Back to Journals » Clinical Epidemiology » Volume 14
Life-Years Lost After Newly Diagnosed Atrial Fibrillation in Patients with Heart Failure
Authors Vinter N , Cordsen P , Lip GYH, Benjamin EJ , Johnsen SP, Frost L , Trinquart L
Received 14 March 2022
Accepted for publication 19 May 2022
Published 31 May 2022 Volume 2022:14 Pages 711—720
DOI https://doi.org/10.2147/CLEP.S365706
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Vera Ehrenstein
Nicklas Vinter,1– 3 Pia Cordsen,3 Gregory YH Lip,4,5 Emelia J Benjamin,6 Søren Paaske Johnsen,3 Lars Frost,1,2 Ludovic Trinquart7– 9
1Diagnostic Centre, University Clinic for Development of Innovative Patient Pathways, Silkeborg Regional Hospital, Silkeborg, Denmark; 2Department of Clinical Medicine, Aarhus University, Aarhus, Denmark; 3Danish Center for Clinical Health Services Research, Department of Clinical Medicine, Aalborg University, Aalborg, Denmark; 4Liverpool Centre for Cardiovascular Science, University of Liverpool and Liverpool Chest & Heart Hospital, Liverpool, UK; 5Department of Clinical Medicine, Aalborg University, Aalborg, Denmark; 6Department of Medicine, Boston University School of Medicine and Department of Epidemiology, Boston University School of Public Health, Boston, MA, USA; 7Tufts Clinical and Translational Science Institute, Tufts University, Boston, MA, USA; 8Institute for Clinical Research and Health Policy Studies, Tufts Medical Center, Boston, MA, USA; 9Department of Biostatistics, Boston University School of Public Health, Boston, MA, USA
Correspondence: Nicklas Vinter, Diagnostic Centre, University Clinic for Development of Innovative Patient Pathways, Silkeborg Regional Hospital, Falkevej 3, Silkeborg, 8600, Denmark, Tel +45 25321675, Email [email protected]
Objective: Prior work estimated excess death rates associated with atrial fibrillation (AF) in heart failure (HF) with hazard ratios (HR). The aim was to estimate the life-years lost after newly diagnosed AF in HF patients.
Methods: Among patients diagnosed with HF in 2008– 2018 in the nationwide Danish Heart Failure Registry, we compared patients with incident AF to referents matched on age, sex, and time since HF. We estimated the marginal hazard ratio (HR) for death and marginal difference in restricted mean survival times (RMST) between AF cases and referents at 10 years after AF diagnosis. We adjusted for sex, age at AF diagnosis, clinical and lifestyle risk factors, and medications.
Results: Among 4463 AF cases and 17,792 referents (mean age 73.7 years, 29% women), the HR was 1.41 (95% CI 1.38; 1.44) but there was evidence of non-proportional hazards. The difference in RMST was − 18.2 months (95% CI − 16.8; − 19.6) at 10 years after AF diagnosis. There were differences in life-years lost between patients diagnosed with AF > 1 year and ≤ 1 year after HF (− 25.7 months, 95% CI − 23.7; − 27.7 vs − 10.4 months, 95% CI − 8.2; − 12.5, p < 0.001), women and men (− 20.3 months, 95% CI − 17.7; − 21.9 vs − 17.2 months, 95% CI − 15.5; − 19.0, p = 0.05), patients with low, medium, and high CHA2DS2-VASc (10.3 months, 95% CI − 4.6; − 16.1 vs − 18.5 months, 95% CI − 16.7; − 20.4 vs 22.1, 95% CI − 18.8; − 22.3, p = 0.002).
Conclusion: HF patients with incident AF lost on average 1.5 life-years over 10 years after AF. Life-years lost were larger among patients diagnosed with AF > 1 year after HF, women, and patients with higher CHA2DS2-VASc.
Keywords: atrial fibrillation, heart failure, RMST, prognosis, mortality, sex
Introduction
Heart failure (HF) is associated with considerable burden worldwide, with high prevalence and mortality rates.1,2 The lifetime risk of HF ranges from 20% to 45% among individuals aged 45 years and older.3,4 In the setting of HF, atrial fibrillation (AF) is common. AF in HF is associated with HF progression, increased rate of stroke, and higher all-cause and cardiovascular mortality rates.5–8 Newly diagnosed AF in HF carries a worse prognosis compared with HF alone and HF with prevalent AF and is associated with an approximately 2-fold increase in death rate.8
AF and HF share common risk factors and AF in HF may be a marker for the duration and intensity of exposure to such risk factors.9 Given the increasing prevalence of HF and AF, it is of public health importance to improve our understanding of the role of AF in HF and to study health interventions to improve the prognosis of these patients.10
In this context, a well-established but underused measure of effects, the difference in restricted mean survival times (RMST), can provide clinically meaningful information to providers and patients.11–13 In HF, the RMST would indicate the expected amount of time an individual will be alive, up to a specified time point. Additionally, the difference in RMST between groups of HF patients with and without AF measures how much longer, on average, individuals without AF live over a fixed time horizon. By giving an absolute measure of effects in the time domain, the difference in RMST complements conventional measures based on relative or absolute risks to communicate clinical evidence and may support shared decision-making. Prior work on AF in HF measured the association by using hazard ratios (HR). However, misinterpretation of HR can lead to exaggeration of the true effect.14 Our objective was to estimate differences in RMST and in survival probabilities between HF patients with and without incident AF over 1, 5, and 10 years.
Materials and Methods
Data Sources
In this nationwide registry-based matched cohort study, we matched HF patients with AF to HF referents without AF. We selected patients diagnosed with incident HF between 2008 and 2018 from the Danish Heart Failure Registry (DFHR).
The DHFR is a nationwide clinical quality database established in 2003 to monitor and improve the quality of care for HF patients in Denmark.15 Registration of HF patients in the DFHR is mandatory for all Danish hospitals. A first-time hospital contact with a primary diagnosis of HF leads to enrollment in the DHFR. The diagnosis is made by a cardiologist using the diagnostic criteria from the Danish National Society for Cardiology and the European Society of Cardiology.15 Enrollment requires both HF symptoms and objective signs of HF at rest, and possibly clinical improvement on HF treatment. Exclusion criteria for the DHFR are previously known HF, HF caused by congenital heart disease, valvular heart disease, or rapid heart rhythm (including AF), HF diagnosed concurrently with a primary diagnosis of acute myocardial infarction, isolated right-sided HF, or HF previously diagnosed and treated by a private practitioner of cardiology. To ensure sufficient time for the registry-based sampling of history of diseases, we further excluded HF patients who lived in Denmark for less than five years before the HF diagnosis. Additionally, we excluded patients with a history of AF (or atrial flutter) who were not excluded by the DHFR (Supplemental Table 1).
We also used the Danish National Patient Registry, the Danish National Prescription Registry, and the Danish Civil Registration System to collect HF patient covariates and outcomes (Supplemental Text 1).
Incident AF Cases and Matched Referents
Among selected patients with HF, we identified cases with incident primary or secondary diagnoses of AF or atrial flutter by using the International Classification of Diseases 10th revision code I48 in the National Patient Registry (Supplemental Table 1). For each AF case, the date of new AF diagnosis defined the index date at which the risk period started. For each AF case, we chose up to 4 HF patients randomly selected among those without AF at the case index date and with the same age at HF diagnosis, age at case index date, and sex as the case (Supplemental Figure 1). We sampled referents with replacement, so an HF patient could be selected as a referent for multiple AF cases, in which case they became at risk at the corresponding index dates (Supplemental Figure 1). Additionally, a referent could become an AF case at an older age, in which case we censored the referent at the time of AF diagnosis.
All-Cause Mortality
We retrieved vital status and date of death from the Danish Civil Registration System. We followed up each AF case from the index date until the earliest of death, heart transplantation, emigration, or end of follow-up (December 31, 2018). We followed up each referent from the matched case’s index date until the earliest of newly diagnosed AF, death, heart transplantation, emigration, or end of follow-up (December 31, 2018). If a referent was later diagnosed with incident AF, we censored this referent at the date of AF diagnosis.
Covariates
We considered sex, age at index date, lifestyle factors, clinical characteristics, comorbidities, and medications (Supplemental Table 2).
Lifestyle factors included alcohol consumption and smoking at the time of HF diagnosis. Elevated alcohol consumption was defined as more than 14 and 21 drinks per week for women and men until July 1, 2015. After that date, it was defined as more than 7 and 14 drinks per week for women and men. Smoking status was categorized into current, former, or never smoker.
Clinical characteristics included left ventricular ejection fraction (LVEF) and New York Heart Association (NYHA) classification. We categorized LVEF into <25%, 25–40%, >40–49%, and ≥50%.16 Patients underwent echocardiography no later than seven days after the diagnosis of HF and up to six months before the diagnosis of HF if the cardiologist considered the examination relevant. Additionally, patients were categorized according to NYHA functional classification into classes I, II, and III/IV at the time of HF diagnosis or up to 12 weeks after the diagnosis.
Comorbidities included history of myocardial infarction, any stroke/transient ischemic attack, diabetes mellitus, chronic obstructive pulmonary disease, hypertension, chronic kidney disease, overweight/obesity (using diagnosis code), and any type of cancer except nonmelanoma skin cancer (Supplemental Table 2).
HF-related treatments included angiotensin-converting enzyme (ACE) inhibitors/angiotensin II receptor blockers (ARBs), beta-blockers, and mineralocorticoid receptor antagonists (MRAs). Antithrombotic treatments included oral anticoagulants and antiplatelet drugs. We assumed that patients were under treatment if they redeemed at least 1 prescription within 6 months before the index date.
Statistical Analyses
We used the Royston–Parmar flexible parametric approach to model the log cumulative hazard of death according to case/referent status and covariates listed in Table 1. We used a restricted cubic spline with 5 degrees of freedom to model the baseline cumulative hazards, and the internal knots were placed at the 20th, 40th, 60th, and 80th centiles of the distribution of the uncensored log survival times.
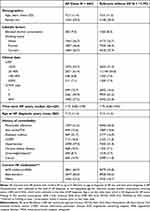 |
Table 1 Characteristics of HF Patients with Incident AF and Matched Referents |
We then used G-computation to obtain marginal survival curves among HF patients with and without AF.17 We used the fitted flexible parametric model to calculate the predicted survival curve for each individual according to their observed covariate pattern. We calculated each individual predicted survival curve under two counterfactuals, by assuming that all HF patients have AF and that all HF patients do not have AF. By averaging all individual curves under each counterfactual, we obtained marginal survival curves for HF patients with and without AF. This enabled a comparison focusing on the difference between the two populations of HF patients with and without AF by forcing the same covariate distribution over all potential confounders included in the model.
Based on the marginal survival curves, we derived marginal HRs. We tested the proportional hazards assumption by a likelihood ratio test comparing the flexible parametric model with and without AF as a time-dependent effect. We also plotted the marginal HR as a function of time. We extended this approach to calculate marginal survival probabilities and marginal RMST. Individual predicted RMST was estimated by the area under the individual predicted survival curve. We averaged over the marginal distribution of potential confounders for all the predicted survival probabilities and RMST obtained for each individual, under each counterfactual. Finally, we calculated the difference in survival probabilities and in RMST at 1, 5, and 10 years. We derived the associated 95% confidence intervals (95% CI) by using the delta method.
We used multiple imputation to account for missing values (Supplemental Text 2). We implemented multiple imputation with the Fully Conditional Specification (multivariate imputation by chained equations), with linear regression for imputation of continuous variables, logistic regression for binary variables, and ordinal logistic regression for categorical variables. We created 10 imputed datasets and combined the estimates with Rubin’s rules. We used Stata Statistical Software (StataCorp. 2019.: Release 16.1. College Station, TX: StataCorp LLC.), the sttocc command to match AF cases to referents, the stpm2 command to fit the flexible parametric model, and the standsurv command to derive marginal survival curves, probabilities, and RMST.
Subgroup Analyses and Sensitivity Analyses
We performed subgroup analyses according to time from HF to AF diagnosis (≤1 year vs >1 year), sex, age (<75 vs ≥75 years), LVEF (≤40%, >40–49%, and ≥50%), and CHA2DS2-VASc score at index date (low, medium, or high). The low CHA2DS2-VASc group included women with a score of 1 or 2 and men with a score of 1. The medium group included women with a score of 3 to 5 and men with a score of 2 to 4. Finally, the high group included women with a score of >5 and men with a score of >4.18 We tested for differences between subgroups by using meta-regression models. Furthermore, we performed two sensitivity analyses to account for the influence of underlying conditions with a high case-fatality rate. First, we excluded patients who died within 30 days after the HF diagnosis. Second, we excluded patients who died within 30 days after the diagnosis of AF.
Ethics
Register-based studies with de-identified data and no active participation by study subjects do not require approval by an ethics committee in Denmark. The Danish Data Protection Agency approved the use of register-based data.
Patient and Public Involvement
No patients were involved in the design of the study or interpretation of the findings, as we did not have patients or members of the public available to us with the level of methodological and statistical experience needed to examine the data.
Results
Patient Characteristics
The study population consisted of 4463 AF cases and 17,792 matched referents (Supplemental Figure 2). The median time from diagnosis of HF to diagnosis of AF was 1.8 years. The cumulative incidence of AF was 23.5% 10 years after the diagnosis of HF when accounting for the competing risk of death (Supplemental Figure 3). The mean age at the diagnosis of AF was 74 years, and 29% were female (Table 1). Hypertension and diabetes were the most prevalent comorbidities among cases and matched referents. The prevalent use of ACE inhibitors/ARBs, beta-blockers, and/or MRA was higher among cases. For oral anticoagulants, 49.4% received treatment after AF at 3 months, and 29.1% received treatment for antiplatelet drugs after AF diagnosis (Supplemental Table 3).
Comparison of Survival Between HF Patients with and without AF
Among AF cases and referents, the median follow-up was 3.4 years from the index date. Among AF cases, 47.9% (N = 2138) of died, and among referents, 26.0% (N = 4663) died. Figure 1A shows the marginal survival curves. The marginal cumulative mortality was higher among HF patients with AF. The absolute difference in marginal cumulative mortality risk between HF patients between with and without AF was 17.0% (95% CI 15.7–18.3) at 10 years (Table 2).
 |
Table 2 Differences in Marginal Cumulative Mortality Risks and Restricted Mean Survival Times Between HF Patients with and without AF |
 |
Figure 1 (A) Marginal survival curves, (B) marginal restricted mean survival times, and (C) difference between marginal restricted mean survival times with 95% confidence interval. Marginal curves adjusted for characteristics in Table 1. |
Figure 1B and Table 2 show the marginal RMST among HF patients with and without AF. The difference in marginal RMST between HF patients with and without AF was −18.2 months (95% CI −16.8 to −19.6) at 10 years after the index date (Table 2 and Figure 1C). Thus, HF patients with AF lose on average 1.5 years of life expectancy over 10 years following their AF diagnosis.
The corresponding marginal HRs were 1.81 (95% CI 1.76–1.85) at 1 year, 1.57 (95% CI 1.53–1.60) at 5 years, and 1.41 (95% CI 1.38–1.44) at 10 years. However, there was evidence of non-proportional hazards (p < 0.001) and the marginal HRs decreased over time (Supplemental Figure 4).
Subgroup and Sensitivity Analyses
At 10 years, we found that the loss in expected lifetime was greater among patients diagnosed with AF more than 1 year after the diagnosis of HF, female patients, and patients with higher CHA2DS2-VASc score (Table 3). We found no substantial difference between age groups or across LVEF groups. All stratified baseline characteristics and detailed results are given in Supplemental Tables 4–14 and Supplemental Figures 5–14. In sensitivity analyses, results were consistent with the main analyses (Supplemental Tables 15 and 16).
 |
Table 3 Difference in Marginal Cumulative Mortality Risks and Restricted Mean Survival Times at 10 Years Between HF Patients with and without AF Among Subgroups |
Discussion
In this nationwide study of patients with incident HF, we found that patients diagnosed with incident AF lose on average 1.5 life-years over 10 years after AF diagnosis. Life-years lost were larger among patients diagnosed with AF >1 year after HF, women, and patients with a high CHA2DS2-VASc score. We found no substantial differences between the age groups or across the LVEF subgroups.
Previous work on AF and HF relied on Cox proportional hazards models to assess associations.5,6 The results were generally expressed in terms of HRs, which measure relative and not absolute effects. It can then be difficult to interpret the clinical significance of the magnitude of the excess mortality risk. HRs are not meaningful when there is a departure from proportional hazards. In our analysis, the HR decreased from ~1.9 down to ~1.4 over time. We quantified the association between newly diagnosed AF and mortality among HF patients in terms of absolute risks and mean survival times, which do not rely on the proportional hazards assumption. These measures are easily interpretable and may support clinical decision-making. Additionally, the considerable loss in life expectancy highlights the need to prevent the development of AF to improve the prognosis in the high-mortality risk population of HF patients.19
In our study sample, the cumulative incidence of AF at 10 years after the diagnosis of incident HF was 24%, comparable with cumulative incidence of 20% after 8 years among patients with prevalent HF in the Framingham Heart Study (FHS).6 Several community-based studies previously examined the association between incident AF and mortality rates among HF patients. The FHS and the Olmsted County Cohort reported that incident AF in HF was associated with an HR of 1.89 (95% CI 1.51–2.38) and 2.22 (95% CI 1.93–2.57) for all-cause mortality, respectively.5,6 A recent Danish study reported a higher mortality rate ratio of 4.32 (95% CI 4.86–5.02).20 However, Barillas-Lara et al were unable to adjust for clinical characteristics, such as LVEF and NYHA class. Identification of HF patients used the general patient registry instead of the DHFR. In our analysis, the HRs for AF and all-cause death in HF were consistent with results from the FHS and Olmsted County Cohort; however, we found that the association decreased over time, from an HR of 1.81 at 1 year to an HR of 1.41 at 10 years after AF. A decline in mortality risk over time may suggest that AF is often diagnosed in connection with symptomatic or hemodynamic deterioration or another acute HF-related event with high short-term mortality such as acute myocardial infarction. We found consistent findings in a sensitivity analysis after excluding patients dying within 30 days after AF.
The expected loss of lifetime was markedly increased among patients diagnosed with AF beyond 1 year after the diagnosis of HF compared to patients diagnosed within 1 year after HF. Data from the Olmsted County Cohort highlighted a similar difference (3-fold increased rate vs 1.5-fold increased rate).5 A possible explanation may be that incident AF beyond 1 year is a marker of HF progression and/or frailty in HF.7,21 Our sex-specific analysis revealed that incident AF was associated with a greater loss in expected lifetime among women than men.22 AF is associated with higher mortality among women in the non-HF setting, and our data support recent work that shows higher mortality among women in an HF-setting.20 There was no evidence of difference between age groups with regard to life-years lost, which is consistent with the study by Barillas-Lara et al.20 However, the difference in mortality risk between HF patients with and without AF was larger among those <75 years. Interactions depend on the analysis scale, and interaction on the cumulative risk scale is mathematically possible without interaction on the RMST scale.23 Similarly, there was no evidence of difference across the three LVEF groups. The FHS study and the Olmsted County cohort study reported similar findings of no substantial difference between HF with reduced and preserved LVEF,5,6 and a meta-analysis supported this finding.8
Recent trials on the management of patients with AF and HF may indicate treatment strategies relating to how the number of lost life-years to AF may be reduced. Compared to medical therapy, catheter ablation was associated with a lower rate of the composite end point of all-cause mortality or hospitalization for worsening HF.24 Early rhythm-control therapy was associated with a lower risk of adverse cardiovascular outcomes than usual care among patients with early AF and HF.25 Recent evidence suggest that electrical cardioversion of AF in concomitant HF with reduced LVEF improves LVEF rapidly.26 Catheter ablation may be superior to medical rhythm control in improving LVEF in the long term.27 However, no studies have so far quantified the improvement in absolute life-years concerning catheter ablation and/or early rhythm control. Multidisciplinary structured care specifically addressing patients with both HF and AF has to be developed and tested using usual care as a comparator.
Limitations
We may have missed patients with prior AF whose diagnosis was not recorded or not recognized in the registries. We were unable to clinically evaluate the patients for undiagnosed AF, use systematic Holter-based screening, examine electrocardiograms to validate the diagnosis, and classify the type of AF. Studies that validated the AF diagnosis coded in the registry have shown positive predictive values of 92% and 95%.28,29 However, non-differential misclassification of AF registration is possible.
A small percentage of participants had missing covariate values and, considering that the missing-at-random or missing-completely-at-random assumptions were plausible, we performed multiple imputation analyses. We did not explore how large a missing-not-at-random mechanism would need to be to influence our findings, and we did not explore nonignorable imputation model to potentially adjust these analyses.
In the analysis stratified by LVEF group, we excluded patients with missing LVEF. These patients included mainly women and patients of higher age. As female patients may have the greatest loss of expected lifetime associated with AF, we may have underestimated the difference in RMST. We adjusted for several relevant covariates but cannot rule out residual confounding. For instance, we had no data on body mass index or genetics. Furthermore, some of the clinical characteristics data, such as LVEF, were ascertained at the diagnosis of HF and may have changed over years.
The somehow restrictive inclusion and exclusion criteria of the DHFR and the fact that the population consisted mainly of European ancestry may reduce the generalizability of our results. Furthermore, the prevalence of HF with preserved LVEF was lower compared to most population-based studies.30 Hence, the generalizability of our findings to HF with preserved LVEF, which excessively affects women, is uncertain. We examined newly diagnosed AF after HF, and we cannot generalize the findings to patients in whom AF was the cause of HF.
Conclusions
This nationwide study shows that HF patients diagnosed with incident AF lose on average 1.5 life-years over 10 years after AF diagnosis. The mean number of life-years lost was larger among HF patients diagnosed with AF more than 1 year after HF, female patients, and patients with higher CHA2DS2-VASc score.
Abbreviations
ACE, angiotensin-converting enzyme; AF, atrial fibrillation; ARB, angiotensin II receptor blockers; DHFR, Danish Heart Failure Registry; FHS, Framingham Heart Study; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonists; NYHA, New York Heart Association; RMST, restricted mean survival time; 95% CI, 95% confidence interval.
Data Sharing Statement
Permission to access the data used in this study can be obtained following approval from the Danish Health Authority.
Funding
This project was supported by grants from the Health Research Fund of Central Denmark Region (R38-A1385-B1044) and The Danish Heart Foundation (16-R107-A3987). Dr Benjamin is supported in part by 2R01 HL092577; 1R01 HL141434; 2U54HL120163; 1R01AG066010; 1R01AG066914; American Heart Association, AHA_18SFRN34110082. Dr Frost is supported by the Health Research Foundation of Central Denmark Region. Dr Trinquart is supported by the American Heart Association 18SFRN34150007.
Disclosure
GYHL is a consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are received personally. LF is a member of Advisory Boards for Pfizer, BMS and MSD. The authors report no other conflicts of interest in this work.
This project was presented at the AHA Scientific Sessions 2021 Conference as a poster presentation with interim findings. The poster’s abstract was published as Abstract 13838 in Circulation. 2021;144(Suppl_1):A13838-A13838.
References
1. Bragazzi NL, Zhong W, Shu J, et al. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur J Prev Cardiol. 2021;28(15):1682–1690. doi:10.1093/eurjpc/zwaa147
2. Virani SS, Alonso A, Aparicio HJ, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–e743. doi:10.1161/CIR.0000000000000950
3. Huffman MD, Berry JD, Ning H, et al. Lifetime risk for heart failure among white and black Americans: cardiovascular lifetime risk pooling project. J Am Coll Cardiol. 2013;61(14):1510–1517. doi:10.1016/j.jacc.2013.01.022
4. Bleumink GS, Knetsch AM, Sturkenboom MC, et al. Quantifying the heart failure epidemic: prevalence, incidence rate, lifetime risk and prognosis of heart failure The Rotterdam Study. Eur Heart J. 2004;25(18):1614–1619. doi:10.1016/j.ehj.2004.06.038
5. Chamberlain AM, Redfield MM, Alonso A, Weston SA, Roger VL. Atrial fibrillation and mortality in heart failure: a community study. Circ Heart Fail. 2011;4(6):740–746. doi:10.1161/CIRCHEARTFAILURE.111.962688
6. Santhanakrishnan R, Wang N, Larson MG, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484–492. doi:10.1161/CIRCULATIONAHA.115.018614
7. Aleong RG, Sauer WH, Davis G, Bristow MR. New-onset atrial fibrillation predicts heart failure progression. Am J Med. 2014;127(10):963–971. doi:10.1016/j.amjmed.2014.06.006
8. Odutayo A, Wong CX, Williams R, Hunn B, Emdin CA. Prognostic importance of atrial fibrillation timing and pattern in adults with congestive heart failure: a systematic review and meta-analysis. J Card Fail. 2017;23(1):56–62. doi:10.1016/j.cardfail.2016.08.005
9. Lubitz SA, Benjamin EJ, Ellinor PT. Atrial fibrillation in congestive heart failure. Heart Fail Clin. 2010;6(2):187–200. doi:10.1016/j.hfc.2009.11.001
10. Al-Khatib SM, Benjamin EJ, Albert CM, et al. Advancing research on the complex interrelations between atrial fibrillation and heart failure: a report from a us national heart, lung, and blood institute virtual workshop. Circulation. 2020;141(23):1915–1926. doi:10.1161/CIRCULATIONAHA.119.045204
11. Staerk L, Preis SR, Lin H, et al. Novel risk modeling approach of atrial fibrillation with restricted mean survival times: application in the Framingham heart study community-based cohort. Circ Cardiovasc Qual Outcomes. 2020;13(4):e005918. doi:10.1161/CIRCOUTCOMES.119.005918
12. Vinter N, Huang Q, Fenger-Grøn M, Frost L, Benjamin EJ, Trinquart L. Trends in excess mortality associated with atrial fibrillation over 45 years (Framingham Heart Study): community based cohort study. BMJ. 2020;370:m2724. doi:10.1136/bmj.m2724
13. Conner SC, Sullivan LM, Benjamin EJ, LaValley MP, Galea S, Trinquart L. Adjusted restricted mean survival times in observational studies. Stat Med. 2019;38(20):3832–3860. doi:10.1002/sim.8206
14. Weir IR, Marshall GD, Schneider JI, et al. Interpretation of time-to-event outcomes in randomized trials: an online randomized experiment. Ann Oncol. 2019;30(1):96–102. doi:10.1093/annonc/mdy462
15. Schjodt I, Nakano A, Egstrup K, Cerqueira C. The Danish heart failure registry. Clin Epidemiol. 2016;8:497–502. doi:10.2147/CLEP.S99504
16. Bozkurt B, Coats AJS, Tsutsui H, et al. Universal definition and classification of heart failure: a report of the heart failure society of America, heart failure association of the European society of cardiology, Japanese heart failure society and writing committee of the universal definition of heart failure: endorsed by Canadian heart failure society, heart failure association of India, the cardiac society of Australia and New Zealand, and the Chinese Heart Failure Association. Eur J Heart Fail. 2021;23(3):252–380.
17. Sjölander A. Regression standardization with the R package stdReg. Eur J Epidemiol. 2016;31(6):563–574. doi:10.1007/s10654-016-0157-3
18. Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2020;42(5):373–498.
19. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? Eur Heart J. 2015;36(46):3250–3257. doi:10.1093/eurheartj/ehv513
20. Barillas-Lara MI, Monahan K, Helm RH, et al. Sex-specific prevalence, incidence, and mortality associated with atrial fibrillation in heart failure. JACC Clin Electrophysiol. 2021;7(11):1366–1375. doi:10.1016/j.jacep.2021.02.021
21. Pulignano G, Del Sindaco D, Tinti MD, et al. Atrial fibrillation management in older heart failure patients: a complex clinical problem. Heart Int. 2016;11(1):e41–e49. doi:10.5301/heartint.5000230
22. Magnussen C, Niiranen TJ, Ojeda FM, et al. Sex differences and similarities in atrial fibrillation epidemiology, risk factors, and mortality in community cohorts: results from the BiomarCaRE consortium (biomarker for cardiovascular risk assessment in Europe). Circulation. 2017;136(17):1588–1597. doi:10.1161/CIRCULATIONAHA.117.028981
23. Rothman KJ, Lash TL, VanderWeele TJ, Haneuse S. Modern Epidemiology.
24. Marrouche NF, Brachmann J, Andresen D, et al. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378(5):417–427. doi:10.1056/NEJMoa1707855
25. Kirchhof P, Camm AJ, Goette A, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383(14):1305–1316. doi:10.1056/NEJMoa2019422
26. Müller-Edenborn B, Minners J, Allgeier J, et al. Rapid improvement in left ventricular function after sinus rhythm restoration in patients with idiopathic cardiomyopathy and atrial fibrillation. Europace. 2019;21(6):871–878. doi:10.1093/europace/euz013
27. Sugumar H, Prabhu S, Costello B, et al. Catheter ablation versus medication in atrial fibrillation and systolic dysfunction: late outcomes of CAMERA-MRI study. JACC Clin Electrophysiol. 2020;6(13):1721–1731. doi:10.1016/j.jacep.2020.08.019
28. Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi:10.2147/CLEP.S91125
29. Sundboll J, Adelborg K, Munch T, et al. Positive predictive value of cardiovascular diagnoses in the Danish National Patient Registry: a validation study. BMJ Open. 2016;6(11):e012832. doi:10.1136/bmjopen-2016-012832
30. Pfeffer MA, Shah AM, Borlaug BA. Heart failure with preserved ejection fraction in perspective. Circ Res. 2019;124(11):1598–1617. doi:10.1161/CIRCRESAHA.119.313572
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
