Back to Journals » OncoTargets and Therapy » Volume 12
LINC00958-MYC positive feedback loop modulates resistance of head and neck squamous cell carcinoma cells to chemo- and radiotherapy in vitro
Authors Huang S, Zhan Z, Li L, Guo H, Yao Y, Feng M, Deng J, Xiong J
Received 11 March 2019
Accepted for publication 6 July 2019
Published 24 July 2019 Volume 2019:12 Pages 5989—6000
DOI https://doi.org/10.2147/OTT.S208318
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjay Singh
Shanshan Huang,* Zhengyu Zhan,* Li Li, Hui Guo, Yangyang Yao, Miao Feng, Jun Deng, Jianping Xiong
Department of Oncology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, People’s Republic of China
*These authors contributed equally to this work
Background: Aberrant long non-coding RNA (lncRNA) expression contributes cancer development and resistance to therapy. This study first assessed expression of lncRNA LINC00958 in a variety of human cancers using GEPIA database data and then associated it with prognosis of head and neck squamous cell carcinoma (HNSCC) and investigated LINC00958 interaction with c-Myc and the c-Myc-related gene interplay in HNSCC cells.
Materials and methods: A cohort of 48 HNSCC vs normal tissues was collected for qRT-PCR analysis of LINC00958 and c-Myc expression and statistical analyses. HNSCC cell lines were subjected to transfection with LINC00958 and c-Myc siRNAs or cDNA and their negative control siRNA or empty vector for qRT-PCR, Western blot, cell viability, colony formation, luciferase reporter, chromatin immunoprecipitation, and RNA immunoprecipitation assays.
Results: The data showed that LINC00958 expression was upregulated in HNSCC tissues and cell lines, upregulation of which was associated with poor tumor differentiation, advanced tumor stage, and shorter overall survival of patients. In vitro, LINC00958 expression induced HNSCC cell viability and colony formation, whereas knockdown of LINC00958 expression enhanced HNSCC cell sensitivity to ionizing radiation and cisplatin treatment. Mechanistically, LINC00958 is a direct target of c-Myc and can enhance the transcriptional activity of c-Myc, thus to form a positive feedback gene network in HNSCC cells, and in turn to modulate HNSCC cell resistance to chemo- and radiotherapy.
Conclusion: This study demonstrated the LINC00958 interplay with c-Myc as a feedback loop facilitated HNSCC development and resistance to chemo- and radiotherapy. Targeting of such a network could be further evaluated as a novel therapeutic strategy for HNSCC patients.
Keywords: head and neck squamous cell carcinoma, LINC00958, c-Myc, chemo- and radiotherapy
Introduction
Head and neck squamous cell carcinoma (HNSCC) accounts for the sixth most common malignancy in the world and the third most common cancer in developing countries1 and the eighth leading cause of cancer deaths in the world.2 HNSCC risk factors include tobacco smoking, alcohol consumption, infection of Epstein-Barr virus (EBV), human papilloma virus (HPV), or consumption of salted fish and meats.3–5 These risk factors induce genetic instability6 and alter expression of different genes to activate oncogenes, but inactivate tumor suppressor genes to transform normal HN epithelium to tumor cells.7,8 To date, concurrent chemoradiotherapy is a standard treatment option in the management of locally advanced HNSCC,9 although numbers of HNSCC patients showed eventual resistance to chemotherapy and radiotherapy, which leads to dismal treatment response and relapse and poor prognosis.10 Thus, search and identification of the molecular mechanisms of HNSCC carcinogenesis could provide us to better understand HNSCC pathogenesis and novel strategies to effectively control HNSCC clinically.
Towards this end, long noncoding RNAs (lncRNAs), a class of non-protein coding transcripts with more than 200 nucleotides in length, function biologically in cell to regulate expression of miRNAs and protein-coding genes.11–13 Altered expression of lncRNAs was reported to promote human carcinogenesis.14–16 For example, LncRNA CRNDE increased the growth of non-small cell lung cancer cells by activation of the PI3K/AKT signaling,17 while lncRNA CCAT2 could regulate the TGF-β signaling pathway in breast cancer18 and lnc34a induced colorectal cancer cell proliferation by silencing of miR-34a expression.19 LncRNA LINC00958 has been reported to play as an oncogene in bladder cancer,20 glioma21 and pancreatic cancer22 as well as associated with metastasis and poor prognosis in gastric cancer.23 However, the expression pattern, clinical significance, biological function and molecular mechanism of LINC00958 in HNSCC are still unknown. We performed a bioinformatical analysis of LINC00958 alteration in HNSCC or other human cancers and found that LINC00958 expression was upregulated in various human cancers, including HNSCC. Thus, in this study, we first assessed the expression of lncRNA LINC00958 in a variety of human cancers using GEPIA database data and then associated LINC00958 expression with prognosis of HNSCC. After that, we performed a bioinformatic analysis to identify LINC00958-related gene and selected c-Myc for further investigation for their LINC00958 interaction in the regulation of HNSCC cell sensitivity to chemo- and radiotherapy in HNSCC cell lines. We expected to provide novel insightful information regarding targeting of the LINC00958-c-Myc signaling as a novel strategy in future control of HNSCC.
Materials and methods
Patient samples
This study prospectively collected 48 cases of paired HNSCC and adjacent normal tissues from The First Affiliated Hospital of Nanchang University (Nanchang, China) between January 2017 and July 2018. These patients were histologically diagnosed with HNSCC and underwent surgical tumor resection and they didn’t receive any chemoradiotherapy prior to surgery. The study was conducted in accordance with the Declaration of Helsinki. All patients provided written consent for the use of their specimens and disease information for future investigation according to the Ethics Committee of the First Affiliated Hospital, Nanchang University. The fresh tissue samples were then collected from the surgery room, snap-frozen and stored in liquid nitrogen until use, while our experienced pathologists confirmed HNSCC diagnosis using the H&E stained tissue sections. Clinicopathological data from these 48 patients were retrieved from their medical record (Table 1) and statistically analyzed.
 |
Table 1 Relationship between LINC00958 expression and clinicopathological variables (n=48) |
RNA isolation and quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total cellular RNA was isolated from frozen tissue samples and cell lines using a Trizol reagent (Invitrogen, Carlsbad, CA, USA) and reversely transcribed into cDNA using a miScript SYBR Green PCR kit (Invitrogen, Carlsbad, CA, USA) according to the manufacturers’ instructions. qPCR was then conducted in an ABI 7500 real-time PCR system (Applied Biosystems, Foster City, CA, USA) with the STBR Green Mix from Applied Biosystems according to the kit’s provided protocol and the primer-instructed annealing temperature. The qPCR conditions were an initial 95 °C for 2 min and 40 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 40 s. The primer sequences were CDK2, 5ʹ-GCTAGCAGACTTTGGACTAGCCAG-3ʹ and 5ʹ-GCTCGGTACCACAGGGTCA-3ʹ; CDK4, 5ʹ-CTGGTGTTTGAGCATGTAGACC-3ʹ and 5ʹ-AAACTGGCGCATCAGATCCTT-3ʹ; CDC25B, 5ʹ-TCCAGGGAGAGAAGGTGTCT-3ʹ and 5ʹ-TGTCCACAAATCCGTCATCT-3ʹ; CDC45, 5ʹ-TGGACTGCACACGGATCT-3ʹ and 5ʹ-AACCTGGCTGCGGTATAG-3ʹ; CDC20, 5ʹ-GGCACCAGTGATCGACACATTCGCAT-3ʹ and 5ʹ-GCCATAGCCTCAGGGTCTCATCTGCT-3ʹ; CyclinA2, 5ʹ-CTGCATTTGGCTGTGAACTAC-3ʹ and 5ʹ-ACAAACTCTGCTACTTCTGGG-3ʹ; GAPDH, 5ʹ-GGCATCCTGGGCTACACTGA-3ʹ and 5ʹ-GAGTGGGTGTCGCTGTTGAA-3ʹ; LINC00958, 5ʹ-AGAAGGAGGAGAAGCAA-3ʹ and 5ʹ-TGTGAAGTGCAGGGAGGA-3ʹ; U6, 5ʹ-GCTTCGGCAGCAGCACATATACTAAAAT-3ʹ and 5ʹ-CGCTTCACGAATTTGCGTGTCAT-3ʹ. The relative level of mRNA expression was calculated using the comparative 2-ΔΔCt method according to a previous study.24 The experiments were in duplicate and repeated three times.
Cell lines and culture
Human HNSCC cell lines (Detroit 562, Cal27, SCC-9, SCC-15, and Fadu) and a normal oral epithelial cell line (HOK) were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). HEK-293T cell line was obtained from the Shanghai Institute of Life Science, Chinese Academy of Sciences (Shanghai, China). Detroit 562 and Fadu cell lines were grown in the Eagle’s Minimum Essential Medium (EMEM) supplemented with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Inc., Waltham, MA, USA), penicillin and streptomycin and SCC-9 and SCC-15 cells were cultured in F12/Dulbecco’s Modified Eagle’s Medium (DMEM; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS, penicillin and streptomycin, while Cal27 and HEK-293T cells were maintained in DMEM supplemented with 10% FBS, penicillin and streptomycin. HOK cells were cultured in Oral Keratinocyte Medium (iXCells Biotechnologies, California, USA). All the cell lines were cultured in a humidified incubator with 5% CO2 at 37 °C.
siRNA and cell transfection
LINC00958 siRNA (si-LINC00958 #1 and si-LINC00958 #2), c-Myc siRNA (si-c-Myc #1 and si-c-Myc #2), negative control siRNA (si-NT), pcDNA3.1-MYC carrying c-Myc cDNA, pcDNA3.1-LINC00958 carrying LINC00958 cDNA, and a control vector pcDNA3.1 were all purchased from GenePharma Corporation (Shanghai, China). The oligonucleotide sequences targeting si-LINC00958 #1 were 5ʹ-GTGACTAGCTTAAACTAAATT-3ʹ; targeting si-LINC00958 #2, 5ʹ-GAGGTACCCAATAGTTTCATT-3ʹ; si-c-Myc #1, 5ʹ-TACGGAACTCTTGTGCGTAAGC-3ʹ; and si-c-Myc #2, 5ʹ-GCTTCACCAACAGGAACTATGC-3ʹ. For cell transfection, various cell lines were grown and transiently transfected with each siRNA or plasmid using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions and analyzed.
Western blot
Cells with or without gene transfection were lysed using a radioimmunoprecipitation assay buffer (RIPA) containing a protease inhibitors cocktail (Roche, Basel, Switzerland). The protein concentrations were assessed using the bicinchoninic acid (BCA) protein assay kit (Beyotime Biotechnology, Haimen, China) according to the manufacturer’s protocol and equal amount of these protein samples were separated in sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels and transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore Corp., Bedford, MA, USA). The Western blotting was performed as described previously25 using an anti-CDK4 (1:1000, Cell Signaling, Beverly, MA, USA), c-Myc (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), or GAPDH (1:1000, Santa Cruz Biotechnology) antibody.
Cell viability CCK-8 assay
Changed cell viability was assayed using a cell counting kit-8 (CCK-8) kit (Beyotime Biotechnology). In brief, Fadu and Detroit 562 cells were grown and transiently transfected with indicated siRNA and/or plasmid for 48 h, and then re-seeded into 96-well plates at a density of 2×104 cells/well and exposed to various doses of ionizing radiation (0–10 Gy) or treated with different doses of cisplatin (0–25 µM) for 48 h. At the end of each experiment, cell culture was added with 10 µL of the CCK-8 reagent and cells were further cultured for 4 h and the optical density of cell culture was then measured at 450 nm with a spectrophotometer (BioTEK, Winooski, USA). Cell viability was determined in terms of the proportion of cell survival, compared with control.
Colony formation assay
For colony formation assay, cells were grown and transiently transfected with indicated siRNA and/or plasmid for 48 h, and then re-seeded into 6-well plates (500 cells in each well) and exposed to 2 Gy ionizing radiation each or 5 µM cisplatin for 2 weeks. The cell growth medium was refreshed every three days. After that, the cells were fixed in 100% methanol and subsequently stained with 0.4% crystal violet (Beyotime Biotechnology) and cell colonies with 50 cells or more were counted under an inverted microscope (Olympus, NY, USA).
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was performed using the EZ ChIP™ Chromatin Immunoprecipitation Kit from Millipore according to the manufacturer’s instructions. Briefly, after cells underwent gene transfection and treatment, cells were subjected to cross-linking of the chromatin, sonication and then immunoprecipitation of DNA-protein complex using an anti-c-Myc antibody (Santa Cruz Biotechnology) according to the kit’s protocol. After that, the immunoprecipitated DNA-protein complex was extracted for DNA and amplified using qPCR according to a standard qPCR protocol.26
Luciferase reporter assay
In this study, we performed two different sets of luciferase reporter assays, ie, 1). To assess whether LINC00958 modulates c-Myc transcriptional activity, we first obtained the c-Myc-responsive 4x-Ebox reporter construct from GenePharma and then grew HNSCC cells for co-transfection with this c-Myc responsive luciferase reporter and indicated siRNAs/cDNA; and 2). To confirm LINC0958 is a transcriptional target of c-Myc, we constructed the LINC00958 sequences containing a putative MYC-binding site or mutated site and inserted into a luciferase reporter vector. We then grew HEK293T cells for co-transfection with pGL3-LINC00958 or pGL3-LINC00958 mutant and indicated siRNAs. After that, we performed the luciferase reporter assays using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s protocol.
RNA immunoprecipitation (RIP) assay
We performed the RIP assay to assess protein-RNA interaction in the cells using the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore) following the manufacturer’s protocol with an anti-c-Myc antibody and normal IgG (as a negative control; both from Santa Cruz Biotechnology). Gene transfected and treated HEK-293T cells were subjected to immunoprecipitation with the kit reagents and the co-precipitated RNA samples were subjected to qRT-PCR analysis of different mRNAs (see qRT-PCR section) to identify these gene involvements in the immune-complex.
Statistical analysis
In this study, all experiments were performed in triplicate and repeated three times and the data were then summarized as the mean ± standard deviation (SD; n=3) and statistically analyzed by using SPSS version 18.0 software (SPSS, Inc., Chicago, Il, USA). Difference between two groups of samples was assessed by Student’s t-test or one-way analysis of variance (ANOVA). A P-value equal to or less than 0.05 was considered statistically significant.
Results
LINC00958 expression is upregulated and associated with poor prognosis of HNSCC patients
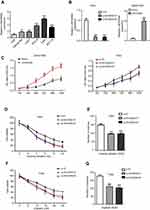
In this study, we first searched the GEPIA database and identified aberrant expression of tumor-related lncRNAs and found that LINC00958 expression was significantly higher in different tumor tissues compared with that of non-tumor tissues (Figure 1A). We then analyzed the level of LINC00958 expression in our HNSCC tissue samples and found that LINC00958 expression was higher in our HNSCC tissues than the paired non-HNSCC normal tissues from 48 patients (Figure 1B). Furthermore, we associated LINC00958 expression with clinicopathological data from these 48 HNSCC patients by dividing patients into high and low LINC00958 expression based on the median value. Our data showed that high LINC00958 expression was associated with poor tumor differentiation (P=0.001) and advanced tumor American Joint Committee on Cancer (AJCC) stage (P=0.003; Table 1). Survival analysis of patients using the GEPIA database (http://gepia.cancer-pku.cn/) (Figure 1C) and Kaplan-Meier plotter website data (http://kmplot.com/analysis/index.php?p=service&cancer=pancancer_rnaseq) (Figure 1D) showed that patients with low LINC00958 expressed HNSCC had better overall survival vs high LINC00958 expressed one.
LINC00958 induces HNSCC cell viability, colony formation, and decreases HNSCC cell sensitivity to ionizing radiation or cisplatin
We then performed in vitro experiments to confirm the tumorigenic effects of LINC00958 in HNSCC cell by first measured LINC00958 levels in various HNSCC cell lines and an immortalized oral epithelial cell line (HOK). Our data showed that upregulation of LINC00958 expression in HNSCC vs HOK cells (Figure 2A). We then selected HNSCC Detroit 562 cells with relative low LINC00958 expression for overexpression of LINC00958 and Fadu cells to knockdown of LINC00958 expression (Figure 2B). Next, we assessed the changed cell viability in these two cell lines and found that LINC00958 overexpression induced Detroit 562 cell viability, whereas knockdown of LINC00958 expression reduced Fadu cell viability (Figure 2C). Moreover, we have subjected these cell lines to radiotherapy or chemotherapy and found that compared to the controls, LINC00958 silenced Fadu cells were more sensitive and had a dose-dependent inhibitory effect on cell viability after the treatment with ionizing radiation or cisplatin (Figure 2D–F). The colony formation assays showed that, in the presence of ionizing radiation or cisplatin, Fadu cells formed less colonies after transfected with LINC00958 siRNAs (Figure 2E–G).
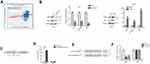
LINC00958 is a downstream gene of c-Myc in HNSCC cells
We then performed a bioinformatical analysis and identified that c-Myc could target LINC00958. Indeed, c-Myc is an oncogene and alteration of the lncRNA-MYC network has been observed in different human cancer.27,28 We then first confirmed their association in our HNSCC tissue samples showing a positive co-expression in tumor tissues (Figure 3A). After that, we knocked down c-Myc expression using c-Myc siRNA in Fadu cells and found that expression of c-Myc-downstream CDK4 and LINC00958 was decreased by c-Myc siRNAs, while overpressing c-Myc by transfecting c-Myc plasmid in Detroit cells increased the expression of CDK4 and LINC00958, indicating that LINC00958 indeed, a potential c-Myc target (Figure 3B). To further confirm it, we analyzed the genomic sequences of LINC00958 cDNA using the JASPAR database and identified a putative MYC binding site in the upstream region of LINC00958 gene (Figure 3C). We then conducted a ChIP assay to assess their binding and found that endogenous c-Myc was able to bind to the LINC00958 promoter, but not to a negative control (Figure 3D). In addition, we constructed luciferase reporter plasmids by cloning a wild-type or mutant LINC00958 promoter region (without putative c-Myc binding site) into pGL3 vector (Figure 3E). Luciferase reporter assay showed that c-Myc siRNAs significantly decreased the luciferase activity of wild-type pGL3-LINC00958 compared to the mutant control (Figure 3F). Taken together, our current data demonstrated that LINC00958 is a direct target of c-Myc, and the Myc-binding site cluster is responsible for c-Myc-induced LINC00958 transcription.
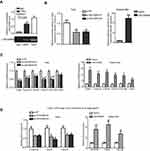
LINC00958 activates the transcriptional activity of c-Myc to form a positive feedback loop
To further investigate the interaction of LINC00958 with c-Myc, we performed the RIP assay to first pull down c-Myc protein from HEK293T cells and then isolated RNA from the immunoprecipitated complex for qRT-PCR analysis of LINC00958. Our data showed that LINC00958 was highly enriched in c-Myc complex (Figure 4A). Our luciferase reporter assay further showed that ectopic expression of LINC00958 induced luciferase activity of the c-Myc responsive construct, whereas knockdown of LINC00958 expression had an inverse effect in HNSCC cells (Figure 4B). Furthermore, LINC00958-overexpressing Detroit cells had higher levels of c-Myc-downstream CDK4, CDC45, and CyclinA2 mRNAs, whereas low LINC00958-expressed Fadu cells showed reduced levels of these transcripts (Figure 4C). In addition, our ChIP assay data further verified these observations showing that the ectopic LINC00958 expression in Detroit562 cells significantly enhanced the c-Myc occupancy in three c-Myc-targeting gene promoters (CDK4, CDC45, and CyclinA2), whereas low LINC00958-expressed Fadu cells showed the inverse data (Figure 4D). These findings suggest that c-Myc could induce LINC00958 expression, while LINC00958 expression further promoted the transcriptional activity of c-Myc and the expression of c-Myc-downstream genes; therefore, to form a positive feedback loop.
LINC00958 induction of HNSCC cell chemo- and radiation resistance is modulated by interplay with c-Myc
So far, we demonstrated the interaction of LINC00958 with c-Myc in HNSCC cells and then explored whether such an interaction could modulate HNSCC cell chemo- and radiation sensitivity in vitro. We transfected LINC00958 silenced Fadu cells with c-Myc cDNA and then assessed the changed tumor cell phenotypes, like cell viability and colony formation. The transfection efficiency was checked by performing Western blot analysis (Figure 5A). And we found that HNSCC cells were regained the resistance to ionizing radiation (Figure 5B and C) and cisplatin (Figure 5D and E) after c-Myc overexpression compared to those of LINC00958-knocked down Fadu cells. This piece of data indicates that LINC00958-c-Myc interaction is the mechanism of LINC00958-related HNSCC cell resistance to chemo- and radiotherapy.
Discussion
Dysregulation of lncRNA expression has been shown to contribute to cancer development and detection of their expression could be useful as therapeutic and/or prognostic biomarkers for different human cancers.29,30 In this study, we first assessed expression of lncRNA LINC00958 in a variety of human cancers using GEPIA database data and then associated LINC00958 upregulation with clinicopathological features and prognosis of head and neck squamous cell carcinoma (HNSCC). We then investigated LINC00958 interaction with c-Myc and their interplay in regulation of HNSCC cell sensitivity to chemo- and radiotherapy in vitro. We found that LINC00958 expression was upregulated in various human cancers and in HNSCC and upregulated LINC00958 expression was associated with poor tumor differentiation and advanced tumor AJCC stage as well as shorter survival of HNSCC patients. Moreover, LINC00958 expression was associated with HNSCC cell resistance to ionizing radiation or cisplatin, whereas knockdown of LINC00958 expression reduced HNSCC cell viability and colony formation and enhanced tumor cell sensitivity to ionizing radiation or cisplatin. At the gene level, LINC00958 is a downstream target of c-Myc in HNSCC cells and LINC00958 enhances the transcriptional activity of c-Myc and the expression of c-Myc transcripts to form a positive feedback loop. This positive feedback loop induced HNSCC cell chemo- and radiation resistance in vitro. In conclusion, the data from our current study revealed a novel mechanism by which LINC00958 interplays with c-Myc to form a feedback loop to promote HNSCC progression and resistance to chemo- and radiotherapy. Future study will further evaluate this network as a novel therapeutic strategy for HNSCC patients.
To date, there are only a few studies reporting the role of LINC00958 in human cancers; for example, LINC00958 was shown to be upregulated in gastric cancer and glioma tissues and associated with tumor metastasis and unfavorable prognosis of patients21,23 and LINC00958 expression was significantly upregulated in lymph node-metastatic bladder cancer and LINC00958 overexpression induced lymphangiogenesis and lymph node metastasis in both bladder cancer cell lines and mouse models,31 whereas silencing of LINC00958 expression suppressed pancreatic cancer progression in vitro.22 Our current study surely confirmed these data showing that LINC00958 expression was upregulated and LINC00958 upregulation was associated with poor prognosis of HNSCC patients, whereas knockdown of LINC00958 expression induced sensitivity of HNSCC cell to chemo- and radiotherapy.
Furthermore, c-Myc, as a transcription factor, is a proto-oncogene and in human cancers, c-Myc is frequently constitutively expressed, which results in enhanced expression of various cell growth-related and apoptosis-resistant genes to promote carcinogenesis.32 Previous studies showed that a remarkable association of aberrant lncRNA expression with c-Myc.7,28,33–35 These publications demonstrated that c-Myc promoted normal cells to neoplastic transformation by affecting a drastic change in the number and the type of gene expression, including various lncRNAs in different human cancers.28 Many of lncRNAs affecting tumor cell viability and proliferation can be found differentially regulated by MYC.28 For example, lncRNA NEAT1, essential to form the nuclear body paraspeckles, was transcriptionally regulated by c-Myc and played a crucial role in imatinib-induced apoptosis in chronic myeloid leukemia cells.35 Transcriptional suppression of c-Myc-altered LncRNA CCAT1 expression contributed to tumorigenesis and metastasis of pancreatic cancer.36 Besides, c-Myc-repressed lncRNA IDH1-AS1 links the function of c-Myc and HIF1a to regulate the Warburg effect in cancer cells.37 However, to date, there is no report showing the interplay of LINC00958 with c-Myc in HNSCC. Our current study is the first to demonstrate that c-Myc promoted LINC00958 expression and LINC00958 promoted the transcriptional activity of c-Myc to form a positive feedback loop in HNSCC and that such network promoted HNSCC resistance to chemo- and radiotherapy.
Based on the concept showing that lncRNA molecules are able to fold into a tridimensional structure and serve as a protein-binding domain to regulate expression of various genes in cells.28,38 And lncRNA can bind to chromatin modifying factors or other transcriptional factors.18,25 Previous studies revealed that different lncRNAs are able to directly bind to c-Myc and affect c-Myc transcriptional activity.7,28,33,34 For instance, lncRNA MINCR was shown to bind and affect c-Myc transcriptional activity by recruiting an MYC co-activator to c-Myc-targeted gene promoters for activation of their transcription in cells,39 while lncRNA PDIA3P26 and PCGEM140 showed to bind to c-Myc to induce gene expression. Other studies revealed lncRNAs can bind to mRNAs and then, in turn, regulate expression of protein-coding genes.11–13 In our current study, we performed the RIP assay and found that LINC00958 level was highly enriched in c-Myc protein-RNA complex, while our luciferase assay data revealed that LINC00958 affected c-Myc transcriptional activity by showing that LINC00958-knocked down Fadu cells had a lower c-Myc responsive luciferase reporter activity, whereas LINC00958-overexpressed Detroit 562 cells exhibited a high c-Myc responsive luciferase reporter activity. However, future study is needed to validate whether LINC00958 oncogenic activity in HNSCC solely depends the c-Myc signaling or other genes and gene pathways are also involved in. To date, as we know, c-Myc plays an important role in the mediation of chemoradiation resistance and increase in c-Myc expression promoted the activity of poly(ADP-ribose) polymerase (PARP)-dependent DNA repair pathways and contributed to chemoresistance.41 c-Myc also induced expression of CHK1 and CHK2 to subsequently activate the DNA-damage-checkpoint response in radioresistance of nasopharyngeal carcinoma cells,42 whereas knockdown of c-Myc expression induced Fas-mediated apoptosis to sensitize lung cancer cells to radiation43 Thus, in any events, targeting of the LINC00958-c-Myc network could induce sensitivity of HNSCC cells to cisplatin and ionizing radiation.
In conclusion, our current study assessed and validated the oncogenic activity of lncRNA LINC00958 in HNSCC and identified the novel LINC00958-c-Myc positive feedback loop in the mediation of HNSCC progression and resistance to chemo- and radiotherapy. This finding could provide a novel molecular therapeutic target for future treatment of HNSCC.
Acknowledgments
This study was supported in part by grants from the National Natural Science Foundation of China (#81860427, #81760432, #81660402, and #81660405), Jiangxi Provincial General Project (#20164BCD40097, #20171BBG70121, and #2017BBH80027), The Youth Scientific Funds-Youth Fund Project (#2018ACB21037 and #20171BAB215041), Jiangxi Provincial Education Fund Project (#700653002), Department of Health of Jiangxi Province Projects (#20181041 and #20195081), Jiangxi Province Postgraduate Special Innovation Funds (#YC2017-B023 and CX2018174), and The Overseas Scholarship of Nanchang University to S. Huang.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Economopoulou P, Kotsantis I, Psyrri A. The promise of immunotherapy in head and neck squamous cell carcinoma: combinatorial immunotherapy approaches. ESMO Open. 2016;1(6):e000122. doi:10.1136/esmoopen-2016-000122
2. Braakhuis BJ, Leemans CR, Visser O. Incidence and survival trends of head and neck squamous cell carcinoma in the Netherlands between 1989 and 2011. Oral Oncol. 2014;50(7):670–675. doi:10.1016/j.oraloncology.2014.03.008
3. McGuire S. World cancer report 2014. Geneva, Switzerland: World Health Organization, international agency for research on cancer, WHO press, 2015. Adv Nutr. 2016;7(2):418–419. doi:10.3945/an.116.012211
4. Andre K, Schraub S, Mercier M, Bontemps P. Role of alcohol and tobacco in the aetiology of head and neck cancer: a case-control study in the Doubs region of France. Eur J Cancer B Oral Oncol. 1995;31B(5):301–309.
5. Kiprian D, Czarkowska-Paczek B, Wyczalkowska-Tomasik A, Paczek L. Human cytomegalovirus and Epstein-Barr virus infections increase the risk of death in patients with head and neck cancers receiving radiotherapy or radiochemotherapy. Medicine. 2018;97(51):e13777. doi:10.1097/MD.0000000000013777
6. Moon JJ, Lu A, Moon C. Role of genomic instability in human carcinogenesis. Exp Biol Med. 2019;1535370219826031. doi:10.1177/1535370219826031
7. Li S, Zhang S, Chen J. c-Myc induced upregulation of long non-coding RNA SNHG16 enhances progression and carcinogenesis in oral squamous cell carcinoma. Cancer Gene Ther. 2019. doi:10.1038/s41417-018-0072-8
8. Xu XC, Ro JY, Lee JS, Shin DM, Hong WK, Lotan R. Differential expression of nuclear retinoid receptors in normal, premalignant, and malignant head and neck tissues. Cancer Res. 1994;54(13):3580–3587.
9. Arunsingh M, Vaidyanathan S, Dyker KE, Sen M, Scarsbrook AF, Prestwich RJD. Accuracy of response assessment positron emission tomography-computed tomography following definitive radiotherapy without chemotherapy for head and neck squamous cell carcinoma. Clin Oncol. 2018. doi:10.1016/j.clon.2018.11.036
10. Elicin O, Cihoric N, Vlaskou Badra E, Ozsahin M. Emerging patient-specific treatment modalities in head and neck cancer - a systematic review. Expert Opin Investig Drugs. 2019;28:365–376. doi:10.1080/13543784.2019.1582642
11. Huan J, Xing L, Lin Q, Xui H, Qin X. Long noncoding RNA CRNDE activates Wnt/beta-catenin signaling pathway through acting as a molecular sponge of microRNA-136 in human breast cancer. Am J Transl Res. 2017;9(4):1977–1989.
12. Chen T, Xue H, Lin R, Huang Z. MiR-34c and PlncRNA1 mediated the function of intestinal epithelial barrier by regulating tight junction proteins in inflammatory bowel disease. Biochem Biophys Res Commun. 2017;486(1):6–13. doi:10.1016/j.bbrc.2017.01.115
13. Yang XJ, Huang CQ, Peng CW, Hou JX, Liu JY. Long noncoding RNA HULC promotes colorectal carcinoma progression through epigenetically repressing NKD2 expression. Gene. 2016;592(1):172–178. doi:10.1016/j.gene.2016.08.002
14. Xue D, Lu H, Xu HY, Zhou CX, He XZ. Long noncoding RNA MALAT1 enhances the docetaxel resistance of prostate cancer cells via miR-145-5p-mediated regulation of AKAP12. J Cell Mol Med. 2018;22(6):3223–3237. doi:10.1111/jcmm.13604
15. Tsai KW, Lo YH, Liu H, et al. Linc00659, a long noncoding RNA, acts as novel oncogene in regulating cancer cell growth in colorectal cancer. Mol Cancer. 2018;17(1):72. doi:10.1186/s12943-018-0821-1
16. Chen L, Dzakah EE, Shan G. Targetable long non-coding RNAs in cancer treatments. Cancer Lett. 2018;418:119–124. doi:10.1016/j.canlet.2018.01.042
17. Liu XX, Xiong HP, Huang JS, Qi K, Xu JJ. Highly expressed long non-coding RNA CRNDE promotes cell proliferation through PI3K/AKT signalling in non-small cell lung carcinoma. Clin Exp Pharmacol Physiol. 2017;44(8):895–902. doi:10.1111/1440-1681.12780
18. Wu ZJ, Li Y, Wu YZ, et al. Long non-coding RNA CCAT2 promotes the breast cancer growth and metastasis by regulating TGF-beta signaling pathway. Eur Rev Med Pharmacol Sci. 2017;21(4):706–714.
19. Wang L, Bu P, Ai Y, et al. A long non-coding RNA targets microRNA miR-34a to regulate colon cancer stem cell asymmetric division. eLife. 2016;5. doi:10.7554/eLife.14620
20. Seitz AK, Christensen LL, Christensen E, et al. Profiling of long non-coding RNAs identifies LINC00958 and LINC01296 as candidate oncogenes in bladder cancer. Sci Rep. 2017;7(1):395. doi:10.1038/s41598-017-00327-0
21. Guo E, Liang C, He X, et al. Long noncoding RNA LINC00958 accelerates gliomagenesis through regulating miR-203/CDK2. DNA Cell Biol. 2018;37(5):465–472. doi:10.1089/dna.2018.4163
22. Chen S, Chen JZ, Zhang JQ, et al. Silencing of long noncoding RNA LINC00958 prevents tumor initiation of pancreatic cancer by acting as a sponge of microRNA-330-5p to down-regulate PAX8. Cancer Lett. 2019;446:49–61. doi:10.1016/j.canlet.2018.12.017
23. Wang W, Song ZJ, Wang Y, Zhong WF, Kang P, Yang Y. Elevated long non-coding RNA LINC00958 was associated with metastasis and unfavorable prognosis in gastric cancer. Eur Rev Med Pharmacol Sci. 2019;23(2):598–603. doi:10.26355/eurrev_201901_16872
24. Liu Z, Huang S, Cao Y, et al. YAP1 inhibits circRNA-000425 expression and thus promotes oncogenic activities of miR-17 and miR-106. Biochem Biophys Res Commun. 2018;503(4):2370–2375. doi:10.1016/j.bbrc.2018.06.163
25. Huang S, Zhu L, Cao Y, et al. Significant association of YAP1 and HSPC111 proteins with poor prognosis in Chinese gastric cancer patients. Oncotarget. 2017;8(46):80303–80314. doi:10.18632/oncotarget.17932
26. Yang X, Ye H, He M, et al. LncRNA PDIA3P interacts with c-Myc to regulate cell proliferation via induction of pentose phosphate pathway in multiple myeloma. Biochem Biophys Res Commun. 2018;498(1):207–213. doi:10.1016/j.bbrc.2018.02.211
27. Wang Z, Yang B, Zhang M, et al. lncRNA epigenetic landscape analysis identifies EPIC1 as an oncogenic lncRNA that interacts with MYC and promotes cell-cycle progression in cancer. Cancer Cell. 2018;33(4):706–20 e9. doi:10.1016/j.ccell.2018.03.006
28. Iaccarino I. lncRNAs and MYC: an intricate relationship. Int J Mol Sci. 2017;18(7):1497. doi:10.3390/ijms18071497
29. Liu Z, Yao Y, Huang S, et al. LINC00662 promotes gastric cancer cell growth by modulating the Hippo-YAP1 pathway. Biochem Biophys Res Commun. 2018;505(3):843–849. doi:10.1016/j.bbrc.2018.09.191
30. Si X, Zang R, Zhang E, et al. LncRNA H19 confers chemoresistance in ERalpha-positive breast cancer through epigenetic silencing of the pro-apoptotic gene BIK. Oncotarget. 2016;7(49):81452–81462. doi:10.18632/oncotarget.13263
31. He W, Zhong G, Jiang N, et al. Long noncoding RNA BLACAT2 promotes bladder cancer-associated lymphangiogenesis and lymphatic metastasis. J Clin Invest. 2018;128(2):861–875. doi:10.1172/JCI96218
32. Gabay M, Li Y, Felsher DW. MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb Perspect Med. 2014;4(6):a014241. doi:10.1101/cshperspect.a014241
33. Li F, Li H, Zhang L, et al. X chromosome-linked long noncoding RNA lnc-XLEC1 regulates c-Myc-dependent cell growth by collaborating with MBP-1 in endometrial cancer. Int J Cancer. 2019. doi:10.1002/ijc.32166
34. Xiao X, Gu Y, Wang G, Chen S. c-Myc, RMRP, and miR-34a-5p form a positive-feedback loop to regulate cell proliferation and apoptosis in multiple myeloma. Int J Biol Macromol. 2019;122:526–537. doi:10.1016/j.ijbiomac.2018.10.207
35. Zeng C, Liu S, Lu S, et al. The c-Myc-regulated lncRNA NEAT1 and paraspeckles modulate imatinib-induced apoptosis in CML cells. Mol Cancer. 2018;17(1):130. doi:10.1186/s12943-018-0884-z
36. Yu Q, Zhou X, Xia Q, et al. Long non-coding RNA CCAT1 that can be activated by c-Myc promotes pancreatic cancer cell proliferation and migration. Am J Transl Res. 2016;8(12):5444–5454.
37. Xiang S, Gu H, Jin L, Thorne RF, Zhang XD, Wu M. LncRNA IDH1-AS1 links the functions of c-Myc and HIF1alpha via IDH1 to regulate the Warburg effect. Proc Natl Acad Sci U S A. 2018;115(7):E1465–E1474. doi:10.1073/pnas.1711257115
38. Moskalev EA, Schubert M, Hoheisel JD. RNA-directed epigenomic reprogramming: an emerging principle of a more targeted cancer therapy? Genes Chromosomes Cancer. 2012;51(2):105–110. doi:10.1002/gcc.20943
39. Doose G, Haake A, Bernhart SH, et al. MINCR is a MYC-induced lncRNA able to modulate MYC’s transcriptional network in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 2015;112(38):E5261–E5270. doi:10.1073/pnas.1505753112
40. Hung CL, Wang LY, Yu YL, et al. A long noncoding RNA connects c-Myc to tumor metabolism. Proc Natl Acad Sci U S A. 2014;111(52):18697–18702. doi:10.1073/pnas.1415669112
41. Ganesan S. MYC, PARP1, and chemoresistance: BIN there, done that? Sci Signal. 2011;4(166):pe15. doi:10.1126/scisignal.2001946
42. Wang WJ, Wu SP, Liu JB, et al. MYC regulation of CHK1 and CHK2 promotes radioresistance in a stem cell-like population of nasopharyngeal carcinoma cells. Cancer Res. 2013;73(3):1219–1231. doi:10.1158/0008-5472.CAN-12-1408
43. Zhang J, Zhou L, Nan Z, et al. Knockdown of cMyc activates Fas-mediated apoptosis and sensitizes A549 cells to radiation. Oncol Rep. 2017;38(4):2471–2479. doi:10.3892/or.2017.5897
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.