography-guided pelvic biopsy revealed no malignant cells.
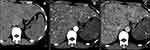 |
Figure 1 Before injection (A). After injection, nodules in the liver and spleen appeared hyper-enhanced in the arterial phase (B), washed out in the venous phase (C). |
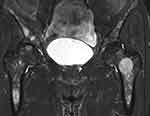 |
Figure 2 Pelvic MRI showed multifocal lesions in the pelvis and femur. |
The patient was indicated for ultrasound-guided liver biopsy, hepatic arteriography, and transarterial embolization by digital subtraction angiography with the following steps: 1) local anesthesia to open the right common femoral artery, and sheath 5F was placed in the right femoral artery; 2) Celiac artery angiography showed a number of nodules in the liver and spleen, and selectively accessing the left hepatic blood supplies for the masses and left liver nodules by using a progreat 2.7F catheter; 3) Placing the ultrasound probe to locate the biopsy in the left liver, an ultrasound-guided biopsy of the left liver was performed (Figure 3A); 4) selective embolization of the left hepatic artery (subsegment S3) by spongel (Figure 3B); and 5) branch exams showed no pseudoaneurysm or drug drainage, the sheath was withdrawn from the right femoral artery, and compression bandage.
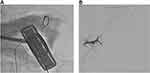 |
Figure 3 (A) Ultrasound-guided biopsy of the left liver; (B) selective embolization of the left hepatic artery by spongel. |
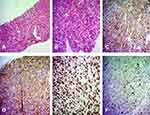
Histopathological diagnosis was performed by two methods, including Hematoxylin and Eosin, and Periodic acid-Schiff. Histopathological results showed that the tumor cells from biopsies taken from the liver and tumor tissues were rhombus-shaped with a large nucleus, alkaline, quite homogenous, and most of them were arranged in clusters or bundles, while were suggestive of a blood vessel structure. There was a relatively high proliferation rate, stromal fibrous proliferation, and chronic inflammatory infiltrates (Figure 4A and B). Immunohistochemistry results revealed CD34 positivity (Figure 4C), factor VIII positivity (Figure 4D), ERG positive (Figure 4E), and Ki-67 positivity of 10% (Figure 4F). The results of both histopathology and immunohistochemistry showed liver angiosarcoma.
The patient had diffuse lesions in both the liver and spleen. The liver biopsy showed angiosarcoma in the liver only, and no evidence of angiosarcoma was found in other organs. Liver resection, liver transplantation, and radiation were not indicated due to diffuse lesions in the bilateral liver and the suspicion of metastasis in the bone and spleen. Chemotherapy with doxorubicin at a dose of 60 mg/m2 and intravenous infusion once every 21 days was administered. After the first dose infusion on day 1, the patient was tired, exhausted, had a poor appetite, had oral mucosal ulcers, and blood tests showed red blood cells 2.70 T/L, platelets 30G/L, white blood cells 3.5 G/L, total protein 46 g/L, blood albumin 26.5 g/L, GGT 613 U/l, and AEP 2.36 ng/mL. Therefore, the second dose was discontinued. In the 4th month from the time of diagnosis, abdominal aspiration was performed every 2 weeks, and 1 liter of clear yellow fluid was removed each time. In the 5th month, abdominal aspiration was performed weekly, and blood fluid was collected. In the 6th month, the patient had severe abdominal pain and shortness of breath and eventually died.
Discussion
The previous publications have shown that liver angiosarcoma has a poor prognosis, and combining hepatectomy with post-surgery chemotherapy is believed to be the best approach, which brings the best prognosis with long-term survival. Meanwhile, liver transplantation is not recommended because of its very poor prognosis.
In 2012, Chien et al reported a case of an 83-year-old female hospitalized with shock due to a rupture of an angioma.5 She received emergent transarterial embolization for hemostasis (TAE) and partial hepatectomy two weeks later. The pathological result confirmed liver angiosarcoma. At 4 months post-surgery, the patient was good with no signs of recurrence or metastasis.
A study by Huang NC in 2015 reported nine patients (five men and four women) with liver angiosarcoma.6 In women, three died before two years, and only one 70-year-old woman who underwent liver resection and adjuvant chemotherapy survived at 37 months. The author suggested that combining surgery and adjuvant chemotherapy could achieve long-term survival in a patient with liver angiosarcoma.
The case reported by Cawich SO is a 52-year-old woman with pain in the right upper quadrant.7 The abdominal CT scan presented a malformed tumor in the liver with a diameter of 15 cm firmly attached to the diaphragm. Subsequently, she underwent resection of the tumor and the diaphragm. Histopathological examination revealed a primary liver angiosarcoma. The authors also suggested that complete liver resection should be indicated because it brought the best long-term prognosis and survival among treatment approaches.
In 2020, Rujeerapaiboon et al reported that, out of 3 female cases with liver angiosarcoma, one presented with abdominal pain and weight loss.8 The abdominal CT scan detected diffused multiple arterial enhancing infiltrating nodules varying in size, scattered throughout the entire liver. Laparoscopic liver biopsy confirmed liver angiosarcoma. She received chemotherapy for five cycles. Peritoneal metastases were found at 14 months, and she died at 19 months from the diagnosis. Another case was admitted due to acute rupture of a liver tumor and underwent embolization to stop bleeding. Four weeks after embolization, she underwent extended right hepatectomy with a tumor size 28×20 × 14 cm. The immunochemical results confirmed liver angiosarcoma. However, she also showed peritoneal metastases and passed away four months later. The last case had left upper quadrant pain for two months and weight loss. CT scan presented numerous nodules and masses across the entire liver. A real-time sonographic guidance percutaneous tumor biopsy confirmed liver angiosarcoma. Two weeks after diagnosis, a ruptured tumor was detected, and she passed away one week later.
Primary liver angiosarcoma is a rare, highly aggressive malignant tumor with rapid progression and poor prognosis, accounting for approximately 0.1–2% of all primary malignancies in the liver. It is common in men aged 60–70 years. Its causes are unclear, but several etiological factors have been identified, including vinyl chloride, arsenic, and industrial materials.1,2 Liver angiosarcoma is caused by long-term exposure to such etiological factors, resulting in a long latent period of 10–40 years.1,2 Unfortunately, our patient was a 61-year-old woman with a history of herbicide exposure for 15–20 years before disease diagnosis. Although there is no clear evidence, long-term herbicide exposure may be a risk factor for angiosarcoma. It is crucial to monitor herbicide exposure since Vietnam is an underdeveloped agricultural country in which herbicides are commonly used in agricultural activities without using standard personal protective equipment.
The clinical symptoms of liver angiosarcoma were poor, vague, and non-specific, thus the accurate diagnosis of angiosarcoma proves to be challenging.4 In our case, our patient was previously healthy but with symptoms of fatigue, anorexia, weight loss, and severe pain in the right upper quadrant for two weeks prior. Clinical examination showed a firm 4-cm hepatomegaly below the right costal margin and grade I splenomegaly. Abdominal ultrasonography and CT revealed diffuse lesions in the entire liver parenchyma, spreading to the spleen, while MRI showed signs of bone metastasis. Blood tests showed elevated transaminase enzymes, especially GGT (501 U/L); thrombocytopenia; no anemia; and other tumor markers such as AFP, CEA, and CA19-9 were within normal limits. On CT images, the dots and nodules in the liver and spleen appeared hyperenhanced in the arterial phase and washout in the venous phase. The pattern of enhancement observed to be centripetal to the peripheral areas suggested liver angiosarcoma rather than peripheral nodular with a centripetal filling enhancement pattern of hemangiomas. However, we believe that these test results did not play a decisive role, but were only indicative of a malignant lesion in the liver. The diagnosis of liver angiosarcoma should be correlated with the findings of imaging and blood tests, and the final decision should be based on a liver biopsy for histopathology. This is consistent with previous reports in the literature.9,10
The diagnosis of liver angiosarcoma still faces many obstacles and challenges owing to non-specific clinical symptoms, laboratory tests, indecision imaging, and high bleeding risk due to liver biopsy. According to Tsunematsu’s report, a case was not performed a biopsy of a liver tumor that was suspected to be malignant based on pre-operative imaging, but liver angiosarcoma was diagnosed only after surgical resection of the liver tumor.10 Averbukh et al performed the first ultrasound-guided liver biopsy and detected no evidence of malignancy, but a second biopsy after two months revealed a liver angiosarcoma at an advanced stage.4 Laparoscopic liver biopsy has certain advantages, including direct tumor detection, selection of specimen collection sites, and post-biopsy bleeding control. However, in our case, after discussing with our patient, she was very worried and disagreed to perform a laparoscopic biopsy to avoid surgery. Therefore, we did not choose laparoscopic biopsy as reported by Mauricio Millan,11 but decided on the ultrasound-guided liver biopsy and selective embolization of the left hepatic artery after collecting specimens to prevent bleeding complications.
The preparation for safe liver biopsy in this case requires caution. If there is no liver biopsy for histopathology, there would be no definitive diagnosis to choose the appropriate treatment approaches. Additionally, a wrong-site biopsy will yield inaccurate results, and if biopsied in lesion nodules, the risk of bleeding will be very high. If bleeding occurred, the patient was more likely to undergo unnecessary surgery. Eventually, our biopsy technique helped prevent bleeding complications and identify the origin of the tumor.
Liver angiosarcoma has a rapid clinical progression and poor prognosis, especially in inoperable cases. Most patients could die within six months of diagnosis from liver failure or bleeding. Even in cases with surgery, only about 3% of them survive for over 2 years.1 Extensive tumor resection and postoperative chemotherapy are the most effective treatment approaches for solitary tumors, but they are not always feasible. Kim et al reported five patients with abdominal pain were diagnosed with liver angiosarcoma.12 However, all these five patients were diagnosed at an advanced stage with distant metastases that were no longer resectable Four patients received chemotherapy, in which two died three months after diagnosis, and the rest survived more than six months. It was indicative of the malignancy of a liver angiosarcoma.
Millan et al reported a case of a 37-year-old man with liver angiosarcoma who underwent left hepatectomy and postoperative chemotherapy, and the results showed a good prognosis,11 which was also seen in several previous studies.6,7,12 For example, a retrospective study by Tripke et al in nine patients with liver angiosarcoma who underwent hepatectomy.3 The median follow-up period was 15.5 months (3–144 months). At the last follow-up, four out of nine patients were alive, and the remaining showed signs of recurrence and died within 3 to 17 months after surgery. It suggested that hepatectomy could maximize the chance of long-term survival. Furthermore, total hepatectomy and liver transplantation are not recommended in these cases due to the high recurrence rate and rapid disease progression. According to Orlando et al, for patients with liver angiosarcoma who received liver transplantation, the 12-month survival rate was 24% and the 24-month mortality rate was 100%.13 Tran Minh et al also suggested that liver transplantation is a contraindication for liver angiosarcoma.14 To date, there is no standard and uniform chemotherapy regimen, and the effectiveness of the treatment combination of 5-FU and doxorubicin is unclear among patients with hepatic angiosarcoma with unresectable liver and metastasis. Embolization chemotherapy is recommended for palliative care or control bleeding.1,2,15
In our study, the patient was diagnosed with liver angiosarcoma, which spread to the spleen and metastasized to the bone; therefore, hepatectomy, liver transplantation, and radiation therapy were not indicated. We decided to administer chemotherapy with the hope of reducing symptoms and prolonging life. Unfortunately, during the first dose of chemotherapy with doxorubicin, side effects appeared; thus, the second dose was discontinued. Since the disease developed continuously and uncontrollably, the patient was subsequently exhausted, anemic, presented peritoneal fluid, and eventually died of intra-abdominal bleeding.
Due to the small number of cases, the diagnosis of liver angiosarcoma is difficult because of non-specific clinical symptoms. In addition, it has a poor prognosis owing to rapid disease progression, high recurrence rate, and resistance to chemotherapy. Therefore, surgical resection remains the definitive treatment, but is not always feasible.
Conclusion
For the diagnosis of liver angiosarcoma, ultrasound-guided liver biopsy could be applied for safe and effective histopathology, and selective embolization of the hepatic artery is necessary to prevent bleeding complications. The disease has a very poor prognosis, and if chemotherapy does not respond, the patient can die within six months of diagnosis.
Abbreviations
MRI, magnetic resonance imaging; CT, computed tomography.
Data Sharing Statement
This case report contains clinical data from the medical records in the Bach Mai hospital. Additional information is available from the first author upon reasonable request.
Ethics Approval and Informed Consent
Writing and publishing this case report was approved by Bach Mai hospital.
Consent for Publication
Written informed consent was obtained from the patient for publication of this case report and the accompanying images. A copy of the written consent is available upon request.
Acknowledgment
The authors would like to thank our patient and her family for their support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This case report involved no source of funding for any of the authors.
Disclosure
The authors declare that they have no competing interests.
References
1. Kumar A, Sharma B, Samant H. Liver angiosarcoma. In: StatPearls. StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022.
2. Chaudhary P, Bhadana U, Singh RA, Ahuja A. Primary hepatic angiosarcoma. Eur J Surg Oncol. 2015;41(9):1137–1143. doi:10.1016/j.ejso.2015.04.022
3. Tripke V, Heinrich S, Huber T, et al. Surgical therapy of primary hepatic angiosarcoma. BMC Surg. 2019;19(1):5. doi:10.1186/s12893-018-0465-5
4. Averbukh LD, Mavilia MG, Einstein MM. Hepatic angiosarcoma: a challenging diagnosis. Cureus. 2018;10(9):e3283. doi:10.7759/cureus.3283
5. Chien C-Y, Hwang -C-C, Yeh C-N, et al. Liver angiosarcoma, a rare liver malignancy, presented with intraabdominal bleeding due to rupture- A case report. World J Surg Oncol. 2012;10(1):23. doi:10.1186/1477-7819-10-23
6. Huang NC, Kuo YC, Chiang JC, et al. Hepatic angiosarcoma may have fair survival nowadays. Medicine. 2015;94(19):e816. doi:10.1097/md.0000000000000816
7. Cawich SO, Thomas D, Ragoonanan V, Naraynsingh V. The hanging manoeuver to complete liver resection for a locally advanced angiosarcoma: a case report. Int J Surg Case Rep. 2015;16:52–55. doi:10.1016/j.ijscr.2015.09.006
8. Rujeerapaiboon N, Wetwittayakhlang P. Primary hepatic angiosarcoma: a rare liver malignancy - varying manifestations but grave prognosis. Case Rep Gastroenterol. 2020;14(1):137–149. doi:10.1159/000506928
9. Yi LL, Zhang JX, Zhou SG, et al. CT and MRI studies of hepatic angiosarcoma. Clin Radiol. 2019;74(5):
10. Tsunematsu S, Muto S, Oi H, et al. Surgically diagnosed primary hepatic angiosarcoma. Intern Med. 2018;57(5):687–691. doi:10.2169/internalmedicine.9318-17
11. Millan M, Delgado A, Caicedo LA, et al. Liver Angiosarcoma: rare tumour associated with a poor prognosis, literature review and case report. Int J Surg Case Rep. 2016;28:165–168. doi:10.1016/j.ijscr.2016.09.044
12. Kim HR, Rha SY, Cheon SH, Roh JK, Park YN, Yoo NC. Clinical features and treatment outcomes of advanced stage primary hepatic angiosarcoma. Ann Oncol. 2009;20(4):780–787. doi:10.1093/annonc/mdn702
13. Orlando G, Adam R, Mirza D, et al. Hepatic hemangiosarcoma: an absolute contraindication to liver transplantation–the European Liver Transplant Registry experience. Transplantation. 2013;95(6):872–877. doi:10.1097/TP.0b013e318281b902
14. Tran Minh M, Mazzola A, Perdigao F, Charlotte F, Rousseau G, Conti F. Primary hepatic angiosarcoma and liver transplantation: radiological, surgical, histological findings and clinical outcome. Clin Res Hepatol Gastroenterol. 2018;42(1):17–23. doi:10.1016/j.clinre.2017.02.006
15. Lee S-W, Song C-Y, Gi Y-H, et al. Hepatic angiosarcoma manifested as recurrent hemoperitoneum. World J Gastroenterol. 2008;14(18):2935–2938. doi:10.3748/wjg.14.2935
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.