Back to Journals » OncoTargets and Therapy » Volume 9
Lipid-rich carcinoma of the breast that is strongly positive for estrogen receptor: a case report and literature review
Authors Oba T, Ono M, Iesato A, Hanamura T, Watanabe T, Ito T, Kanai T, Maeno K, Ito K , Tateishi A, Yoshizawa A, Takayama F
Received 17 May 2015
Accepted for publication 30 November 2015
Published 18 March 2016 Volume 2016:9 Pages 1641—1646
DOI https://doi.org/10.2147/OTT.S88726
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Daniele Santini
Takaaki Oba,1 Mayu Ono,1 Asumi Iesato,1 Toru Hanamura,1 Takayuki Watanabe,1 Tokiko Ito,1 Toshiharu Kanai,1 Kazuma Maeno,1 Ken-ichi Ito,1 Ayako Tateishi,2 Akihiko Yoshizawa,2 Fumiyoshi Takayama3
1Division of Breast, Endocrine and Respiratory Surgery, Department of Surgery, Shinshu University School of Medicine, Matsumoto, Nagano, 2Department of Laboratory Medicine, Shinshu University Hospital, 3Imaging Center, Ichinose Neurosurgical Hospital, Matsumoto, Japan
Abstract: Lipid-rich carcinoma (LRC) of the breast is a rare breast cancer variant that accounts for <1% of all breast malignancies. It has been reported that LRCs are negative for estrogen receptor. Here, we report a case of LRC of the breast that was strongly positive for estrogen receptor and treated with endocrine adjuvant therapy. A 52-year-old postmenopausal female noticed a lump in her right breast by self-examination and presented to our hospital. Physical examination revealed an elastic 30 mm ×20 mm hard mass in the upper medial part of her right breast. The findings obtained using ultrasonography, mammography, and contrast-enhanced magnetic resonance imaging suggested breast cancer. Core needle biopsy resulted in the diagnosis of invasive carcinoma. The patient underwent mastectomy and sentinel lymph node biopsy. Histopathologically, the tumor cells were abundant in foamy cytoplasm. Because the presence of marked cytoplasmic lipid droplets was confirmed by Sudan IV staining and electron microscopic examination of the tumor and the lipid droplets were negative for periodic acid–Schiff staining, the tumor was diagnosed as an LRC. Immunohistochemically, estrogen and progesterone receptors of the tumor were strongly positive, human epidermal growth factor receptor type 2 was negative, and the ratio of Ki-67-positive cells was ~30%. After surgery, the patient underwent combination chemotherapy with anthracycline, cyclophosphamide, and 5-fluorouracil, followed by docetaxel. Thereafter, the pateint was treated with letrozole and has remained well for 24 months with no signs of recurrence.
Keywords: breast cancer, estrogen receptor, endocrine therapy
Introduction
Lipid-rich carcinoma (LRC) of the breast is a rare form of breast cancer that accounts for <1% of all breast malignancies according to the World Health Organization’s classification of breast tumors1 and is considered to be aggressive with a poor prognosis.2 Although LRC is thought to be negative for estrogen receptors (ERs),2,3 we recently experienced a case of breast LRC that was strongly positive for ERs. To the best of our knowledge, our case is the second report of ER-positive LRC, which was treated with endocrine therapy. Here, we report a case of LRC strongly positive for ER and review the literature. Written informed consent was obtained from the patient to use her data for publication. Approval was obtained from the ethics committee of Shinshu University, School of Medicine.
Case report
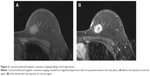
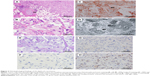
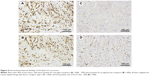
A 52-year-old postmenopausal female noticed a painless lump in her right breast on self-examination and presented to our hospital. The patient had no past or family history of serious disease and received no past hormonal therapy. Physical examination revealed a firm lump in the upper medial quadrant of the right breast. The tumor was 30 mm ×20 mm in size. No nipple discharge or swelling of the axillary lymph node was observed. Blood examination results, including tumor markers, were within normal limits. Mammography showed an irregularly shaped mass with a microlobulated border. Ultrasonography showed a hypoechoic 25.8 mm ×22.9 mm mass with an irregular and indistinct border (Figure 1). Contrast-enhanced magnetic resonance imaging revealed an irregularly shaped mass with strong enhancement in the early phase. No intraductal spread of the tumor was detected (Figure 2). Core needle biopsy of the tumor led to a diagnosis of invasive carcinoma. 18F-Fluorodeoxyglucose positron emission tomography revealed no sign of distant metastasis. The patient was diagnosed as stage IIA breast cancer and underwent mastectomy and sentinel lymph node biopsy. Histopathologically, the tumor cells were abundant in foamy cytoplasm (Figure 3A and B). Sudan IV staining (Figure 3C) and electron microscopic examination of the tumor (Figure 3D) demonstrated the presence of marked cytoplasmic lipid droplets, which were negative for periodic acid–Schiff (PAS) staining (Figure 3E). Furthermore, gross cystic disease fluid protein-15 (GCDFP-15) staining (Figure 3F) was negative in the cytoplasm of the tumor cells. On the other hand, cytokeratin (CK) 5/6 staining was weakly positive (Figure 3G), while p53 staining was positive (Figure 3H). Based on these findings, the tumor was diagnosed as LRC. Immunohistochemically, ERs and progesterone receptors (PgRs) of the tumor were strongly positive (Figure 4A and B), human epidermal growth factor receptor type 2 (HER2) was negative (Figure 4C), and the ratio of Ki-67-positive cells was ~30% (Figure 4D). No lymph node metastasis was detected in the axilla. Taking into consideration the high ratio of Ki-67-positive cells and aggressiveness of LRC, the patient received standard adjuvant chemotherapy of anthracycline, cyclophosphamide, and 5-fluorouracil, followed by docetaxel. Thereafter, the patient received letrozole and has remained well for 24 months with no signs of recurrence.
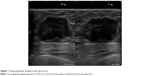  | Figure 1 Ultrasonographic findings of the right breast. |
Discussion
LRC is histopathologically characterized by cells with numerous optically free vacuoles of various sizes in the cytoplasm that are positive for Sudan IV staining.1 Although it is important to differentiate LRC from other vacuolated or clear cell tumors, such as glycogen-rich and apocrine carcinomas, these tumors are appropriately differentiated by special staining or immunohistochemical staining. For example, glycogen-rich and apocrine carcinomas are positive for PAS, whereas LRC is negative. With regard to GCDFP-15 staining, apocrine carcinoma is positive and LRC is negative.1,4,5 In our case, the tumor cells were negative for PAS and GCDFP-15. Furthermore, an electron microscopic examination revealed the presence of cytoplasmic lipid droplets that were positive for Sudan IV staining, which met the criteria of LRC.
The clinical characteristics of LRC are not well known because only ~70 cases have been reported in the English literature to date. However, Shi et al2 analyzed the clinicopathological data of 49 LRCs among 3,206 patients with breast cancer and reported lymph nodes metastases in 38 (78%) and that the 2- and 5-year survival rates of patients with LRC were 64.6% and 33.2%, respectively. Thus, LRC is generally considered to be an aggressive phenotype of breast cancer.
Because of the rarity of LRC, the association between the subtype and clinical features of LRC has not been extensively studied. LRC cases reported in the English literature are summarized in Table 1.2,3,6,7–14 Guan et al3 investigated the clinicopathological features of 17 patients with LRC and reported that none were ER positive, whereas one (5.9%) was PgR positive; however, all patients were HER2 positive. Shi et al2 studied 49 cases of LRC and reported that none were ER positive, five (10.2%) were PgR positive, and 35 (71.4%) were HER2 positive. The ratios of HER2-positive cancer in both studies were higher than the general average of 20%–30%.6,15 In addition, >30% of Ki-67-positive tumor cells were detected in 27 (55.1%) cases in the study by Shi et al.2 Moreover, LRC was ER negative in 12 of the 14 case reports.5,8–14 Thus, all LRCs except for two cases13,14 have been reported to be ER negative, and there has been a tendency for LRC to be HER2 positive and contain highly proliferative cells. Hence, the aggressiveness of LRC may be attributed to these biological features. However, the significance of Ki-67 levels in LRC has been not elucidated. Shi et al2 reported that Ki-67 status was not associated with overall survival among 49 patients with LRC. However, more clinical data are required to elucidate the significance of Ki-67 in the prognosis of LRC.
Treatment of LRC is typically performed on the basis of standard treatment protocols for breast cancer. However, considering the aggressiveness of LRC, systemic therapy should be an important part of the treatment regimen and appropriate systemic therapies according to the subtype and stage of each tumor should be administered. Shi et al2 performed an in vitro chemosensitivity assay and found that lipid-rich tumors were sensitive to paclitaxel, carboplatin, and cisplatin. Thus, chemotherapy including paclitaxel or platinum agents may have the potential to improve the prognosis of recurrent LRC. On the other hand, because all LRCs except for two previously reported cases13,14 have been ER negative, the effect of endocrine therapy on LRC has not been established. In the present case, endocrine therapy is expected to be effective because the tumor was strongly ER positive.
Conclusion
The present case is the second report of LRC that was positive for ER expression and treated with endocrine therapy. Although LRC is generally considered to be negative for ERs, our case suggests that a small percentage of LRCs are ER positive.
Acknowledgment
Written informed consent was obtained from the patient to use her data for publication.
Disclosure
The authors report no conflicts of interest in this work.
References
Eusebi V, Ichihara S, Vincent-Salomon A, et al. Exceptionally rare types and variants. In: Lakhani SR, Ellis IO, Schnitt SJ, editors. WHO Classification of Tumours of the Breast. 4th ed. Lyon: IARC Press; 2012:74. | ||
Shi P, Wang M, Zhang Q, Sun J. Lipid-rich carcinoma of the breast. A clinicopathological study of 49 cases. Tumori. 2008;94:342–346. | ||
Guan B, Wang H, Cao S, et al. Lipid-rich carcinoma of the breast clinicopathologic analysis of 17 cases. Ann Diagn Pathol. 2011;15:225–232. | ||
Satoh F, Umemura S, Osamura RY. Immunohistochemical analysis of GCDFP-15 and GCDFP-24 in mammary and non-mammary tissue. Breast Cancer. 2000;7:49–55. | ||
Machalekova K, Kajo K, Bencat M. Unusual occurrence of rare lipid-rich carcinoma and conventional invasive ductal carcinoma in the one breast: case report. Case Rep Pathol. 2012;2012:387045. | ||
Kaptain S, Tan LK, Chen B. Her-2/neu and breast cancer. Diagn Mol Pathol. 2001;10:139–152. | ||
Wrba F, Ellinger A, Reiner G, Spona J, Holzner JH. Ultrastructural and immunohistochemical characteristics of lipid-rich carcinoma of the breast. Virchows Arch A Pathol Anat Histopathol. 1988;413:381–385. | ||
Umekita Y, Yoshida A, Sagara Y, Yoshida H. Lipid-secreting carcinoma of the breast: a case report and review of the literature. Breast Cancer. 1998;5:171–173. | ||
Varga Z, Robl C, Spycher M, Burger D, Caduff R. Metaplastic lipid-rich carcinoma of the breast. Pathol Int. 1998;48:912–916. | ||
Russo S, Coppola D, Vinaccia P. Lipid-rich histology in a basal-type immuno-profile breast carcinoma: a clinicopathological histochemical and immunohistochemical analysis of a case. Rare Tumors. 2009;1:e41. doi:10.4081/rt.2009.e41. | ||
Kimura A, Miki H, Yuri T, Hatano T, Tsubura A. A case report of lipid-rich carcinoma of the breast including histological characteristics and intrinsic subtype profile. Case Rep Oncol. 2011;4:275–280. | ||
Nagata Y, Hanagiri T, Ono K, et al. A non-invasive form of lipid-secreting carcinoma of the breast. Breast Cancer. 2012;19:83–87. | ||
Kurisu Y, Tsuji M, Shibayama Y. Intraductal lipid-rich carcinoma of the breast with a component of glycogen-rich carcinoma. J Breast Cancer. 2012;15:135–138. | ||
Cong Y, Lin J, Qiao G, et al. Lipid-rich carcinoma of the breast: a report of two cases and a literature review. Oncol Lett. 2015;9:1729–1732. | ||
Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.