Back to Journals » Infection and Drug Resistance » Volume 15
Linezolid-Associated Neuropathy in Patients with MDR/XDR Tuberculosis in Shenzhen, China
Authors Zhang P , Li W , Liu M, Zhan S, Zhang H, Deng G , Chen X
Received 7 March 2022
Accepted for publication 12 May 2022
Published 23 May 2022 Volume 2022:15 Pages 2617—2624
DOI https://doi.org/10.2147/IDR.S365371
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Peize Zhang,1,2 Wei Li,3 Miaona Liu,3 Senlin Zhan,2 Hailin Zhang,2 Guofang Deng,2 Xiaoyou Chen1
1Beijing Tuberculosis and Thoracic Tumor Institute, Beijing, People’s Republic of China; 2Department of Pulmonary Medicine and Tuberculosis, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China; 3Department of Pharmacy, The Third People’s Hospital of Shenzhen, Shenzhen, Guangdong, People’s Republic of China
Correspondence: Guofang Deng; Xiaoyou Chen, Email [email protected]; [email protected]
Objective: Linezolid is one of the key drugs for the treatment of multidrug-resistant/extensively drug-resistant tuberculosis (MDR/XDR-TB). We aimed to describe the incorporation of the Michigan Neuropathy Screening Instrument (MNSI) and serum trough concentration as screening tools for neurotoxicity in the management of MDR/XDR-TB patients receiving a linezolid-based treatment regimen in Shenzhen, China.
Methods: A total of 73 patients on a linezolid-containing anti–MDR/XDR-TB regimen were prospectively enrolled. The MNSI was used for peripheral neuropathy screening. Optic neuropathy was diagnosed by ophthalmologists. Serum trough concentration was recorded and its relationship with neuropathy analyzed.
Results: Of all patients, neuropathy was observed in 40% (29) during anti-TB treatment. Of these, 20 (69%) had peripheral neuritis, seven (24%) optic neuritis, and two (7%) both. Serum trough concentration > 2 mg/L was observed in 17 (59%) patients with neuropathy and 13 (30%) patients without neuropathy. There was a significant statistical difference between the two groups (P=0.013). Time to onset of neuropathy from initiation of the linezolid-containing regimen was within 2 months for eight (28%) patients, 2– 6 months for 18 (62%) patients, and > 6 months for three (10%) patients. Sixteen (55%) patients were adjusted to a lower dose of 300 mg linezolid daily. Four (14%) patients had linezolid permanently removed from their regimen.
Conclusion: Neuropathy is a commonly reported adverse event associated with long-term use of linezolid. MNSI and serum trough–concentration monitoring can be adopted as simple screening tools for early detection of neuropathy to balance linezolid efficacy and tolerability.
Keywords: linezolid, MDR/XDR-TB, neuropathy, MNSI, serum trough concentration
Introduction
An increasing incidence of multidrug-resistant tuberculosis (MDR-TB) and extensively drug-resistant TB (XDR-TB) worldwide calls for focused efforts by the End TB program. China has one of the highest TB burdens.1 Disease management has been a challenge, as treatment options are limited. The efficacy and favorable treatment outcomes of linezolid, a group A antimicrobial endorsed by the WHO for MDR/XDR-TB in 2018,2,3 have been well documented in the treatment of DR-TB in numerous studies. However, adverse events have also been reported in patients with prolonged use of the drug,4–7 with neurotoxicity the most frequent.
Some studies have shown that dosage of linezolid is directly correlated with the risk of adverse events and trough concentration of linezolid associated with the risk of neurotoxicity.2,5,8–10 Peripheral neuropathy has been reported to be as high as 81% in patients at a dose of up to 1,200 mg/day.5 A randomized control trial of 33 MDR/XDR-TB patients on an initial daily dose of 1,200 mg linezolid for 4–6 weeks followed by 300–600 mg at the continuation phase reported significantly higher odds of adverse events of peripheral (24.2%) and optic (18.2%) neuropathy in patients on linezolid than a control group.6 A meta-analysis reported development of neuropathy in 30% of patients during anti–MDR-TB treatment.4 Neuropathy is one of the key causes of early or permanent discontinuation of linezolid in a regimen.7
Traditional diagnostic methods for peripheral neuropathy include nerve-conduction studies and electromyography, and optical examinations performed by an ophthalmologist in cases of optic neuropathy.6,11,12 However, these are expensive, and have to be performed by specialized personnel. Referrals are usually necessary, as the equipment is not readily available in all clinical settings. As such, it would be beneficial if an easy-to-use screening tool could be adopted to monitor drug toxicity to allow early interventions in optimizing treatment outcome and completion.
Self-administered screening tools have been introduced to detect early development of neuropathic disorder. The Subjective Peripheral Neuropathy Screen was developed to monitor painful sensory neuropathy in HIV-positive patients.13 The Michigan Neuropathy Screening Instrument (MNSI) was designed to provide a straightforward self-administered tool to screen for early sensorimotor polyneuropathy in diabetic patients.14 With its simple and noninvasive nature and sensitivity of up to 80%, the MNSI has been translated into over ten languages and is used in different countries for screening of early peripheral neuropathic symptoms.15,16 No study has described the use of the MNSI in screening linezolid-induced peripheral neuropathy in TB patients. In this study, we describe in more detail the neuropathic adverse effects associated with linezolid and evaluate the occurrence and management of neuropathy among MDR/XDR-TB patients receiving a linezolid-based treatment regimen.
Methods
Study Setting and Participants
We prospectively studied patients aged >18 years with clinically confirmed rifampicin-resistant TB (RR-TB), MDR-TB, and XDR-TB during January 1, 2020 to January 1, 2021 who were on a linezolid-based regimen and had agreed to have their trough linezolid concentration monitored during treatment. Exclusion criteria were HIV-positivity, severe cardiovascular, liver, or blood-system disease, other serious illness, and pregnance or lactation. All participants were admitted to the Third People’s Hospital of Shenzhen, China when treatment was initiated and then followed up in the outpatient unit for >12 months after treatment completion.
An anti-TB regimen was tailored for each patient based on their results on drug-susceptibility testing and after review and approval by the MDR-TB care team in the hospital for compliance with guidelines and recommendations of the WHO and Antituberculosis Committee of China. Management of adverse events were based on physician consensus and guidelines from the Antituberculosis Committee of China. Demographic, clinical, and laboratory data were obtained from medical records for all eligible patients. All 73 participants provided written consent to take part (ChiCTR190002662). This study was approved by the ethics committee of the Third People’s Hospital. The hospital undertook that in using these statistics, no personal information of any patient would be revealed. It also complied with the Declaration of Helsinki in regard to confidentiality and ethics.
Patient Care and Key Definitions
Molecular testing and phenotypic susceptibility testing were used to determine drug-resistance profiles of Mycobacterium tuberculosis isolates. RR-TB is defined as resistance of M. tuberculosis to rifampicin only, MDR-TB as resistance to at least rifampicin and isoniazid, pre–XDR-TB as resistance to isoniazid and rifampicin and either a fluoroquinolone or a second-line injectable agent, but not both, and XDR-TB as MDR-TB plus resistance to at least one fluoroquinolone and a second-line injectable agent.3
Linezolid was a common agent in regimen compositions of all participants in this study. Dosage was set at 600 mg, administered once daily on treatment initiation for all participants, except one on dialysis, who was administered a lower dose of 300 mg once daily. To screen for the presence of neuropathy during treatment, the MNSI was adopted, and patients were assessed by their attending clinicians for peripheral and optic neuropathy on a monthly basis. Referral to a neurologist and ophthalmologist was arranged when deemed necessary. The MNSI consists of two parts. The first is a self-administered questionnaire for patients on foot sensation, with higher scores indicating more neuropathic symptoms. The second part is a physical examination performed by a health professional that includes assessment of the patient’s feet on appearance and ulceration, ankle reflexes, and vibration perception using a 128 Hz tuning fork. A patient is considered peripheral neuropathic when their score is ≥2 (on an 8-point scale).
Optic neuropathy includes visual acuity loss, blurred vision, or diminution of vision. Patients presenting with any susceptible neuropathic symptoms would be referred to an ophthalmologist for thorough clinical assessment. For this analysis, only adverse events associated with peripheral and optic neuropathy were included. Dosage adjustment and follow-up interval were determined by the physician depending on the severity of neuropathy. Trough concentration of linezolid was measured at least once during treatment. Planned time points to measure serum trough concentration were at the end of 1, 3, and 6 months of treatment or when neuropathy was highly suspected. Time from treatment initiation to onset of neuropathy was classified as ≤2 months, >2 to ≤6 months, and >6 months.
Pharmacokinetics
Venous blood (2 mL) was collected 0–30 minutes before oral linezolid administration for linezolid trough concentrations. Plasma was separated and analyzed within 1 hour. High Perfermance Liquid Chromatography (HPLC) was used for measuring serum concentration. As the reference for linezolid trough concentration is <2 mg/L,10 we considered linezolid trough concentration >2 mg/L at least once at baseline or during treatment to be high and associated with neuropathy.
Statistical Analysis
Data analysis was conducted using SAS 9.2. Chi-squared tests were used to measure differences between the two groups. P<0.05 was regarded as statistically significant.
Results
Patient Characteristics and Incidence of Neuropathy in RR/MDR/XDR-TB Patients
Of the 73 patients, 36% (26) were female and 64% (47) male. Mean age was 37.6±13.93 years, seven (10%) had a history of treated TB, 56 (77%) were infected with an MDR strain of M. tuberculosis: ten (14%) with an RR strain, five (7%) with a pre-XDR strain, and two (3%) with an XDR strain. Six (8%) were coinfected with HBV, five (7%) with type 2 diabetes mellitus, and two (3%) had had a kidney transplantation. A total of 66 patients had pulmonary TB, six had pulmonary and extrapulmonary TB (bone, lymph node, urinary, or genital), and 40% (29) were diagnosed with neuropathy (Table 1). A list of medicines administered for >1 month for the 73 patients in this study is given in Table 2.
 |
Table 1 Participant demographic and clinical characteristics |
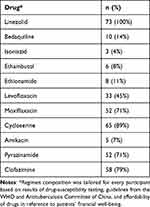 |
Table 2 Regimen composition with duration >1 month |
Serum Linezolid Trough Concentration in Patients with and without Neuropathy
Thirty patients were observed to have >2 mg/L trough concentration at least once in the study period. Based on results of the MNSI and optic neurological examination, we divided patients into two groups: neuropathic and nonneuropathic. Comparison between these two groups showed that linezolid trough concentration >2 mg/L had a higher prevalence in patients with neuropathy (59%, 17) than those without neuropathy (30%, 13). There is a significant statistical difference between the two groups (P=0.013, Table 3). Another comparison using baseline characteristics showed that serum linezolid trough concentration had no relationship to age, weight, albumin level, eGFR, or coexisting diabetes on the first visit (Supplement Figure 1). We further compared trough concentration between patients on daily linezolid dosages of 300 mg and 600 mg and found that trough concentration was lower in patients on a 300 mg/day dose, but the difference was not statistically significant (P=0.26; Figure 1).
 |
Table 3 Univariate analysis of determinants of neuropathic development |
 |
Figure 1 Serum trough concentration (Cmin) in patients on daily linezolid dosages of 300 mg and 600 mg. |
Types of Neuropathy and Dosage Adjustment
We further analyzed presentation among types of neuropathy. Of the 29 patients with neuropathy, 20 (69%) had peripheral neuritis, seven (24%) had optic neuritis, and two (7%) had both. For patients with peripheral neuropathy, five (25%) reported neuropathy within 2 months, 13 (65%) 2–6 months, and two (10%) after 6 months. Ten (50%) patients were adjusted to a lower dose of 300 mg linezolid daily. Four (20%) patients had linezolid permanently removed from their regimen. For patients with optic neuropathy, three (43%) reported neuropathy within 2 months and four (57%) 2–6 months. Three (43%) patients were kept on the original dose and four (57%) were adjusted to a lower dose of 300 mg linezolid daily. None of them had linezolid permanently removed from their regimen. The median duration from linezolid withdrawal to recovery from neuropathy was 4–6 months (Table 4).
 |
Table 4 Clinical characteristics of linezolid-associated neuropathy |
Discussion
Peripheral and optic neuropathy is a common toxic effect reported with long-term use of linezolid for treatment of MDR/XDR-TB.17 In the existing literature, prescribed daily linezolid doses range from 300 mg to 1,200 mg. All dosages have been proven to be effective and suit patients’ specific conditions with precise monitoring. Nonetheless, adverse events have been reported, and they seem to be linearly correlated with dosage. Daily administration of 1,200 mg has been reported to be associated with the presence of peripheral neuropathy in >80% of patients.5 A lower dose of 300 mg/day is believed to induce less toxicity; however, acquired drug resistance would become another concern with prolonged use.9,11 To balance regimen efficacy and safety, a daily dose of 600 mg for 12–18 months is recommended for MDR/XDR-TB,18 but this does not rule out the development of neuropathy completely. Forty percent of MDR/XDR-TB patients in our study showed neuropathy susceptible to be linezolid-associated. This was a little higher than the reported incidence of 30% (95% CI 20.53%–40.25%) in a systematic review in 2016.4 A recent study by Marie et al. reported an incidence of 32% in linezolid-related peripheral neuropathy confirmed by electromyography and nerve conduction–velocity testing.12 A confirmed diagnosis of peripheral neuropathy requires professional device and knowledge, which may result in delayed diagnosis.
We suggest the introduction of a screening tool to identify neuropathy at an early stage and allow effective intervention. The MNSI is designed for peripheral neuropathy screening in patients with diabetes. It has been proven to be a simple and reliable assessment tool in the early diagnosis of diabetic peripheral neuropathy.15 In our study, we extended the use of the MNSI to cover TB patients in monitoring the use of linezolid, which has been reported to be effective but limited by side effects. We expect the incorporation of a systematic assessment tool can assist with early identification of drug toxicity and provide data to assist physicians with close therapeutic monitoring and treatment management to optimize therapy efficacy and balance the drug’s side-effect profile.
Optic neuropathy is another adverse event reported with extended use of linezolid.6,19 In our study, seven patients developed optic neuropathy alone and two had both optic and peripheral neuropathy. As diagnosis of optic neuropathy is more complicated and involves optical examination by a professional, referral to ophthalmologists is needed in any susceptible cases, so it is not included in the MNSI. Elimination of linezolid from the body occurs by both renal and nonrenal mechanisms. Nonrenal clearance accounts for 65% of the linezolid dose, and 30% of the dose is excreted as unchanged drug in the urine. It has been shown that renal dysfunction is associated with high linezolid trough concentration.20 Our findings showed demographic characteristics and comorbidities did not seem to be associated with the development of neuropathy or serum trough concentration. The only dialysis patient in our study was prescribed a lower linezolid dose of 300 mg/day. His serum trough concentration was 1.5 mg/L, and a diagnosis of neuropathy was confirmed at 6 months of treatment. This reminds us that more attention should be paid to prioritizing high-risk patients to minimize complications.
Peripheral neuropathy is a common complication of diabetes, but the five diabetic patients in our study did not develop peripheral or optic neuropathy. It seems that linezolid does not increase the incidence of neuropathy in diabetes patients. Until now, no published studies have described higher incidence of neuropathy or neuropathy deterioration being associated with long-term use of linezolid in MDR-TB patients with diabetes. As it is difficult to identify whether neuropathy is induced by diabetes itself or by drugs, we suggest closer monitoring of the onset of neuropathy and precise hyperglycemic control in MDR-TB patients with diabetes.
Whether linezolid trough concentrations >2 mg/L can be used as an indicator for the occurrence of neuropathy is still debated.20 An early study by Song et al demonstrated a direct correlation between trough concentration and clinical toxicity.10 In a retrospective study, Bolhuis et al. speculated that peripheral neuropathy was mediated by cumulative dose and days of exposure to linezolid.21 A recent study by Jaspard et al. in France reported no significant association between trough concentration and neurologic toxicity.12 In our study, serum trough concentration >2 mg/L was more commonly observed in patients with peripheral and optic neuropathy. There was no significant difference in serum trough concentration among patients who developed peripheral or optic neuropathy or both. Most had onset of neuropathy within 6 months. Patients who did not develop neuropathy were observed to have good tolerability to linezolid over a treatment course >12 months, but a few patients showed neuropathy within 2 months. The rapid onset of neuropathy reminds us that mitochondrial genetic heterogeneity may contribute to the development of neurotoxicity.22–24 Trough concentration can be used as an indicator of linezolid accumulation in the body, and explains some side effects of the drug to a certain extent.
Although neurotoxicity is one of the major factors leading to the temporary or permanent withdrawal of linezolid for some patients, numerous studies have described the relief of neuropathic symptoms after linezolid has been discontinued.19 Persistent irreversible neuropathy has also been reported.25 A majority of patients with neuropathy in our study also reported gradual relief of symptoms after cessation of linezolid regimen. Consistently with other studies, the median time for elimination of neuropathic symptoms in our study was about 6 months.12 Only two patients complained of pain and numbness persisting >1 year following the completion of linezolid therapy.
There are several limitations to our study. Firstly, it was monocentric with a limited number of participants, though it was of prospective design and the clinical data of the patients were recorded in detail. Secondly, how trough concentration reflected the accumulation of linezolid over time could not be established, as measurement, of trough concentration for all patients were not taken at identical time intervals over the whole treatment. Thirdly, optic neuropathy was diagnosed according to clinical examinations by an ophthalmologist, resulting in a lack of a systematic algorithm data set, and so can be biased as to the exact number of patients being affected.
In summary, we have described the characteristics of neuropathy in patients with long-term use of linezolid. We illustrated that the MNSI can be adopted as a simple and reliable screening tool for peripheral neuropathy. Systematic monitoring of serum trough concentration can be used as a supportive indicator for toxicity and therapy management. In the event of suspected neuropathy, dose adjustment or withdrawal can be considered to balance therapy efficacy and safety. Further studies are needed to explore the possible genetic factors associated with linezolid tolerability and predict the rapid onset of severe adverse effects.
Acknowledgments
We appreciate our patients and their families for their trust and consent to participate in this study. We thank all the physicians involved in the management and care of our patients, particularly the ophthalmic team, for their professional opinions. We also thank Youli Jiang for helping us with the manuscript.
Funding
This study was funded by the Yangfan Foundation (2018KYJJ003), Summit Plan for Foshan High-level Hospital Construction (No. FSSYKF-2020001), Project funded by Shenzhen Third People's Hospital (No. G2021023) and the Technology Project of Guangdong Province (2020B1111170014), which are government funding schemes for the research of tuberculosis treatment and control.
Disclosure
The authors declare no conflicts of interest in this study.
References
1. World Health Organization. Global Tuberculosis Report 2021. Geneva, Switzerland: World Health Organization; 2021.
2. Singh B, Cocker D, Ryan H, Sloan DJ. Linezolid for drug-resistant pulmonary tuberculosis. Cochrane Database Syst Rev. 2019;3:CD012836. doi:10.1002/14651858.CD012836.pub2
3. World Health Organization. Rapid communication: key changes to treatment of multidrug- and rifampicin-resistant tuberculosis (MDR/RR-TB). Available from: http://www.who.int/tb/publications/2018/rapid_communications_MDR.
4. Agyeman AA, Ofori-Asenso R. Efficacy and safety profile of linezolid in the treatment of multidrug-resistant (MDR) and extensively drug-resistant (XDR) tuberculosis: a systematic review and meta-analysis. Ann Clin Microbiol Antimicrob. 2016;15(1):41. doi:10.1186/s12941-016-0156-y
5. Conradie F, Diacon AH, Ngubane N, et al. Treatment of highly drug-resistant pulmonary tuberculosis. N Engl J Med. 2020;382(10):893–902. doi:10.1056/NEJMoa1901814
6. Tang S, Yao L, Hao X, et al. Efficacy, safety and tolerability of linezolid for the treatment of XDR-TB: a study in China. Eur Respir J. 2015;45(1):161–170. doi:10.1183/09031936.00035114
7. Borisov S, Danila E, Maryandyshev A, et al. Surveillance of adverse events in the treatment of drug-resistant tuberculosis: first global report. Eur Respir J. 2019;54:1901522. doi:10.1183/13993003.01522-2019
8. Koh WJ, Kwon OJ, Gwak H, et al. Daily 300 mg dose of linezolid for the treatment of intractable multidrug-resistant and extensively drug-resistant tuberculosis. J Antimicrob Chemother. 2009;64(2):388–391. doi:10.1093/jac/dkp171
9. Koh WJ, Kang YR, Jeon K, et al. Daily 300 mg dose of linezolid for multidrug-resistant and extensively drug-resistant tuberculosis: updated analysis of 51 patients. J Antimicrob Chemother. 2012;67(6):1503–1507. doi:10.1093/jac/dks078
10. Song T, Lee M, Jeon HS, et al. Linezolid trough concentrations correlate with mitochondrial toxicity-related adverse events in the treatment of chronic extensively drug-resistant tuberculosis. EBioMedicine. 2015;2(11):1627–1633. doi:10.1016/j.ebiom.2015.09.051
11. Lee M, Lee J, Carroll MW, et al. Linezolid for treatment of chronic extensively drug-resistant tuberculosis. N Engl J Med. 2012;367(16):1508–1518. doi:10.1056/NEJMoa1201964
12. Jaspard M, Butel N, El helali N, et al. Linezolid-associated neurologic adverse events in patients with multidrug-resistant tuberculosis, France. Emerg Infect Dis. 2020;26(8):1792–1800. doi:10.3201/eid2608.191499
13. Cherry CL, Wesselingh SL, Lal L, McArthur JC. Evaluation of a clinical screening tool for HIV-associated sensory neuropathies. Neurology. 2005;65(11):1778–1781. doi:10.1212/01.wnl.0000187119.33075.41
14. Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi:10.2337/diacare.17.11.1281
15. Mohammad MT, Muhaidat J, Momani MS, et al. Translation and psychometric properties of the Arabic version of Michigan neuropathy screening instrument in type 2 diabetes. J Diabetes Res. 2019;2019:2673105. doi:10.1155/2019/2673105
16. Herman WH, Pop-Busui R, Braffett BH, et al. Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med. 2012;29(7):937–944. doi:10.1111/j.1464-5491.2012.03644.x
17. Kishor K, Dhasmana N, Kamble SS, Sahu RK. Linezolid induced adverse drug reactions - an update. Curr Drug Metab. 2015;16(7):553–559. doi:10.2174/1389200216666151001121004
18. Bolhuis MS, Akkerman OW, Sturkenboom MGG, et al. Linezolid-based regimens for multidrug-resistant tuberculosis (TB): a systematic review to establish or revise the current recommended dose for TB treatment. Clin Infect Dis. 2018;67(suppl_3):S327–S335. doi:10.1093/cid/ciy625
19. Dempsey SP, Sickman A, Slagle WS. Case report: linezolid optic neuropathy and proposed evidenced-based screening recommendation. Optom Vis Sci. 2018;95(5):468–474. doi:10.1097/OPX.0000000000001216
20. Morata L, De la Calle C, Gómez-Cerquera JM, et al. Risk factors associated with high linezolid trough plasma concentrations. Expert Opin Pharmacother. 2016;17(9):1183–1187. doi:10.1080/14656566.2016.1182154
21. Bolhuis MS, Tiberi S, Sotgiu G, et al. Linezolid tolerability in multidrug-resistant tuberculosis: a retrospective study. Eur Respir J. 2015;46(4):1205–1207. doi:10.1183/13993003.00606-2015
22. Abou Hassan OK, Karnib M, El-Khoury R, Nemer G, Ahdab-Barmada M, BouKhalil P. Linezolid toxicity and mitochondrial susceptibility: a novel neurological complication in a Lebanese patient. Front Pharmacol. 2016;7:325. doi:10.3389/fphar.2016.00325
23. Ye X, Huang A, Wang X, Wen C, Hu L, Lin G. Linezolid inhibited synthesis of ATP in mitochondria: based on GC-MS metabolomics and HPLC method. Biomed Res Int. 2018;2018:3128270. doi:10.1155/2018/3128270
24. Engvall M, Kawasaki A, Carelli V, et al. Case report: a novel mutation in the mitochondrial MT-ND5 gene is associated with Leber Hereditary Optic Neuropathy (LHON). Front Neurol. 2021;12:652590. doi:10.3389/fneur.2021.652590
25. Lee S, Kang BH, Ryu WY, Um SJ, Roh MS, Son C. Is severe and long-lasting linezolid-induced optic neuropathy reversible? Intern Med. 2018;57(24):3611–3613. doi:10.2169/internalmedicine.1344-18
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
