Back to Journals » International Journal of General Medicine » Volume 15
Lipopolysaccharide-Induced lncRNA TMC3-AS1 is Highly Expressed in Osteoporosis and Promotes Osteoblast Apoptosis by Suppressing the Formation of Mature miR-708
Received 18 November 2021
Accepted for publication 10 January 2022
Published 25 March 2022 Volume 2022:15 Pages 3345—3352
DOI https://doi.org/10.2147/IJGM.S350081
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Sheng Chen, Min Dai
Orthopedics Department, the First Affiliated Hospital of Nanchang University, Nanchang City, Jiangxi Province, 330006, People’s Republic of China
Correspondence: Min Dai, Tel +86 0791-88692748, Email [email protected]
Background: LncRNA TMC3-AS1 expression is affected by lipopolysaccharide (LPS), a contributor to osteoporosis (OS). Therefore, we hypothesized that TMC3-AS1 could inhibit osteoblast apoptosis and interact with miR-708, a regulator of osteoblast apoptosis in OS.
Methods: Differential expression of TMC3-AS1 and miR-708 (mature and premature) in OS patients and controls was analyzed using RT-qPCR. Subcellular location of TMC3-AS1 in osteoblasts was analyzed using subcellular fractionation assay. The direct interaction between TMC3-AS1 and premature miR-708 was analyzed using RNA pulldown assay. The role of TMC3-AS1 and miR-708 in the expression of each other was explored with overexpression assays. Cell apoptosis induced by LPS was analyzed using cell apoptosis assay.
Results: TMC3-AS1 and premature miR-708 were highly expressed in OS and were upregulated by LPS in osteoblasts. In contrast, mature miR-708 was under-expressed in OS and downregulated by LPS. TMC3-AS1 directly interacted with premature miR-708 and was detected in both the nuclear and cytoplasm fractions. TMC3-AS1 decreased premature miR-708 level and increased mature miR-708 level. Moreover, TMC3-AS1 increased LPS-induced cell apoptosis and suppressed the role of miR-708 in cell apoptosis.
Conclusion: TMC3-AS1 is highly expressed in OS and promotes LPS-induced osteoblast apoptosis by reducing miR-708 maturation.
Keywords: osteoporosis, TMC3-AS1, miR-708, osteoblast
Background
As a common systemic skeletal disorder, osteoporosis (OS) is characterized by bone fragility caused by reduced bone mass and deteriorated bone tissue micro-architecture.1,2 Porous bones in OS patients may cause bone fractions, leading to a high morbidity rate.3 OS mainly affects postmenopausal women. The latest global statistics estimated that nearly 25% of females and 8% of males older than 50 years are currently suffering from OS.4,5 There are strategies to strengthen the bones of OS patients, thereby slowing bone breakdown and only spurring new bone growth, in rare cases.6,7 However, none of these approaches can achieve a cure.8 Therefore, more effective approaches need to be developed to improve the treatment of OS.
Recent advances in elucidating the molecular mechanism of OS have revealed potential targets to treat OS by regulating gene expression.9,10 For instance, regulating strontium signaling provides novel insights into the treatment of OS.11 However, the development of OS-targeted molecular therapy still requires the identification of novel targets with high efficiency and safety. Altered expression of lncRNAs is frequently observed in OS, and certain lncRNAs play critical roles in the progression of OS,12,13 indicating that lncRNAs are potential therapeutic targets for OS. LncRNA TMC3-AS1 is affected by lipopolysaccharide (LPS),14 which contributes to OS.15 Therefore, we hypothesized that TMC3-AS1 is involved in OS and interacts with miR-708, which could inhibit osteoblast apoptosis,16 and explored the interaction between TMC3-AS1 and miR-708 in OS.
Materials and Methods
Patients and Cells
The present study enrolled 60 OS patients and 60 controls who were willing to donate blood samples at The First Affiliated Hospital of Nanchang University. The study was approved by the Ethics Committee of The First Affiliated Hospital of Nanchang University (Approval No. 72355). All experimental procedures were performed in accordance with the Declaration of Helsinki. Patients diagnosed with OS by dual-energy x-ray absorptiometry and willing to participate were included. Patients complicated with metabolic diseases and severe diseases, such as cancers and heart diseases, and previously had OS were excluded from the study. All controls exhibited normal physiological parameters except obesity. All participants signed informed consent. Patients’ clinical data are presented in Table 1. OS-affected osteoblasts derived from an adult OS patient (406–05A, Sigma-Aldrich) were used in this study and cultured in DMEM containing FBS (10%), L-glutamine (2 mM), and penicillin/streptomycin (1000 units) at 37°C in an incubator with 5% CO2 and 95% humidity. LPS was added to the medium to final concentrations of 0, 0.5, 1, 2, and 4 μg/mL, followed by cell culture for 48h to achieve LPS treatment.
 |
Table 1 Clinical Data of Both OS Patients and Controls |
Blood Isolation and Plasma Preparations
Overnight fasten blood samples were withdrawn from all controls and OS patients and immediately transferred to BDVacutainer Plastic Blood Collection Tubes. After centrifugation 1200g for 12 min, the plasma samples were collected and stored in liquid nitrogen prior to RNA isolations.
Cell Transfection
TMC3-AS1 and/or miR-708 were overexpressed in osteoblasts by transiently transfecting TMC3-AS1 expression vector (pcDNA3.1) and/or miR-708 mimic (RiboBio) using Neon™ Transfection System (Thermo Fisher Scientific). Untransfected cells were cultured until the end of experiments and served as the control (C).
RNA Preparations
Total RNAs were isolated from both plasma samples and osteoblasts using RNAstorm™ RNA Isolation Kit (Biotium) and treated with DNase I (NEB) until a ratio of OD 260/280 above 1.8 was reached. All RNA samples were analyzed using Bioanalyzer to check their RNA concentrations and ensure they had a RIN value higher than 8.0.
RT-qPCRs
Total RNAs (5000ng) were reverse transcribed into cDNA samples. With cDNA samples as templates, expression levels of TMC3-AS1 and miR-708 (both mature and premature) were determined using qPCRs with GAPDH and U6 as the internal controls, respectively. Ct values of targeted genes were normalized to their controls using the 2−ΔΔCt method. Primer sequences were 5’-CTGAAGTCCCGAGACCACAACCC-3’ (forward) and 5’-TCTGCCCAAGACCTGGACACC-3’ (reverse) for TMC3-AS1, 5’-ATGGTGAAGGTCGGTGTGAA-3’ (forward) and 5’-CGCTCCTGGAAGATGGTGAT-3’ (reverse) for GAPDH, 5’-GCTTCGGCAGCACATATACTAA-3’ (forward) and 5’-CGCTTCACGAATTTGCGTGT-3’ (reverse) for U6, and 5’-AAGGAGCTTACAATCTAGC-3’ (forward) and poly(T) (reverse) for miR-708.
Subcellular Distribution of TMC3-AS1
Cytoplasm and nucleus samples of TMC3-AS1 in osteoblast cells were prepared using the Cytoplasmic and Nuclear RNA Purification Kit (Cat. 21000, BioVision) through centrifugation. The separated cytoplasm sample was directly used for RNA isolation, while the nucleus fraction was further subjected to nucleus isolation and used for RNA isolation. The isolated RNA samples were reverse transcribed into cDNAs and used for PCRs to amplify TMC3-AS1. The PCR products were separated on agarose gels (2%) and stained using EB to visualize DNA bands under MyECL analyzer.
RNA-RNA Pull-Down Assay
Both negative control (NC) and TMC3-AS1 transcripts were prepared using T7 RNA polymerase in vitro and labeled at 3’ end with biotin using Pierce™ RNA 3’ End Biotinylation Kit (Fisher Scientific). The labeled RNAs were named Bio-NC and Bio-TMC3-AS1 and transiently transfected into osteoblasts. At 48h of post-transfection, cells were lysed on ice. The labeled RNAs were pulled down with magnetic beads, isolated, and subjected to RT-qPCR to determine TMC3-AS1 and miR-708 levels.
Cell Apoptosis Assay
Transfected osteoblasts were harvested at 48h of post-transfection and further incubated in the medium with 4 μg/mL LPS for 48h. After that, cells were digested with 0.25% trypsin, washed with PBS, resuspended in binding buffer, and stained with FITC and PI staining. Cell apoptosis was analyzed using flow cytometry.
Statistical Analysis
All experiments were performed in triplicates. Data were expressed as mean ± SD values. Differences among more than two independent groups and between two independent groups were compared using ANOVA Tukey’s test and unpaired t test, respectively. P < 0.05 was statistically significant.
Results
Differential Expression of TMC3-AS1 and miR-708 in OS
The study included 60 OS patients and 60 controls. OS patients had body mass index (BMI) in the range from 24.9 to 34.8, with a mean of 30.8± 3.8, height in the range from 153 cm to 182 cm, with a mean of 168.8± 9.7 cm, and weight in the range from 62.6 to 103.4 kg, with a mean of 86.8 ± 7.8 kg. The expression levels of TMC3-AS1 and miR-708 in plasma samples donated by the 60 OS patients and 60 controls were determined using RT-qPCR. The results showed that TMC3-AS1 (Figure 1A, p < 0.01) and premature miR-708 (Figure 1B, p < 0.01) were highly expressed in OS. In contrast, mature miR-708 was lowly expressed in OS (Figure 1C, p < 0.01). Pearson’s correlation coefficient analysis showed that TMC3-AS1, premature miR-708, and mature miR-708 levels were not closely correlated to patients’ BMI, height, and weight (p > 0.05, data not shown).
Osteoblasts were treated with LPS (0, 0.5, 1, 2, and 4 μg/mL) for 48h, and the levels of both premature (Figure 1E) and mature miR-708 (Figure 1F) and TMC3-AS1 (Figure 1D) were determined. TMC3-AS1 and premature miR-708 were upregulated by LPS in osteoblasts, while mature miR-708 was downregulated by LPS (p < 0.05).
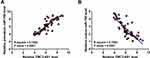
Correlation of TMC3-AS1 with miR-708
Correlations of TMC3-AS1 with miR-708 at both premature (Figure 2A) and mature (Figure 2B) levels across OS samples were analyzed with Pearson’s correlation coefficient. The data revealed that TMC3-AS1 level was positively correlated with premature miR-708 level and inversely correlated with mature miR-708 level.
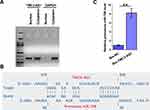
Subcellular Location of TMC3-AS1 in Osteoblasts and Direct Interaction Between TMC3-AS1 and Premature miR-708
The subcellular location of TMC3-AS1 in osteoblasts was analyzed with the subcellular fractionation assay. Different from GAPDH, which is a cytoplasmic marker, TMC3-AS1 was detected in both nuclear and cytoplasm samples from osteoblasts (Figure 3A). IntaRNA 2.0 predicted that TMC3-AS1 and premature miR-708 might form multiple base pairings (Figure 3B). RNA-RNA pulldown assay showed that premature miR-708 level was significantly higher in Bio-TMC3-AS1-pulldown samples than in Bio-NC-pulldown samples, confirming the direct interaction between TMC3-AS1 and premature miR-708 (Figure 3C, p < 0.01).
Regulation of miR-708 Maturation by TMC3-AS1
TMC3-AS1 and miR-708 were overexpressed in osteoblasts. Their overexpression was confirmed every 24h until 96h (Figure 4A, p < 0.05). TMC3-AS1 overexpression increased premature miR-708 level (Figure 4B, p < 0.05) and decreased mature miR-708 level (Figure 4C, p < 0.05).
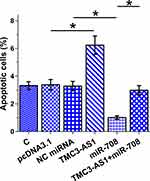
Regulation of Osteoblast Apoptosis by TMC3-AS1 and miR-708
Apoptosis of osteoblast with TMC3-AS1 and miR-708 overexpression induced by LPS (4 μg/mL LPS) for 48h was analyzed with cell apoptosis assay. TMC3-AS1 overexpression increased LPS-induced osteoblast apoptosis and suppressed the role of miR-708 in inhibiting osteoblast apoptosis (Figure 5, p < 0.05).
Discussion
This study characterized the role of TMC3-AS1 in OS. Interestingly, the present study illustrated that TMC3-AS1, a possible LPS-regulated lncRNA, may participate in OS by regulating osteoblast apoptosis through regulating miR-708 maturation.
Ye et al recently reported that TMC3-AS1 expression is regulated by LPS, and LPS-regulated TMC3-AS1 may negatively regulate IL-10 expression.14 The present study confirmed TMC3-AS1 upregulation by LPS in osteoblast and, for the first time, reported TMC3-AS1 overexpression in OS. It is known that increased LPS level in OS contributes to the disease progression.15 Therefore, increased TMC3-AS1 expression in OS is likely induced by LPS. Interestingly, the present study showed that TMC3-AS1 overexpression increased osteoblast apoptosis induced by LPS. In addition, IL-10 is a well-characterized anti-inflammatory cytokine and can reduce the production and secretion of proinflammatory cytokines.17 Therefore, TMC3-AS1 may also promote inflammatory responses in OS to accelerate disease progression. Future studies may focus on the role of TMC3-AS1 in the inflammation pathways involved in OS.
It has been reported that miR-708 could inhibit H2O2-induced MC3T3-E1 cell injury and apoptosis by targeting PTEN.16 Interestingly, the present study showed that LPS reduced the mature miR-708 level and increased its premature level. Moreover, miR-708 was lowly expressed at the mature level and highly expressed at the premature level in OS patients. Therefore, LPS may suppress miR-708 maturation in OS. Moreover, overexpression of mature miR-708 suppressed osteoblast apoptosis induced by LPS. Therefore, miR-708 overexpression may serve as a potential target to treat OS.
Furthermore, TMC3-AS1, which is localized at both nuclear and cytoplasm fractions of osteoblasts, suppresses miR-708 maturation in osteoblasts and directly interacts with premature miR-708. We speculated that TMC3-AS1 might sponge premature miR-708 in the nucleus of the osteoblasts to suppress premature miRNA translocation from the nucleus to the cytoplasm, thereby inhibiting miR-708 maturation. However, this research is limited by the small sample numbers. Moreover, the in vivo interaction between TMC3-AS1 and miR-708 is not explored.
In conclusion, TMC3-AS1 is overexpressed in OS. TMC3-AS1 may sponge premature miR-708 to suppress its maturation, thereby increasing LPS-induced osteoblast apoptosis. However, the conclusions remain to be further verified. The present study characterizes a novel TMC3-AS1/miR-708 axis in OS and indicates that this axis may be targeted to treat OS. However, clinical trials are needed to test this hypothesis.
Disclosure
The authors declare that they have no competing interests in this work.
References
1. Sözen T, Özışık L, Başaran N. An overview and management of osteoporosis. Eur J Rheumatol. 2017;4(1):46–56.
2. Akkawi I, Zmerly H. Osteoporosis: current concepts. Joints. 2018;6(2):122–127.
3. Mughal N, Inderjeeth AJ, Inderjeeth CA. Osteoporosis in patients with dementia is associated with high morbidity and mortality: findings from a single orthogeriatric unit. Australian j General Practice. 2019;48(1–2):53–58.
4. Alswat KA. Gender disparities in osteoporosis. J Clin Med Res. 2017;9(5):382–387.
5. De Martinis M, Sirufo MM, Polsinelli M, Placidi G, Di Silvestre D, Ginaldi L. Gender differences in osteoporosis: a single-center observational study. World J Men’s Health. 2020;1:76.
6. Iconaru L, Smeys C, Baleanu F, et al. Osteoporosis treatment gap in a prospective cohort of volunteer women. Osteoporosis Int. 2020;31(7):1377–1382.
7. Ström O, Lauppe R, Ljunggren Ö, et al. Real-world effectiveness of osteoporosis treatment in the oldest old. Osteoporosis Int. 2020;31(8):1525–1533.
8. Sugiyama T, Torio T, Miyajima T, Kim YT, Oda H. Romosozumab and blosozumab: alternative drugs of mechanical strain-related stimulus toward a cure for osteoporosis. Front Endocrinol. 2015;6:54.
9. Li H, Xiao Z, Quarles LD, Li W. Osteoporosis: mechanism, molecular target and current status on drug development. Curr Med Chem. 2021;28(8):1489–1507.
10. Khosla S, Hofbauer LC. Osteoporosis treatment: recent developments and ongoing challenges. Lancet Diabetes Endocrinol. 2017;5(11):898–907.
11. Saidak Z, Marie PJ. Strontium signaling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol Ther. 2012;136(2):216–226.
12. Yang Y, Yujiao W, Fang W, et al. The roles of miRNA, lncRNA and circRNA in the development of osteoporosis. Biol Res. 2020;53(1):40.
13. Chen RS, Zhang XB, Zhu XT, Wang CS. LncRNA Bmncr alleviates the progression of osteoporosis by inhibiting RANML-induced osteoclast differentiation. Eur Rev Med Pharmacol Sci. 2019;23(21):9199–9206.
14. Ye M, Xie M, Zhu J, Wang C, Zhou R, Li X. LPS-Inducible lncRNA TMC3-AS1 Negatively Regulates the Expression of IL-10. Front Immunol. 2020;11:1418.
15. Chandra A, Rajawat J. Skeletal Aging and Osteoporosis: mechanisms and Therapeutics. Int J Mol Sci. 2021;22(7):98579.
16. Zhang W, Cui SY, Yi H, Zhu XH, Liu W, Xu YJ. MiR-708 inhibits MC3T3-E1 cells against H(2)O(2)-induced apoptosis through targeting PTEN. J Orthop Surg Res. 2020;15(1):255.
17. Vernal R, Velásquez E, Gamonal J, Garcia-Sanz JA, Silva A, Sanz M. Expression of proinflammatory cytokines in osteoarthritis of the temporomandibular joint. Arch Oral Biol. 2008;53(10):910–915.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.