Back to Journals » Drug Design, Development and Therapy » Volume 14
Levonorgestrel Ameliorates Adenomyosis via lncRNA H19/miR-17/TLR4 Pathway
Authors Liang N, Zhang W, Wang H, Shi W, Wang L, Ma L
Received 2 February 2020
Accepted for publication 26 June 2020
Published 24 August 2020 Volume 2020:14 Pages 3449—3460
DOI https://doi.org/10.2147/DDDT.S248095
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianbo Sun
Na Liang,1,* Wenfeng Zhang,2,* Hongjiang Wang,3 Wei Shi,4 Li Wang,2 Lijuan Ma2
1Department of Traditional Chinese Medicine, The First Affiliated Hospital of Shandong First Medical University, Shandong Provincial Qianfoshan Hospital, Shandong University, Jinan City, Shandong Province, People’s Republic of China; 2Department of Obstetrics and Gynecology, The First Affiliated Hospital of Shandong First Medical University, Shandong Provincial Qianfoshan Hospital, Jinan City, Shandong Province, People’s Republic of China; 3TCM Gynecology Department, Maternal and Child Health Hospital, Dezhou City, Shandong Province, People’s Republic of China; 4Department of Gynecology, Affiliated Hospital of Shandong University of Traditional Chinese Medicine, Jinan City, Shandong Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Li Wang; Lijuan Ma
Department of Obstetrics and Gynecology, The First Affiliated Hospital of Shandong First Medical University, Shandong Provincial Qianfoshan Hospital, No. 16766, Jingshi Road, Lixia District, Jinan City, Shandong Province, People’s Republic of China
Tel +86-15969718268; +86-13791056924
Email [email protected]; [email protected]
Purpose: To explore the mechanism of levonorgestrel (LNG)-ameliorating adenomyosis through long non-coding RNA H19 (lncRNA H19)/miR-17/Toll-like receptor 4 (TLR4) pathway.
Patients and Methods: A total of 71 cases of adenomyosis and 54 cases of normal endometrium were sampled. Quantitative polymerase chain reaction (qPCR) was employed to quantify lncRNA H19, miR-17, and TLR4 mRNA, while Western blot (WB) was used to quantify TLR4 protein. Effects of LNG on normal endometrial stromal cells (ESCs) were evaluated. Suppression/over-expression vectors of lncRNA H19, miR-17, and TLR4 were constructed to observe their effects on ESCs.
Results: MiR-17 and TLR4 mRNA were up-regulated and lncRNA H19 was down-regulated in adenomyosis. After LNG treatment, lncRNA H19 was up-regulated while miR-17 and TLR4 were down-regulated. LNG, up-regulation of lncRNA H19, and down-regulation of miR-17 and TLR4 portend increased apoptosis, G1-arrested cells, as well as inhibited inflammation. Dual-luciferase reporter (DLR) assay conformed the targeting relation of lncRNA H19/miR-17/TLR4 pathway.
Conclusion: LNG ameliorates adenomyosis via lncRNA H19/miR-17/TLR4 pathway.
Keywords: adenomyosis, levonorgestrel, lncRNA H19/miR-17/TLR4 pathway
Introduction
The invasion of endometrial glands and stroma into the myometrium is known as adenomyosis.1,2 Although there is no evidence that adenomyosis triggers infertility directly, it interferes with the success rate of the in-vitro fertilization.3 The development of adenomyosis is closely related to concentration and activity of estrogen.4,5 Failure to treat adenomyosis in time is likely to lead to premature delivery, preeclampsia, placental dislocation, as well as endometrial adenocarcinoma.6–8 The diagnostic indexes of adenomyosis are unclear,9,10 so it may be a new idea to find one from the perspective of genetics.
MicroRNAs (miRNA) are a kind of most studied gene regulatory factors in molecular biology, which are regulated after transcription by pairing and binding with mRNAs. MiR-17, a 84 bp long miRNA located on human chromosome 13, is an essential regulatory tool for many diseases. In coronary heart disease, miR-17 inhibits apoptosis of vascular endothelial cells and increases cell survival by down-regulating Insulin-like Growth Factor 1 (IGF1).11 Elevated miR-17 accelerates cyst growth and leads to intensified mitochondrial metabolism in polycystic kidney.12,13 Abnormal expression of miR-17 is also associated with colorectal cancer, cervical cancer, pancreatic cancer, as well as non-small cell lung cancer.14–18 Besides, miR-17 may be involved in endometriosis and adenomyosis.19,20 Long non-coding RNAs (lncRNAs) are important upstream regulators of miRNA regulating physiological process by binding to miRNA. Previous studies have shown that lncRNA H19 is involved in the regulation of expression of many miRNAs.21–24 Liu et al25 stated that up-regulated lncRNA H19 participates in the regulation of proliferation and apoptosis of endometrial stromal cells (ESCs), so lncRNA H19 may be related to adenomyosis.
Levonorgestrel (LNG) is the preferred treatment for adenomyosis due to its low side effects and high success rate.10 In this study, up-regulated lncRNA H19 and down-regulated miR-17 were found when ESCs were treated with LNG. Therefore, we explored this to seek the molecular mechanism of LNG in the treatment of adenomyosis and to provide reliable data for improving the curative effect of LNG.
Patients and Methods
Patients with Adenomyosis
Seventy-one samples of endometrium from patients diagnosed with adenomyosis clinically and pathologically between January 2015 and March 2017 were collected in The First Affiliated Hospital of Shandong First Medical University. The average age of the patients was (42.12±3.14) years. Inclusion criteria: patients diagnosed with adenomyosis and willing to cooperate with the therapist. Exclusion criteria: patients receiving preoperative chemotherapy; patients with chronic diseases such as coronary heart disease and hypertension; patients with a long history of drug therapy. Another 54 samples of myometrium form patients undergoing hysteroscopic uterine septum were allocated in a control group. The patients in the group had no history of hormone therapy, no underlying chronic diseases, and no mental diseases. All participants were fully informed and signed the written informed consent in accordance with the declaration of helsinki. And the study received the approval from the The First Affiliated Hospital of Shandong First Medical University ethics Committee. Tissue sections were stored in −80°C liquid nitrogen for testing.
Cell Isolation and Transfection
ESCs were extracted from myometrium of patients with adenomyosis. Components of culture medium (50 mL): Dulbecco’s Modified Eagle Medium (DMEM)/F12K (Gibco) + 10% fetal bovine serum (FBS, Gibco), 2 mmol glutamine + 1% penicillin/streptomycin solution (100X, Solarbio). Immunohistochemistry was employed to detect cytokeratin and vimentin, and interstitial cells detected were cultured. Cells were inoculated into T25 flasks (Thermo Fisher). Preheated medium (5 mL, 37°C) was added to the flasks. The cells were cultured in a 37°C and 5%CO2 incubator (Binder) to their good growth. Before transfection, the medium was replaced with FBS-free medium. During transfection, 1×105 cells per well were inoculated into 6-well plates. LncRNA H19-ad (over-expression vector), miR-17 inhibitor/mimics, TLR4 siRNA, NC vector were all purchased from Shanghai Sangon Bioengineering Co., Ltd. Cell lines were transfected using the Lipofectamine 2000 transfection kit (invitrogen, USA). After transfection for 8 h, fresh culture medium was used to remove dead cells.
LNG-Treated Cells
Preparation of LNG mother liquor: LNG was dissolved in dimethyl sulphoxide (DMSO) (Solarbio), and the concentration of LNG was controlled to be 1×10−2 mol/L. The prepared LNG solution was added into the medium, and the final concentration of LNG was 5×10−5 mol/L. The effect of LNG on cells was monitored. Another group was added with the same amount of DMSO as negative control to eliminate the influence of DMSO.
Quantitative Polymerase Chain Reaction (qPCR)
Trizol was applied to extract total RNA from tissues or cells. The optical density (OD) value at 260–280nm was obtained by an ultraviolet spectrophotometer, and those with the value of OD260/OD280 >1.8 were used for subsequent qPCR. FastKing one-step reverse transcription-fluorescence quantitative kit (Beijing Tiangen Biotech Co., Ltd.) and ABI PRISM 7000 (Applied Biosystems) were employed for RNA quantification. LncRNA H19 and miR-17 primers were designed and synthesized by Shanghai Sangon Bioengineering Co., Ltd. LncRNA H19, F: 5ʹ-ATC GGT GCC TCA GCG TTC GG-3ʹ, R: 5ʹ-CTG TCC TCG CCG TCA CAC CG-3ʹ. miR-17, F: 5ʹ-CAA AGT GCT TAC AGT GCA GGT A-3ʹ, R: 5ʹ-GCA CCT TAG AAC AAA AAG CAC T-3ʹ. TLR4, F: 5′-CCG CTT TCA CTT CCT CTC AC-3′, R: 5′-CAT CCT GGC ATC ATC CTC AC-3ʹ. The qPCR reaction system (50 μL) contained 1.25 μL upstream primer, 1.25 μL downstream primer, 1.0 μL probe, 10 pg/μg RNA template, 5 μL 50×ROX Reference Dye ROX, and made up to 50 uL with RNase-Free ddH2O. Reaction process: 1 cycle of reverse transcription at 50°C for 30 min, 1 cycle of pre-denaturation at 95°C for 3 min, 40 cycles of denaturation at 95°C for 15 s and annealing at 60°C for 30 s. Results obtained were analyzed with the ABI PRISM 7000. U6 and glyceraldehyde phosphate dehydrogenase (GAPDH) were served as internal reference genes, and the data were normalized by 2−ΔΔCt.
Western Blot (WB)
Protein extract: protein inhibitor (Solarbio) + 20 mM Tris-HCl solution (pH7.5, Solarbio). Adherent cells in flasks were digested and prepared into cell suspension. The suspension was mixed with 1 mL extract, and the mixture was pipetted repeatedly until the complete lysis of cells. The extract was centrifuged in a precooled centrifuge at 4°C for 20 min at 1.6×104 ×g. Protein concentration in the supernatant was determined by bicinchoninic acid (BCA), and the protein was separated by sodium dodecylsulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Afterwards, the separated protein was transferred to a nitrocellulose (NC) membrane and left for 1 h at room temperature (sealed with 5% skim milk-phosphate buffer saline (PBS)). Then testing protein and anti-beta Actin antibody (Abcam) were added, and placed overnight at 4°C. The NC membrane was washed three times with PBS solution, then added with goat anti-rabbit secondary antibody (horseradish peroxidase (HRP) conjugate, Abcam), and left to stand for 1 h at room temperature. Finally, the NC membrane was washed with PBS and visualized with enhanced chemiluminescence (ECL) solution. β-actin was taken as the internal reference protein, and the relative expression of the protein = gray value of test band/gray value of the β-actin band.
Flow Cytometry
Cell suspension with 1×106 cells was prepared. Cells were immobilized in 70% ethanol solution for 30 min, keeping the ambient temperature at 4°C. The ethanol solution was then removed. A mixed solution of disodium propidium iodide (50 ng/mL)/RNase (0.2 mg/mL)/0.1% Triton X-100 was added, and the cells were incubated at indoor temperature for 30 min. A FACScan flow cytometer (Becton Dickinson, USA) was employed to evaluate the apoptosis.
Transwell
The cells (2×104/well) were inoculated into the apical chamber (200 μL of mixed solution containing 10% FBS and 1% DMEM was added in advance), and DMEM (500 μL, containing 10% FBS) was added to the basolateral chamber. The Transwell insert was cultured at 37°C and 5% CO2 for 24 h, and then the liquid in the apical chamber was removed and the cells were wiped off. Cells attached to the outside of the membrane were immobilized in 4% paraformaldehyde for 20 min, stained with crystal violet for 15 min, washed with PBS buffer. The migrated cells were counted in random 3 visual fields using a 200-fold microscope, and the average value was taken. The test was repeated three times. As for the invasion assay, the insert was covered with 8% Matrigel, and the number of cells was increased to 5×104 per well, the rest steps were the same as the migration assay described above.
Dual-Luciferase Reporter (DLR) Assay
Cells were inoculated in 96-well plates and DLR assay was carried out when they were in good condition. GLO-H19-wt, GLO-H19-mut, GLO-TLR4-wt, GLO-TLR4-mut vectors were separately co-transfected with miR-17 mimics and NC mimics. After 48 h, luciferase activity was detected in a DLR assay system (Promega).
Statistics and Analysis
SPSS 20.0 (Asia Analytics Formerly SPSS China) and GraphPad Prism8.0 carried out statistical analysis and graphic processing on the data, respectively. Measurement data were presented as Mean±standard deviation (SD), independent sample t-test was adopted for comparison between two groups, one-way analysis of variance (ANOVA) for comparison among multiple groups, and Fisher’s least significant difference-t-test for post hoc pairwise comparison. All data were analyzed by two-tailed test; 95% was taken as the confidence interval. The difference was statistically significant as P<0.05.
Results
Effects of LNG on lncRNA H19/miR-17/TLR4
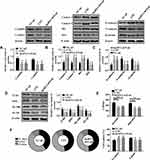
Seventy-one samples of endometrium from patients diagnosed with adenomyosis were collected. qPCR quantified the expression of lncRNA H19, miR-17, TLR4 mRNA. Another 51 healthy endometrium were taken as control group. LncRNA H19 was down-regulated and miR-17 and TLR4 mRNA were up-regulated in adenomyosis (Figure 1A), indicating their involvement in adenomyosis. In this study, cells were treated with LNG, a drug for the treatment of adenomyosis, at a concentration of 5 × 10−5 mol/L to study its therapeutic mechanism, and the cells displayed up-regulation of lncRNA H19 and down-regulation of miR-17 and TLR4 mRNA (Figure 1B), indicating that LNG may improve adenomyosis through lncRNA H19, miR-17, and TLR4. In addition, up-regulation of lncRNA H19 suppressed miR-17 and TLR4, and down-regulation of miR-17 reduced TLR4, which suggests that lncRNA H19/miR-17/TLR4 pathway exists in ESCs.
LNG Hinders Cell Proliferation and Accelerates Apoptosis by Up-Regulating lncRNA H19
To study the effect of LNG on cells, flow cytometry was adopted for determination of cell proliferation, WB for related marker proteins, and Transwell for cell migration and invasion. LncRNA H19-ad group (over-expression group) was set up to study how the up-regulation of lncRNA H19 induced by LNG would affect cell function. It was turned out that LNG and lncRNA H19-ad led to the reduction of CyclinD1 and CyclinE1, decrease of S-phase cells and increase of G1-phase cells (Figure 2A and F, Figure 3). LNG and lncRNA H19-ad up-regulated Caspase 3, Caspase 9, Bax, and down-regulated Bcl2 (Figure 2B). LNG and lncRNA H19-ad inhibited cell migration and invasion, up-regulated E-cadherin, as well as down-regulated N-cadherin and β-catenin (Figure 2C and E). TLR4 mediated signal pathways mainly include TLR4, myeloid differentiation protein-2 (MD2), myeloid differential protein-88 (MyD88) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), so the activity of TLR4 pathway is evaluated by the level of these four proteins. LNG and lncRNA H19-ad down-regulated TLR4, MD2, MyD88, and NF-κB (Figure 2D), indicating that LNG hindered cell proliferation and accelerated apoptosis by up-regulating lncRNA H19.
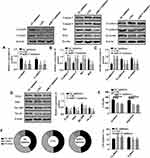
LNG Hinders Cell Proliferation and Accelerates Apoptosis by Down-Regulating miR-17
This part investigated how LNG-induced down-regulation of miR-17 regulates cell function. LNG and down-regulation of miR-17 arrested cells in G1 phase, hindered cell migration and invasion, reduced TLR4 pathway activity, up-regulated Caspase 3, Caspase 9, Bax, E-cadherin, and down-regulated CyclinD1, CyclinE1, Bcl2, N-cadherin and β-catenin (Figures 3 and 4). The above results indicated that LNG hindered cell proliferation and accelerated apoptosis by down-regulating miR-17.
LNG Hinders Cell Proliferation and Accelerates Apoptosis by Down-Regulating TLR4
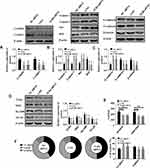
How LNG-induced down-regulation of TLR4 regulates cell function was introduced in this part. LNG and down-regulation of TLR4 arrested cells in G1 phase, hindered cell migration and invasion, reduced TLR4 pathway activity, up-regulated Caspase 4, Caspase 9, Bax, E-cadherin, and down-regulated CyclinD1, CyclinE1, Bcl2, N-cadherin and β-catenin (Figures 5 and 3). These results indicated that LNG hindered cell proliferation and accelerated apoptosis by down-regulating TLR4.
Targeting Relation of lncRNA H19/miR-17/TLR4 Pathway
Since the above results revealed that the lncRNA H19/miR-17/TLR4 pathway may be the mechanism of LNG action, so the targeting relation of the pathway was studied in this part. Targetscan7.2 and Starbase2.0 demonstrated that there were binding loci between lncRNA H19 and TLR4 on miR-17 sequence fragment (Figure 6A). DLR assay verified that lncRNA H19 and TLR4 can be paired and bound with miR-17 (Figure 6B–D). Therefore, the lncRNA H19/miR-17/TLR4 pathway is actually present in ESCs.
LVG Attenuates Inflammatory Response in ESCs
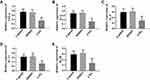
Since LVG inhibited TLR4 pathway, how pro-inflammatory factors tumor necrosis factor-α (TNF-tab α), interleukin −6 (IL-6), IL-8, IL-17, and IL-1β mediated by TLR4 pathway were regulated by LVG was investigated in this section. The results showed that LVG could suppress TNF-α, IL-6, IL-8, IL-17 and IL-1β. Therefore, LVG inhibited the inflammatory response in ESCs by decreasing the activity of TLR4 pathway (Figures 7 and 3).
Discussion
In this study, we found that lncRNA H19 was down-regulated and miR-17 and TLR4 were up-regulated in endometrium of adenomyosis patients. LVG treatment of ESCs resulted in up-regulation of intracellular lncRNA H19 and down-regulation of miR-17 and TLR4. After that, lncRNA H19, miR-17 and TLR4 were regulated by plasmid vectors to observe their effects on ESCs, and it was turned out that up-regulation of lncRNA H19, down-regulation of miR-17 and TLR4 led to decrease of cell proliferation, migration and invasion, increase of apoptosis, as well as reduction of activity of TLR4 signaling pathway. Sequence pairing analysis showed that lncRNA H19 and TLR4 shared common binding loci on the miR-17 sequence, and DLR assay confirmed that lncRNA H19 and TLR4 could bind to miR-17. In addition, we found that LVG played a role in inducing cell apoptosis, hindering cell proliferation, and suppressing TNF-α, IL-6, IL-8, IL-17 and IL-1β. These findings bring us to a conclusion that LVG is capable of accelerating cell apoptosis and hindering cell inflammation via lncRNA H19/miR-17/TLR4 pathway.
TLR4 is an important member of cellular immunity and mediates the release of proinflammatory factors,26 and its abnormal expression may trigger a series of diseases or cancers.27–30 In the case of adenomyosis, the inflammatory response induced by TLR4 promotes the excessive proliferation of ESCs and endows them with invasive phenotypes,31 resulting in the cells to invade to myometrium. According to our findings, LVG reduces the level of TLR4 in ESCs by regulating lncRNA H19/miR-17, thus attenuating the inflammatory response (down-regulation of TNF-α, IL-6, IL-8, IL-17 and IL-1β), and finally suppressing the excessive proliferation and malignant proliferation of ESCs.
Besides, LVG down-regulates the intracellular inflammatory microenvironment through LNCRNAH 19/miR-17/TL R4 pathway, which ultimately inhibits the proliferation and invasion of ESCs. Zhao et al32 stated that LVG inhibits the proliferation of ESCs by regulating gap junctional intercellular communication, indicating that LVG regulates the growth and metastasis of ESCs by changing the intercellular and intracellular matrix, and also implying that ESCs are strictly controlled by intracellular and extracellular microenvironment. Therefore, the influence of microenvironment-derived proteins on ESCs and how LVG affects these proteins will be further discussed in the following research.
To sum up, LVG relieves inflammatory response and hinders excessive proliferation and malignant proliferation of ESCs through lncRNA H19/miR-17/TLR4 pathway. The potential value of this mechanism is to improve the efficacy of LVG on adenomyosis by up-regulating lncRNA H19, down-regulating miR-17, or down-regulating TLR4. Moreover, lncRNA H19, miR-17, and TLR4 are also expected to become biomarkers of adenomyosis.
Acknowledgments
This study was financially supported by National Natural Science Foundation of China Youth Fund Project (Grant number: 81904243); Shandong Traditional Chinese Medicine Science and Technology Development Plan Project (Grant number: 2017-173); National Natural Science Foundation of China Funded Project (81873330); Shandong Science and Technology Innovation Project (2018CXGC1309); Shandong Province Traditional Chinese Medicine Technology Development Plan Project (2017-070); Shandong Province Key R & D Plan (2017G006 017); Taishan Scholar Project in Shandong Province (No: tsqn201909185).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vargas MV, Huang K. Endometriosis and adenomyosis. Evidence Based Obstet Gynecol. 2019;75–87.
2. Munro MG. Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertil Steril. 2019;111(4):629–640. doi:10.1016/j.fertnstert.2019.02.008
3. Vercellini P, Bonfanti I, Berlanda N. Adenomyosis and infertility: is there a causal link? Expert Rev Endocrinol Metab. 2019;1–3.
4. Artymuk N, Zotova O, Gulyaeva L. Adenomyosis: genetics of estrogen metabolism. Horm Mol Biol Clin Investig. 2019;37(2).
5. Garcia-Solares J, Donnez J, Donnez O, Dolmans MM. Pathogenesis of uterine adenomyosis: invagination or metaplasia? Fertil Steril. 2018;109(3):371–379. doi:10.1016/j.fertnstert.2017.12.030
6. Hashimoto A, Iriyama T, Sayama S, et al. Adenomyosis and adverse perinatal outcomes: increased risk of second trimester miscarriage, preeclampsia, and placental malposition. J Matern Fetal Neonatal Med. 2018;31(3):364–369. doi:10.1080/14767058.2017.1285895
7. Shin YJ, Kwak DW, Chung JH, Kim MY, Lee SW, Han YJ. The risk of preterm births among pregnant women with adenomyosis. J Ultrasound Med. 2018;37(8):1937–1943. doi:10.1002/jum.14540
8. Mahmoud A, Khalifa MD. Adenomyosis as a confounder to accurate endometrial cancer staging. Semin Ultrasound CT MRI. 2019;40(8):358–363. doi:10.1053/j.sult.2019.04.004
9. Vannuccini S, Petraglia F. Recent advances in understanding and managing adenomyosis. F1000Res. 2019;8. doi:10.12688/f1000research.17242.1
10. Struble J, Reid S, Bedaiwy MA. Adenomyosis: a clinical review of a challenging gynecologic condition. J Minim Invasive Gynecol. 2016;23(2):164–185. doi:10.1016/j.jmig.2015.09.018
11. Chen Z, Pan X, Sheng Z, et al. miR-17 regulates the proliferation and apoptosis of endothelial cells in coronary heart disease via targeting insulin-like-growth factor 1. Pathol Res Pract. 2019;215(9):152512. doi:10.1016/j.prp.2019.152512
12. Lee EC, Valencia T, Allerson C, et al. Discovery and preclinical evaluation of anti-miR-17 oligonucleotide RGLS4326 for the treatment of polycystic kidney disease. Nat Commun. 2019;10(1):4148. doi:10.1038/s41467-019-11918-y
13. Yheskel M, Lakhia R, Cobo-Stark P, Flaten A, Patel V. Anti-microRNA screen uncovers miR-17 family within miR-17~92 cluster as the primary driver of kidney cyst growth. Sci Rep. 2019;9(1):1920. doi:10.1038/s41598-019-38566-y
14. Gu J, Wang D, Zhang J, et al. GFRalpha2 prompts cell growth and chemoresistance through down-regulating tumor suppressor gene PTEN via Mir-17-5p in pancreatic cancer. Cancer Lett. 2016;380(2):434–441. doi:10.1016/j.canlet.2016.06.016
15. Xu J, Meng Q, Li X, et al. Long noncoding RNA MIR17HG promotes colorectal cancer progression via miR-17-5p. Cancer Res. 2019;79(19):4882–4895. doi:10.1158/0008-5472.CAN-18-3880
16. Cai N, Hu L, Xie Y, et al. MiR-17-5p promotes cervical cancer cell proliferation and metastasis by targeting transforming growth factor-beta receptor 2. Eur Rev Med Pharmacol Sci. 2018;22(7):1899–1906. doi:10.26355/eurrev_201804_14712
17. Zhang G, An X, Zhao H, Zhang Q, Zhao H. Long non-coding RNA HNF1A-AS1 promotes cell proliferation and invasion via regulating miR-17-5p in non-small cell lung cancer. Biomed Pharmacother. 2018;98:594–599. doi:10.1016/j.biopha.2017.12.080
18. Ast V, Kordass T, Oswald M, et al. MiR-192, miR-200c and miR-17 are fibroblast-mediated inhibitors of colorectal cancer invasion. Oncotarget. 2018;9(85):35559–35580. doi:10.18632/oncotarget.26263
19. Jia SZ, Yang Y, Lang J, Sun P, Leng J. Plasma miR-17-5p, miR-20a and miR-22 are down-regulated in women with endometriosis. Hum Reprod. 2013;28(2):322–330. doi:10.1093/humrep/des413
20. Hu H, Li H, He Y. MicroRNA-17 downregulates expression of the PTEN gene to promote the occurrence and development of adenomyosis. Exp Ther Med. 2017;14(4):3805–3811. doi:10.3892/etm.2017.5013
21. Sun Y, Zhu Q, Yang W, et al. LncRNA H19/miR-194/PFTK1 axis modulates the cell proliferation and migration of pancreatic cancer. J Cell Biochem. 2019;120(3):3874–3886. doi:10.1002/jcb.27669
22. Zhao Y, Feng C, Li Y, Ma Y, Cai R. LncRNA H19 promotes lung cancer proliferation and metastasis by inhibiting miR-200a function. Mol Cell Biochem. 2019;460(1–2):1–8. doi:10.1007/s11010-019-03564-1
23. Liu S, Qiu J, Tang X, Cui H, Zhang Q, Yang Q. LncRNA-H19 regulates cell proliferation and invasion of ectopic endometrium by targeting ITGB3 via modulating miR-124-3p. Exp Cell Res. 2019;381(2):215–222. doi:10.1016/j.yexcr.2019.05.010
24. Pan Y, Zhang Y, Liu W, et al. LncRNA H19 overexpression induces bortezomib resistance in multiple myeloma by targeting MCL-1 via miR-29b-3p. Cell Death Dis. 2019;10(2):106. doi:10.1038/s41419-018-1219-0
25. Liu Z, Liu L, Zhong Y, et al. LncRNA H19 over-expression inhibited Th17 cell differentiation to relieve endometriosis through miR-342-3p/IER3 pathway. Cell Biosci. 2019;9:84. doi:10.1186/s13578-019-0346-3
26. Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42(2):145–151. doi:10.1016/j.cyto.2008.01.006
27. Wang J, Tian XT, Peng Z, et al. HMGB1/TLR4 promotes hypoxic pulmonary hypertension via suppressing BMPR2 signaling. Vascul Pharmacol. 2019;117:35–44. doi:10.1016/j.vph.2018.12.006
28. Lei JR, Tu XK, Wang Y, Tu DW, Shi SS. Resveratrol downregulates the TLR4 signaling pathway to reduce brain damage in a rat model of focal cerebral ischemia. Exp Ther Med. 2019;17(4):3215–3221. doi:10.3892/etm.2019.7324
29. Dickinson SE, Wondrak GT. TLR4 in skin cancer: from molecular mechanisms to clinical interventions. Mol Carcinog. 2019;58(7):1086–1093. doi:10.1002/mc.23016
30. Zandi Z, Kashani B, Poursani EM, et al. TLR4 blockade using TAK-242 suppresses ovarian and breast cancer cells invasion through the inhibition of extracellular matrix degradation and epithelial-mesenchymal transition. Eur J Pharmacol. 2019;853:256–263. doi:10.1016/j.ejphar.2019.03.046
31. Guo J, Chen L, Luo N, et al. LPS/TLR4-mediated stromal cells acquire an invasive phenotype and are implicated in the pathogenesis of adenomyosis. Sci Rep. 2016;6:21416. doi:10.1038/srep21416
32. Zhao X, Tang X, Ma T, et al. Levonorgestrel inhibits human endometrial cell proliferation through the upregulation of gap junctional intercellular communication via the nuclear translocation of Ser255 phosphorylated Cx43. Biomed Res Int. 2015;2015:758684.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.