Back to Journals » Cancer Management and Research » Volume 12
Lidocaine Suppresses Cell Proliferation and Aerobic Glycolysis by Regulating circHOMER1/miR-138-5p/HEY1 Axis in Colorectal Cancer
Authors Du J, Zhang L, Ma H, Wang Y, Wang P
Received 6 January 2020
Accepted for publication 28 May 2020
Published 25 June 2020 Volume 2020:12 Pages 5009—5022
DOI https://doi.org/10.2147/CMAR.S244973
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
This paper has been retracted.
Juan Du,* Liying Zhang,* Hongzhong Ma, Yang Wang, Pengpeng Wang
Department of Anesthesiology, The Affiliated Yantai Yuhuangding Hospital of Qingdao University, Yantai 264000, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hongzhong Ma Tel +86-535-6691999
Email [email protected]
Background: Increasing evidence has uncovered the anticancer activity of lidocaine in many cancers. However, the role and the underlying molecular mechanism of lidocaine in colorectal cancer (CRC) remain poorly understood.
Materials and Methods: Cell viability and apoptosis were measured by cell counting kit-8 assay and flow cytometry. Western blot was used to detect the protein of p53, CyclinD1, Pro-caspase-3, Cleaved-caspase-3, Pro-caspase-9, Cleaved-caspase-9, and hes-related family bHLH transcription factor with YRPW motif 1 (HEY1). Glycolytic metabolism was calculated by measuring the glucose consumption, lactate production and adenosine triphosphate (ATP) contents. The expression of circRNA homer scaffold protein 1 (circHOMER1), microRNA (miR)-138-5p and HEY1 mRNA was detected by quantitative real-time polymerase chain reaction. The interaction between miR-138-5p and circHOMER1 or HEY1 was analyzed using the dual-luciferase reporter assay. In vivo experiments were performed using the murine xenograft model.
Results: Lidocaine suppressed CRC cell viability and aerobic glycolysis but promoted cell apoptosis in vitro as well as hindered tumor growth in vivo. CircHOMER1 was elevated in CRC tissues and cells, while lidocaine decreased circHOMER1 expression in CRC cells. Additionally, circHOMER1 overexpression reversed the anti-tumor activity of lidocaine in CRC cells. miR-138-5p was confirmed to interact with circHOMER1 and HEY1 in CRC cells directly, and circHOMER1 regulated HEY1 expression through repressing miR-138-5p expression. Besides, rescue assay indicated the anti-tumor activity mediated by lidocaine could be regulated by circHOMER1/miR-138-5p/HEY1 axis.
Conclusion: Lidocaine mediated CRC cell viability loss, apoptosis induction and aerobic glycolysis inhibition by regulating circHOMER1/miR-138-5p/HEY1 axis, providing a novel treatment option for lidocaine to prevent the progression of CRC.
Keywords: circHOMER1, miR-138-5p, HEY1, CRC, lidocaine, aerobic glycolysis
Introduction
Colorectal cancer (CRC) is the third most common lethal malignancies worldwide and results in massive cancer-related deaths each year.1 Despite the roughly double of the average survival time in advanced CRC with the improvement of multimodality treatment methods, such as surgical resection combined with chemotherapy or radiotherapy, its 5-year survival rate is lower than 14% once metastasis occurs.2,3 Rapid and sustained proliferation of cancer cells is the linchpin of the malignant enlargement, which results in the organ compression and subsequent migration and invasion.4 Thus, a better understanding of the molecular mechanisms of CRC cell growth is necessary for developing novel therapeutic strategies against CRC.
Retrospective studies have documented multiple cancer patients can benefit from regional anesthesia to improve the long-term survival rate of patients after surgery and decline cancer recurrence.5,6 Lidocaine is a commonly used local anesthetic in clinical.7 However, in addition to multiple anesthetic effects, emerging evidence has identified the antitumor effects of lidocaine in many cancers.8 For example, lidocaine suppressed lung cancer cell proliferation by modulating the inhibition of GOLT1A.4 Lidocaine performed anti-proliferative and apoptotic effects by regulating p53 expression in hepatocarcinoma cells to suppress tumor growth.9 Besides that, Qu et al discovered that lidocaine suppressed proliferation and stimulated apoptosis in CRC cells through regulating miR-520a-3p/EGFR,10 suggesting the possible effects of lidocaine on CRC cell growth. Nevertheless, the function and underlying mechanisms of lidocaine in CRC cells in vitro and in vivo remain vague. So far, it has been revealed that most cancer cells prefer to taking adenosine triphosphate (ATP) through aerobic glycolysis rather than oxidative phosphorylation regardless of oxygen available to meet their metabolic needs for sustained cell proliferation.11,12 Suppression of aerobic glycolysis is an effective therapeutic method to repress tumor growth.13 In addition, a recent study showed propofol, an intravenous anesthetic, could inhibit aerobic glycolysis in CRC cells to impeded tumor growth.14 Therefore, we speculated lidocaine might also involve in the regulation of aerobic glycolysis in CRC.
Increasing studies have shown that circular RNAs (circRNAs) play important roles in a wide variety of cancers through involving in the oncogenesis and the malignant behavior of cancer cells, such as cell migration, cell cycle, proliferation, apoptosis and drug sensitivity.15–17 CircRNA homer scaffold protein 1 (circHOMER1) is a novel identified circRNA molecule in CRC. Li et al investigated that circHOMER1 was up-regulated in CRC tissues, and potentially involved in the pathogenesis of CRC; besides, circHOMER1 could target microRNAs (miRNAs), which implicated in the KEGG pathway of CRC and miRNAs in cancer.18 In addition, some circRNAs have been found to participate in the regulation of aerobic glycolysis to affect tumor cell growth and progression.19,20 However, the role of circHOMER1 in aerobic glycolysis in CRC is still poorly unclear.
In this study, we mainly concentrated on studying the role of lidocaine in CRC cell proliferation and aerobic glycolysis, explored the relationship between lidocaine and circHOMER1 as well as the molecular mechanisms of circHOMER1 in CRC cell tumorigenesis.
Materials and Methods
Clinical Specimens
Tumor tissues and adjacent normal tissues from 30 CRC patients who underwent surgical resection at the Affiliated Yantai Yuhuangding Hospital of Qingdao University were collected and immediately frozen in liquid nitrogen until further analysis. All patients were diagnosed by histopathological examination. Then, the clinical features, including age, gender, tumor site, tumor size, TNM stages, and lymph node metastasis, were collected from the recruited cases. This study was permitted by the Ethics Committee of the Affiliated Yantai Yuhuangding Hospital of Qingdao University and written informed consent had been signed by all subjects.
Cell Culture, Lidocaine Treatment and Transfection
Human CRC cell line (SW480 and LoVo), and normal colon cells (FHC) were obtained from Shanghai Academy of Life Science (Shanghai, China) and grown in the Dulbecco’s modified Eagle’s medium (DMEM; Gibco, Carlsbad, CA, USA) harboring with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (Gibco) with 5% CO2 at 37°C.
Lidocaine (Lido) was obtained from Sigma (St. Louis, MO, USA), followed by dissolved in serum-free medium. SW480 and LoVo cell monolayers were treated with different dosages of lidocaine (50, 100, 500, and 1000 μM) in the viability assay for 24 h, 48 h, or 72 h. SW480 and LoVo cells were exposed with 500 μM lidocaine for 48 h in cell viability analysis, apoptosis analysis, Western blot, and glycolysis analysis. For some experiments, cells were pre-transfected with plasmids or miRNAs listed as followed for 48 h, followed by incubation with (500 μM) lidocaine. Cells treated by the same volume of PBS were considered as blank controls.
Small interfering RNA (siRNA) targeting circHOMER1 (si-circHOMER1), siRNA targeting hes-related family bHLH transcription factor with YRPW motif 1 (HEY1) (si-HEY1), siRNA negative control (si-NC), the empty vector (pcDNA-NC), and pcDNA-circHOMER1 overexpression vector (pcDNA-circHOMER1) were synthesized by Genepharma (Shanghai, China). The mimic or inhibitor targeting miR-138-5p (miR-138-5p or anti-miR-138-5p) and their corresponding control (miR-NC or anti-miR-NC) were generated by RIBOBIO (Guangzhou, China). The transfection was conducted using LipofectamineTM 2000 transfection reagent (Invitrogen, Carlsbad, CA, USA).
Cell Viability
SW480 and LoVo cells were cultivated into a 96-well plate (5,000 cells per well) with 100 μL culture medium and treated with different concentrations of lidocaine, and then 10 μL cell counting kit-8 solution (CCK-8) (Dojindo Molecular Technologies, Japan) was added to per well and incubated with cells at 37°C for 2 h. Finally, the absorbance value at 450 nm was analyzed by a microplate reader.
Western Blot
Total proteins were isolated from transfected and/or treated cells through using the RIPA buffer (Beyotime, Shanghai, China), and quantified with a bicinchoninic acid (BCA) protein quantification kit. Then, approximately 30 μg of protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then transferred onto polyvinylidene fluoride membranes. Subsequently, membranes were interacted with primary antibodies against p53 (1:1000, ab131442, Abcam, Cambridge, MA, USA), CyclinD1 (1:20,000, ab134175, Abcam), Pro-caspase-3 (1:1000, ab32150, Abcam), Cleaved-caspase-3 (1:1000, ab2302, Abcam), Pro-caspase 9 (1:10,000, ab32539, Abcam), Cleaved-caspase-9 (1:1000, ab2324, Abcam), HEY1 (1:5000, ab22614, Abcam), glyceraldehyde 3-phosphate dehydrogenase (GADPH) (1:10,000, ab8245, Abcam) and the secondary HRP-conjugated antibody (1:5000, ab20272, Abcam). Finally, protein bands were detected using electrochemiluminescence.
Cell Apoptosis
Cells with 1 mL culture medium were seeded into a six-well plate and exposed to 500 μM lidocaine for 48 h. After that, cells were collected and resuspended in binding buffer, followed by staining with Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) (10 μL) (BD Biosciences, San Jose, CA, USA) for 15 min in the dark. Finally, the apoptotic cells were measured by FlowJo software with flow cytometry.
Glycolysis Analysis
Cells were cultivated into a 6-well plate. After transfection and/or treatment, supernatants of cell culture media were collected to detect the levels of glucose and lactate using the Glucose Uptake Assay Kit and l-Lactate Assay Kit (Sigma, St Louis, MO, USA) according to the protocol of the manufacturer. Glucose consumption and lactate production were calculated according to the percentage of the control group and normalized by the protein concentration of samples.
The ATP levels in cells were measured by an ATP Assay Kit (Sigma) according to the protocol of the manufacturer. Cells were lysed and then incubated with ATP reaction mix for 30 min. Finally, the optical density at 570 nm was measured by a microplate reader.
Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
Whole-RNA was prepared using TRIzol reagent (Invitrogen) following the standard procedure. Then, isolated RNA was reversely transcribed into complementary DNA (cDNA) by PrimeScript™ RT reagent Kit (Qiagen, Valencia, CA, USA), and then quantitative PCR was carried out with SYBR Premix Ex Taq (Qiagen). The following cycling conditions were used: pre-denaturation at 95°C for 5 min; 50 denaturation cycles at 95°C for 15 s, and then 60°C for 30 s. Relative transcription alterations were detected by 2−ΔΔCt method. GADPH and U6 small nuclear B noncoding RNA (U6) were employed as internal controls. The specific primer sequences were presented as follows: circHOMER1: F 5ʹ- CTCAGAGCCAAGGGCTGAAC-3ʹ, R 5ʹ-GGGTCAATTTGGAAGACATGAGC-3ʹ; miR-138-5p: F 5ʹ- GCTTAAGGCACGCGG-3ʹ, R 5ʹ-GTGCAGGGTCCGAGG-3ʹ; HEY1: F 5ʹ- TGGATCACCTGAAAATGCTG-3ʹ, R 5ʹ-CGAAATCCCAAACTCCGATA-3ʹ; GADPH: F 5ʹ-GATATTGTTGCCATCAATGAC-3ʹ, R 5ʹ-TTGATTTTGGAGGGATCTCG-3ʹ; U6: F 5ʹ-CTCGCTTCGGCAGCACA-3ʹ, R 5ʹ-ACGCTTCACGAATTTGCGT-3ʹ.
Dual-Luciferase Reporter Assay
The wild-type (WT) or mutant (MUT) circHOMER1/HEY1 3ʹUTR containing miR-138-5p binding sequences were amplified and cloned into the pmirGLO Vector (Promega, Shanghai, China) to generate wild-type pmirGLO-circHOMER1-WT/pmirGLO-HEY1 3ʹUTR-WT or the mutated circHOMER1-MUT/HEY1 3ʹUTR-MUT. Then, SW480 and LoVo cells were co-transfected with these constructed vectors and miR-138-5p or miR-NC using Lipofectamine 2000 reagent (Invitrogen). Finally, a dual-luciferase reporter assay kit (Promega) was used to measure the relative luciferase activity.
Xenograft Experiments in vivo
The animal experiment procedures were approved by the Animal Research Committee of the Affiliated Yantai Yuhuangding Hospital of Qingdao University and were performed according to the National Institutes of Health guidelines. BALB/c nude mice (female, 4–6 weeks old, N=6) were purchased from Jinan Pengyue Animal Center (Jinan, China). SW480 cells were treated with 500 μM lidocaine or the same volume of PBS for 48 h, and then were subcutaneously injected into the flanks of the nude mice. After 6 days following the tumor implantation, the tumor volume was measured and calculated every 3 days. On day 21, all mice were killed and tumor masses were weighed and harvested for further molecular analysis.
Statistical Analysis
Significant differences were analyzed using one-way analysis of variance (ANOVA) or Student’s t-test with GraphPad Prism 7 software (GraphPad Inc., San Diego, CA, USA). The correlation analysis was performed using Pearson correlation analysis. Data were presented as the mean ± standard deviation (SD) of the mean of three replicates. P value less than 0.05 was suggested statistically significant.
Results
The Effects of Lidocaine on CRC Cell Viability, Apoptosis and Aerobic Glycolysis in vitro
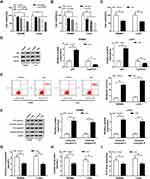
The effects of lidocaine on CRC cell growth were evaluated. First, the favorable concentration of lidocaine and treatment time were analyzed. As shown in Figure 1A, treatment with 50, 100, 500, and 1000 μM lidocaine led to a significant decrease in SW480 and LoVo cell viability, whereas lower concentrations of lidocaine (50 μM) had no effect. Furthermore, Figure 1B exhibits that 500 μM lidocaine treatment for 24, 48 or 72 h reduced the viability of SW480 and LoVo cells. Thus, lidocaine suppressed CRC cell viability in a dose- and time-dependent manner. Subsequently, exposure with 500 μM lidocaine for 48 h declined SW480 and LoVo cell viability to 65 ± 7% and 61 ± 6% (Figure 1C); therefore, 500 μM lidocaine was chosen for further experiments. After that, Western blot analysis showed 500 μM lidocaine treatment increased the expression of anti-proliferative proteins p53, but decreased the level of pro-proliferative protein Cyclin D1 in SW480 and LoVo cells (Figure 1D). Besides that, the apoptotic SW480 and LoVo cells were increased in the presence of 500 μM lidocaine (Figure 1E), and the up-regulation of pro-apoptotic proteins Cleaved-caspase-3 and Cleaved-caspase-9 was observed in SW480 and LoVo cells stimulated with 500 μM lidocaine (Figure 1F). Thus, lidocaine treatment suppressed the proliferation and induced apoptosis in CRC cells. More importantly, 500 μM lidocaine exposure decreased glucose consumption (Figure 1G), lactate production (Figure 1H), and ATP levels (Figure 1I). Therefore, lidocaine treatment inhibited aerobic glycolysis to impeded cell proliferation.
Lidocaine Decreases circHOMER1 Expression in CRC Cells
The expression levels of circHOMER1 in CRC tumor tissues and corresponding normal tissues were detected. We found circHOMER1 was highly expressed in CRC tumor tissues relative to corresponding normal tissues (Figure 2A). Subsequently, patients were divided into two groups according to the median expression level of circHOMER1: a high circHOMER1 expression group and a low circHOMER1 expression group, and the correlation between circHOMER1 expression and clinicopathological features in 30 CRC patients was analyzed. Results showed higher circHOMER1 expression was correlated with Lymph node metastasis and advanced TNM stages (P < 0.05, Table 1). Moreover, by contrast with normal colon FHC cells, circHOMER1 expression was also elevated in SW480 and LoVo cells (Figure 2B), indicating the potential carcinogenic roles of circHOMER1 in CRC. In addition, we also found lidocaine exposure reduced the level of circHOMER1 in CRC cells (Figure 2C), suggesting circHOMER1 decrease induced by lidocaine treatment might be related to the anti-tumor activity of lidocaine in CRC.
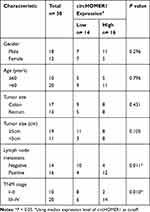 |
Table 1 Association of circHOMER1 Level with Clinicopathologic Features in CRC Patients |
Lidocaine Inhibits CRC Cell Proliferation, Aerobic Glycolysis and Induces Apoptosis by Modulating circHOMER1 Expression
To investigate the impact of circHOMER1 on lidocaine-induced SW480 and LoVo cell viability arrest, apoptosis elevation and aerobic glycolysis inhibition, pcDNA-circHOMER1 was transfected into SW480 and LoVo cells before lidocaine treatment. As expected, circHOMER1 expression was dramatically up-regulated in SW480 and LoVo cells after transfection (Figure 3A). After that, CCK-8 assay displayed that lidocaine-induced inhibition of SW480 and LoVo cell viability was evidently abated by circHOMER1 overexpression (Figure 3B), which was accompanied with the up-regulation of CyclinD1 level and down-regulation of p53 level (Figure 3C). Besides, circHOMER1 overexpression abrogated lidocaine-induced apoptosis of SW480 and LoVo cells (Figure 3D), reflected by the reduction of pro-apoptotic proteins Cleaved-caspase-3 and Cleaved-caspase-9 (Figure 3E and F). What is more, by contrast with lidocaine single group, the glucose consumption (Figure 3G), lactate production (Figure 3H), and ATP levels (Figure 3I) of SW480 and LoVo cells in lidocaine + pcDNA-circHOMER1 group were notably increased. Taken together, the level of circHOMER1 was associated with CRC cell viability loss, apoptosis induction and aerobic glycolysis suppression mediated by lidocaine.
circHOMER1 Is a Sponge of miR-138-5p in CRC Cells
To explore the underlying mechanism of how circHOMER1 participated in lidocaine mediated regulation of CRC malignancy. The target miRNAs of circHOMER1 were investigated. By searching online software program StarBase3.0, miR-138-5p was found to have the potential to bind to circHOMER1 (Figure 4A). Subsequently, the decline of luciferase activity in SW480 and LoVo cells co-transfected with circHOMER1-WT and miR-138-5p confirmed the direct interaction between circHOMER1 and miR-138-5p (Figure 4B and C). Moreover, we also discovered miR-138-5p expression was increased by the down-regulation of circHOMER1 but was decreased by the up-regulation of circHOMER1 in SW480 and LoVo cells (Figure 4D). These data confirmed that circHOMER1 targetedly repressed miR-138-5p expression in CRC cells. After that, the level of miR-138-5p was detected. Results indicated miR-138-5p was decreased in CRC tumor tissues and cell lines relative to corresponding normal tissues and FHC cells (Figure 4E and F), and lidocaine exposure elevated the level of miR-138-5p in CRC cells (Figure 4G), hinting the possible roles of miR-138-5p increase caused by lidocaine in the anticancer activity of lidocaine on CRC.
Lidocaine Exerts Anti-Tumor Activity by Regulating circHOMER1/miR-138-5p Axis in CRC Cells
We further studied the influence of circHOMER1/miR-138-5p axis on lidocaine-stimulated cell viability loss, apoptosis elevation and aerobic glycolysis inhibition in CRC cells. First, SW480 and LoVo cells were transfected with pcDNA-NC, pcDNA-circHOMER1, pcDNA-circHOMER1 + miR-NC, pcDNA-circHOMER1 + miR-138-5p before lidocaine treatment, and qRT-PCR analysis showed miR-138-5p expression was reduced by circHOMER1 overexpression, while was restored by following miR-138-5p mimic transfection (Figure 5A), which indicated the successful transfection. After that, data in Figure 5B exhibited miR-138-5p overexpression reversed the anti-viability effect of circHOMER1 on SW480 and LoVo cells treated with lidocaine, which were accompanied with the increased protein level of p53 and decreased protein level of CyclinD1 (Figure 5C). Additionally, relative to the lidocaine + pcDNA-circHOMER1 + miR-NC group, the number of apoptotic SW480 and LoVo cells was significantly elevated in lidocaine + pcDNA-circHOMER1 + miR-138-5p group (Figure 5D), and Western blot also displayed that Cleaved-caspase-3 and Cleaved-caspase-9 were increased in lidocaine + pcDNA-circHOMER1 + miR-138-5p group in SW480 and LoVo cells (Figure 5E and F). Besides that, miR-372 overexpression attenuated circHOMER1 up-regulation-mediated promotion on glucose consumption (Figure 5G), lactate production (Figure 5H), and ATP levels (Figure 5I) in lidocaine-treated SW480 and LoVo cells. These results illustrated circHOMER1/miR-138-5p axis was concerned with lidocaine-induced CRC cell viability loss, apoptosis induction and aerobic glycolysis suppression.
HEY1 Is a Target of miR-138-5p in CRC Cells
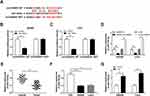
To further study the molecular mechanisms of lidocaine action in CRC cells, the direct target of miR-138-5p was searched using StarBase3.0 program. The predicted results exhibited the binding sites between miR-138-5p and HEY1 (Figure 6A). Immediately, a dual-luciferase reporter assay was performed in CRC cells and results showed the luciferase activity was significantly reduced in SW480 and LoVo cells co-transfected with HEY1 3ʹ-UTR-WT and miR-138-5p, but there was no obvious impact on HEY1 3ʹ-UTR-MUT-type compared with the control group (Figure 6B and C). These results suggested the direct interaction between miR-138-5p and HEY1. Subsequently, the expression of HEY1 was explored and results showed HEY1 was increased in CRC tumor tissues and cell lines at mRNA and protein levels relative to corresponding normal tissues and FHC cells (Figure 6D–G); besides, lidocaine treatment reduced the level of HEY1 in SW480 and LoVo cells (Figure 6H and I). In addition, we also observed that miR-138-5p inhibited HEY1 expression, while this inhibition was rescued by circHOMER1 overexpression in SW480 and LoVo cells (Figure 6J and K), more importantly, a negative correlation between miR-138-5p and HEY1 (r=−0.554, P < 0.0001) (Figure 6M) or circHOMER1 (r=−0.555, P < 0.0001) (Figure 6L), and a positive correlation between HEY1 and circHOMER1 (r=0.625, P < 0.0001) (Figure 6N) were confirmed. Altogether, circHOMER1 could regulate HEY1 expression by directly binding to miR-138-5p in CRC cells.
Lidocaine Mediates CRC Cell Viability Loss, Apoptosis Induction and Aerobic Glycolysis Suppression by Regulating miR-138-5p/HEY1 Axis
The effects of miR-138-5p/HEY1 axis on lidocaine-stimulated inhibition of CRC cell malignant behaviors were further investigated. SW480 and LoVo cells were transfected with anti-NC, anti-miR-138-5p, anti-miR-138-5p + si-NC, or anti-miR-138-5p + si-HEY1 before treatment with lidocaine. Then, we found HEY1 expression was increased by miR-138-5p inhibition but was rescued by following HEY1 knockdown (Figure 7A and B), indicating the successful transfection. Afterwards, functional experiments were conducted. As presented in Figure 7C, miR-138-5p inhibition reversed lidocaine treatment-mediated CRC cell viability loss, and this reversion also was verified by decreased p53 level and increased CyclinD1 level in the lidocaine + anti-miR-138-5p group (Figure 7D). Moreover, results in Figure 7E exhibited lidocaine-stimulated SW480 and LoVo cell apoptosis elevation was notably mitigated by miR-138-5p inhibition, which was accompanied with the decrease of Cleaved-caspase-3 and Cleaved-caspase-9 protein in both SW480 and LoVo cells (Figure 7F and G). Additionally, the inhibition of glucose consumption (Figure 7H), lactate production (Figure 7I), and ATP levels (Figure 7J) in SW480 and LoVo cells induced by lidocaine treatment also was abolished by silencing miR-138-5p. Therefore, we confirmed miR-138-5p inhibition could reverse lidocaine-mediated CRC cell viability loss, apoptosis induction and aerobic glycolysis suppression. However, rescue assay also displayed HEY1 deletion could rescue the pro-tumor activities mediated by miR-138-5p inhibition via repressing the viability (Figure 7C and D) and aerobic glycolysis (Figure 7H–J), as well as enhancing apoptosis (Figure 7E–G) in lidocaine-induced SW480 and LoVo cells. In all, these data confirmed lidocaine performed anti-tumor function by regulating miR-138-5p/HEY1 axis in CRC cells.
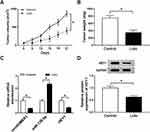
Lidocaine Inhibits Tumor Growth in vivo
The roles of lidocaine in tumor growth in vivo were elaborated by establishing mouse xenograft models. Results in Figure 8A and B displayed that lidocaine suppressed tumor growth in vivo, demonstrated by the decline of tumor volume and weight in lidocaine exposure groups. Furthermore, molecular analysis exhibited that lidocaine treatment reduced the expression of circHOMER1 (Figure 8C) and HEY1 (Figure 8C and D), but elevated the expression level of miR-138-5p (Figure 8C) in the tumor masses. Thus, we concluded lidocaine might hinder tumor growth in vivo by regulating circHOMER1/miR-138-5p/HEY1 axis.
Discussion
Currently, growing researches have confirmed that lidocaine may perform anticancer effects on the therapy of diverse cancers through affecting tumor cell malignant behaviors, such as cell viability, apoptosis, migration and drug resistance.4,8,21,22 In fact, sustaining proliferation and resisting apoptosis are two significant hallmarks of cancer cells that contribute to cancer cell malignant enlargement and progression, and proliferation inhibition and apoptosis promotion of cancer cells are promising methods to hinder the development of human cancers.23
In this study, lidocaine up-regulated the pro-proliferative protein Cyclin D1 and pro-apoptotic proteins cleaved-Caspase-3 and cleaved-Caspase-9, which might result in the proliferation repression and apoptosis induction in CRC cells. p53 is considered as an important tumor suppressor, which can mediate the inhibition of proliferative or survival capacity of cells with DNA damage or inappropriate cell-cycle progression.24 This study also found lidocaine elevated the protein of p53, thus suppressing the CRC cell proliferation and viability. Aerobic glycolysis has been demonstrated to be a hallmark of tumor cells. Tumor cells preferably take energy by aerobic glycolysis, which in turn allows tumor cells to successfully compete with normal cells for glucose uptake to sustain uninterrupted growth.25 In this study, we observed a decrease in glucose consumption, lactate production, and ATP levels in CRC cells treated with lidocaine, indicating the inhibition of CRC cell viability mediated by lidocaine through suppressing aerobic glycolysis. In addition, in vivo xenograft model also confirmed lidocaine impeded tumor growth in vivo.
The impacts of noncoding RNAs (ncRNAs) dysregulation on cancer cell growth and apoptosis have been widely demonstrated.26 Previous studies have shown that lidocaine could participate in the regulation of cancer cell progression by modulating ncRNAs. For example, lidocaine performed anti-proliferation and pro-apoptosis in cervical cancer cells through regulating MEG3/miR-421/BTG1 axis.27 Lidocaine suppressed gastric cancer cell tumorigenesis via increasing miR-145 expression.28 Lidocaine repressed lung cancer cell proliferation and metastasis by modulating miR-539/EGFR pathway.29 In CRC, Qu et al discovered that lidocaine suppressed proliferation and stimulated apoptosis via the regulation of miR-520a-3p/EGFR.10 Therefore, the underlying ncRNAs pathway in the action of lidocaine on CRC cells was investigated.
In this study, we confirmed lidocaine down-regulated the expression of circHOMER1 and HEY1, but increased the expression of miR-138-5p in CRC cells. Besides, we confirmed miR-138-5p directly interacted with circHOMER1 and HEY1 in CRC cells, and circHOMER1 regulated HEY1 expression through repressing miR-138-5p expression. CircHOMER1 has been identified to be associated with the pathogenesis of CRC.18 Zhao et al revealed that miR-138-5p functioned as a tumor suppressor to inhibit CRC cell growth via decreasing PD-L1.30 In this study, we demonstrated, lidocaine-induced cell viability loss, apoptosis induction and aerobic glycolysis suppression in CRC could be attenuated by circHOMER1 overexpression or miR-138-5p inhibition. Moreover, miR-138-5p overexpression reversed circHOMER1 induced carcinogenic effects on lidocaine-induced CRC cells. HEY1 is a downstream effector of Notch signaling, which initiate is related to the modulation of pathological processes.31 Additionally, HEY1 was found to act as an oncogene in the tumorigenesis of CRC.32 In the current study, we also found miR-138-5p/HEY1 axis involved in the anticancer activity of lidocaine on CRC cells.
However, the data presented are based on a limited number of cell or animal experiments, regarding the shortcomings of the present study, the function of lidocaine and circHOMER1 in healthy cell lines should be examined before the application of them to clinical use to ensure the safety and efficiency, and then a larger cohort of the disease is necessary to validate these conclusions.
In conclusion, this study demonstrated that lidocaine inhibited CRC cell viability and aerobic glycolysis, but induced cell apoptosis in vitro and impeded tumor growth in vivo through circHOMER1/miR-138-5p/HEY1 axis, indicating new avenues for the development of anti-cancer therapies in CRC.
Acknowledgment
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67(3):177–193. doi:10.3322/caac.21395
3. Brody H. Colorectal cancer. Nature. 2015;521(7551):S1. doi:10.1038/521S1a
4. Zhang L, Hu R, Cheng Y, et al. Lidocaine inhibits the proliferation of lung cancer by regulating the expression of GOLT1A. Cell Prolif. 2017;50(5):e12364. doi:10.1111/cpr.12364
5. Byrne K, Levins KJ, Buggy DJ. Can anesthetic-analgesic technique during primary cancer surgery affect recurrence or metastasis? Can J Anaesth. 2016;63(2):184–192. doi:10.1007/s12630-015-0523-8
6. Tedore T. Regional anaesthesia and analgesia: relationship to cancer recurrence and survival. Br J Anaesth. 2015;115(Suppl 2):ii34–ii45. doi:10.1093/bja/aev375
7. Ngom PI, Dubray C, Woda A, Dallel R. A human oral capsaicin pain model to assess topical anesthetic-analgesic drugs. Neurosci Lett. 2001;316(3):149–152. doi:10.1016/S0304-3940(01)02401-6
8. Chamaraux-Tran TN, Mathelin C, Aprahamian M, et al. Antitumor effects of lidocaine on human breast cancer cells: an in vitro and in vivo experimental trial. Anticancer Res. 2018;38(1):95–105. doi:10.21873/anticanres.12196
9. Jurj A, Tomuleasa C, Tat TT, Berindan-Neagoe I, Vesa SV, Ionescu DC. Antiproliferative and apoptotic effects of lidocaine on human hepatocarcinoma cells. A preliminary study. J Gastrointestin Liver Dis. 2017;26(1):45–50. doi:10.15403/jgld.2014.1121.261.juj
10. Qu X, Yang L, Shi Q, Wang X, Wang D, Wu G. Lidocaine inhibits proliferation and induces apoptosis in colorectal cancer cells by upregulating mir-520a-3p and targeting EGFR. Pathol Res Pract. 2018;214(12):1974–1979. doi:10.1016/j.prp.2018.09.012
11. Xu XD, Shao SX, Jiang HP, et al. Warburg effect or reverse Warburg effect? A review of cancer metabolism. Oncol Res Treat. 2015;38(3):117–122. doi:10.1159/000375435
12. Kroemer G, Pouyssegur J. Tumor cell metabolism: cancer’s Achilles’ heel. Cancer Cell. 2008;13(6):472–482. doi:10.1016/j.ccr.2008.05.005
13. Li C, Zhang G, Zhao L, Ma Z, Chen H. Metabolic reprogramming in cancer cells: glycolysis, glutaminolysis, and Bcl-2 proteins as novel therapeutic targets for cancer. World J Surg Oncol. 2016;14(1):15. doi:10.1186/s12957-016-0769-9
14. Chen X, Wu Q, Sun P, Zhao Y, Zhu M, Miao C. Propofol disrupts aerobic glycolysis in colorectal cancer cells via inactivation of the NMDAR-CAMKII-ERK pathway. Cell Physiol Biochem. 2018;46(2):492–504. doi:10.1159/000488617
15. Zhao ZJ, Shen J. Circular RNA participates in the carcinogenesis and the malignant behavior of cancer. RNA Biol. 2017;14(5):514–521. doi:10.1080/15476286.2015.1122162
16. Hsiao KY, Lin YC, Gupta SK, et al. Noncoding effects of circular RNA CCDC66 promote colon cancer growth and metastasis. Cancer Res. 2017;77(9):2339–2350. doi:10.1158/0008-5472.CAN-16-1883
17. Zhang J, Liu H, Hou L, et al. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol Cancer. 2017;16(1):151. doi:10.1186/s12943-017-0719-3
18. Li XN, Wang ZJ, Ye CX, Zhao BC, Li ZL, Yang Y. RNA sequencing reveals the expression profiles of circRNA and indicates that circDDX17 acts as a tumor suppressor in colorectal cancer. J Exp Clin Cancer Res. 2018;37(1):325. doi:10.1186/s13046-018-1006-x
19. Wang S, Zhang Y, Cai Q, et al. Circular RNA FOXP1 promotes tumor progression and Warburg effect in gallbladder cancer by regulating PKLR expression. Mol Cancer. 2019;18(1):145. doi:10.1186/s12943-019-1078-z
20. Yu T, Wang Y, Fan Y, et al. CircRNAs in cancer metabolism: a review. J Hematol Oncol. 2019;12(1):90. doi:10.1186/s13045-019-0776-8
21. Yang X, Zhao L, Li M, et al. Lidocaine enhances the effects of chemotherapeutic drugs against bladder cancer. Sci Rep. 2018;8(1):598. doi:10.1038/s41598-017-19026-x
22. Yang W, Cai J, Zhang H, Wang G, Jiang W. Effects of lidocaine and ropivacaine on gastric cancer cells through down-regulation of ERK1/2 phosphorylation In vitro. Anticancer Res. 2018;38(12):6729–6735. doi:10.21873/anticanres.13042
23. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi:10.1016/j.cell.2011.02.013
24. Tonnessen-Murray CA, Lozano G, Jackson JG. The regulation of cellular functions by the p53 protein: cellular senescence. Cold Spring Harb Perspect Med. 2017;7(2):a026112. doi:10.1101/cshperspect.a026112
25. Ganapathy-Kanniappan S. Molecular intricacies of aerobic glycolysis in cancer: current insights into the classic metabolic phenotype. Crit Rev Biochem Mol Biol. 2018;53(6):667–682. doi:10.1080/10409238.2018.1556578
26. Lekka E, Hall J. Noncoding RNAs in disease. FEBS Lett. 2018;592(17):2884–2900. doi:10.1002/1873-3468.13182
27. Zhu J, Han S. Lidocaine inhibits cervical cancer cell proliferation and induces cell apoptosis by modulating the lncRNA-MEG3/miR-421/BTG1 pathway. Am J Transl Res. 2019;11(9):5404–5416.
28. Sui H, Lou A, Li Z, Yang J. Lidocaine inhibits growth, migration and invasion of gastric carcinoma cells by up-regulation of miR-145. BMC Cancer. 2019;19(1):233. doi:10.1186/s12885-019-5431-9
29. Sun H, Sun Y. Lidocaine inhibits proliferation and metastasis of lung cancer cell via regulation of miR-539/EGFR axis. Artif Cells Nanomed Biotechnol. 2019;47(1):2866–2874. doi:10.1080/21691401.2019.1636807
30. Zhao L, Yu H, Yi S, et al. The tumor suppressor miR-138-5p targets PD-L1 in colorectal cancer. Oncotarget. 2016;7(29):45370–45384. doi:10.18632/oncotarget.9659
31. Sethi N, Dai X, Winter CG, Kang Y. Tumor-derived JAGGED1 promotes osteolytic bone metastasis of breast cancer by engaging notch signaling in bone cells. Cancer Cell. 2011;19(2):192–205. doi:10.1016/j.ccr.2010.12.022
32. Han C, Song Y, Lian C. MiR-769 inhibits colorectal cancer cell proliferation and invasion by targeting HEY1. Med Sci Monit. 2018;24:9232–9239. doi:10.12659/MSM.911663
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.