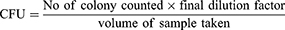Back to Journals » Veterinary Medicine: Research and Reports » Volume 13
Levels of Escherichia coli as Bio-Indicator of Contamination of Fish Food and Antibiotic Resistance Pattern Along the Value Chain in Northwest Ethiopia
Authors Yohans H , Mitiku BA , Tassew H
Received 30 June 2022
Accepted for publication 5 October 2022
Published 2 November 2022 Volume 2022:13 Pages 299—311
DOI https://doi.org/10.2147/VMRR.S373738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Young Lyoo
Halo Yohans, Birhan Agmas Mitiku, Habtamu Tassew
Department of Veterinary Science, School of Animal Science and Veterinary Medicine, College of Agriculture and Environmental Science, Bahir Dar University, Bahir Dar, Ethiopia
Correspondence: Halo Yohans, Department of Veterinary Science, School of Animal Science and Veterinary Medicine, College of Agriculture and Environmental Science, Bahir Dar University, Bahir Dar, Ethiopia, Email [email protected]
Introduction: Microbiological contamination in fish origin foods is the leading risk for public health. Among the range of pathogenic bacterial species that cause fish food borne diseases is Escherichia coli. The pathogenic strains of Escherichia coli cause diarrhea by producing and releasing toxins and can also be the cause of food spoilage in fish.
Methods: A cross-sectional study was conducted to assess hygienic practices of fish handlers, to evaluate bacterial load and antimicrobial resistance patterns of Escherichia coli along the fish value chain in Northwest Ethiopia. Systematic and purposive sampling techniques were used for uncooked and cooked fish samples respectively.
Results: From a total of 180 fish samples, 36 (20%) were positive for Escherichia coli. From 115 uncooked and 65 cooked fish samples examined, 27 (23.5%) and 9 (13.8%) had E. coli respectively. The highest mean bacterial count was observed in raw fish samples (6.13 × 105 cfu/g), followed by cooked fish samples (2.81 × 104 cfu/g). Among the interviewed fish handlers, 83.3%, 76.7% and 80% of respondents had good knowledge and attitude towards using a clean cutting-and-filleting board, storing raw and cooked foods separately and using an apron for reducing the risk of fish contamination, respectively. All 36 isolates were 100% sensitive to ciprofloxacin and gentamycin. Of the Escherichia coli isolates subjected to tetracycline, 55.6% were resistant, 8.3% were intermediate and 36.1% were susceptible.
Conclusion and Recommendation: This study revealed that there was a lack hygienic practice and high Escherichia coli profiles were observed. Hence, it could be wise to advise the fish harvesters, fish traders, hotels and restaurants about fish food safety practices from harvesting to consumption to improve fish food safety practices and quality standards of fish harvested and sold in northwest Ethiopia.
Keywords: Escherichia coli, fish food, hygienic practice, Lake Tana
Introduction
Fish and fishery products are the most necessary nutritious meals all over the world, which represent about 15–20% of all animal protein on a world basis.1 Fish constitutes 19% of animal protein consumption in Africans and performs a special role in supplying a range of micronutrients and especially essential fatty acids. Africa’s fish consumption is 10.8 kg/person/year.2 Ethiopia’s fish consumption is 0.2 kg/person/year.3
The health benefits of fish consumption have been properly demonstrated by numerous studies. These are due to the presence of proteins, minerals and vitamins; and peptides, amino acids, selenium and long-chain n-3 polyunsaturated fatty acids (LC n-3 PUFAs). In addition to nutritional value, the health benefits of fish food consumption have especially been related to protection against cardiovascular disease (CVD); to extended fetal and child development and to really helpful results in protecting various different illnesses and clinical conditions.4 The health-promoting effects have mainly been attributed to the LC n-3 PUFAs, eicosapentanoic acid (EPA) and docosahexaenoic acid (DHA).5 However, alongside the advantages there are associated risks, such as bacterial contamination and other biological, chemical and physical contaminations.2 Among the risks, microbiological contamination is the leading risk in fish foods.6 As a result, fish food is a common source of food poisoning, causing illnesses with various levels of severity, ranging from mild indisposition to persistent or life-threatening illness.7 Microbial contamination, in addition to the negative health effects, causes loss of food. Of the fish captured, 30% is lost via microbial activity alone.8
Foodborne diseases are recognized to regularly take place in developing countries, probably due to poor food handling and hygiene, a lack of implementation of safety measures, a weak regulatory systems, a lack of economic assets to procure safety tools and a lack of education and/or training for different food handlers.7,9 In Ethiopia, animal and fish origin meals are main sources of foodborne ailments due to poor handling conditions and sanitation practices, inadequate food safety laws, weak regulatory structures and lack of training for food handlers.10,11 This low food safety and quality practice in developing countries aggravates fish food spoilage and contamination.
Among the range of pathogenic bacterial species that cause fish food borne diseases is Escherichia coli. The pathogenic strains of Escherichia coli may cause diarrhea by producing and releasing toxins and can also be the cause of food spoilage in fish.12 Currently, six categories of diarrheagenic Escherichia coli have been acknowledged: enterotoxigenic E. coli (ETEC), enteropathogenic Escherichia coli (EPEC), enteroinvasive Escherichia coli (EIEC), enterohemorrhagic Escherichia coli (EHEC, Shiga toxin-producing Escherichia coli or STEC), enteroaggregative Escherichia coli (EAEC or EAggEc) and diffusely adherent Escherichia coli (DAEC). Some strains such as Shiga toxin-producing Escherichia coli (STEC) can cause severe foodborne disease. It is transmitted to humans primarily through consumption of contaminated foods, such as raw or undercooked ground meat products, raw milk, and contaminated raw vegetables. Different strains Escherichia coli cause diseases in gastrointestinal, urinary, or central nervous systems.
The occurrence of this bacterium in food is directly related to fecal contamination. This bacterium is the most abundant facultative anaerobe of the human intestinal micro flora.13 Furthermore, Escherichia coli is broadly present in the intestinal tracts of warm-blooded animals.14 The presence of Escherichia coli in ready-to-eat foods is undesirable because it suggests poor hygienic conditions that lead to contamination or inadequate heat treatment. Ideally, Escherichia coli should not be detected, and as such, a level of <20 cfu/gram has been given as the quality criteria for this organism. In fish origin foods and other foods, levels between 20 and 100 cfu/g are border-line or intermediate, and levels exceeding (>) 100 cfu/g are unacceptable and indicate a stage of contamination.15
The use of antimicrobial agents in the treatment of Escherichia coli infection causes the emergence of antibiotic resistant bacteria, and their resistance genes have turned into a serious, growing issue in current medication.16 Escherichia coli resistance to antimicrobials is creating trouble for the healthcare system worldwide.17 Hence, monitoring of bacterial load and the antibiotic resistance pattern of bacteria, and surveying hygienic practice in fish are of paramount importance in providing useful data regarding the public health risk profile of fish and fish products. The results of these studies will assist planning the right management strategies against fish foodborne diseases. Therefore, the objective of this study was to evaluate bacterial load and antimicrobial resistance patterns of Escherichia coli from fish value chain in the upper Blue Nile watershed.
Methods
Description of Study Area
The study was conducted in the upper Blue Nile river watershed in Northwest Ethiopia. Lake Tana and Bahir Dar city were the selected representative fishing aquatic site and fish food processing city, respectively. Lake Tana and Bahir Dar are the biggest lake and city in the region, respectively (Figure 1). Bahir Dar city is located 580 km north-northwest of Addis Ababa. Geographically, Bahir Dar is located at a latitude of 11.59° north and 37.39° east. Its average elevation is estimated to be 1810 m above sea level.18 Bahir Dar city is one of the leading tourist destinations in Ethiopia, with a variety of attractions in nearby Lake Tana and Blue Nile River.
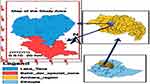 |
Figure 1 Map of study area (Arc GIS software, 2020). |
Lake Tana is the headwaters source of the Blue Nile river and is the main fishing aquatic environment in the Northwest Ethiopia region. The lake provides three commercially important, delicious fish species groups: namely, African catfish (Clarius gariepinus locally called “Ambaza”), Nile tilapia (Oreochromis niloticus, locally called “Kereso”) and Labeobarbus spp. (locally called “Nech Asa”). They are consumed by the larger part of the community, rural and urban, and are traded widely in the region and the country.19
Study Design and Sample Size Determination
A cross-sectional study was conducted from November 2019 to May 2020. The sample size was determined by using a 95% confidence interval and a 5% desired level of precision. No previous studies had been conducted about the presence of Escherichia coli in raw to ready-to-eat fish products in the study area; hence, the expected prevalence of Escherichia coli was taken as 50% and the size was determined by the formula for infinite population given below:20
where: n = required sample size; P = expected prevalence; d = desired absolute precision. Based on the abovementioned formula, the total sample size was 384,21 but due to financial problems related to the laboratory reagents and the cost of cooked fish samples, 180 fish samples were sampled and considered for the study.
Sampling Method for Laboratory Data Collection
Thirty (30) hotels and restaurants were selected by using purposive sampling methods based on their major contribution from the 6 sub-cities in Bahir Dar city. Two (2) landing sites were selected by a purposive sampling method in Lake Tana based on their major fishing practices. In these landing sites, fishing activity was mostly two times per day (morning and afternoon). Fish samples from fish harvesters at landing sites were sampled by using a systematic sampling method.
Based on this, a total of 180 (115 raw and 65 cooked) fish samples were collected. To make sure that samples were taken without being contaminated, sterile inverted plastic bags were used for collection. The inner surface of the bag was used to touch nothing else but the sample. All samples were labeled with the type of the sample, the place, date of sampling and given an identification code and transported to Bahir Dar university veterinary microbiology laboratory in an icebox containing ice packs for microbiological analysis. Upon arrival, the samples were immediately processed or stored at 4°C in a refrigerator until use and processed within 24 h of collection.
Questionnaire Survey and Observation
Direct observation and a questionnaire survey were conducted to assess the cleanliness or uncleanliness of food storage conditions and prevention of cross-contamination of raw and ready-to-eat fish origin foods among retailers of the city. The content of the questionnaire also included questions addressing the educational status, the health status and the personal hygiene, the food handling practices, the food safety knowledge and the attitude of food handlers among fish retailers. The questionnaire was designed in two ways: for fish processors in hotels and restaurants and fish harvesters in landing sites.
Fish retailers (fishermen, filleting processors and fish processors of hotels and restaurants) were included in the questionnaire survey. The questionnaire was completed by a face-to-face interview with one representative fish food handler. The workers were selected purposively based on their major contribution to food processing and handling. The questionnaire and observational checklists were managed in accordance with the standard guidelines of the Codex Alimentarius Commission of food and agriculture organization.1
Data Collection Procedure
Fish samples were aseptically collected from 30 hotels and restaurants in Bahir Dar city and from 2 landing sites in Lake Tana (n = 180). Cooked fish samples (65) and raw fish samples (115) were collected using sterile plastic bags. Fish samples were collected from sampling sites by using an icebox and transporting them to a veterinary microbiology laboratory in Bahir Dar University within 4 h of collection for bacteriological analysis. Raw or fresh fish samples were filleted using sterile knives and forceps so that the skin part was kept with the flesh and then placed on a sterile tray. Laboratory procedures were conducted according to the codex.21
Quality Assurance Mechanisms
The quality of data and the reliability of test results were assured by following standard procedures. The sterility of prepared media was checked by incubating some randomly selected plates for 24–48 h at 37°C. A well-known bacterial culture was used as a positive control for screening tests and confirmatory tests of the bacteria.
Microbiological Isolation and Characterization of Escherichia coli
Isolation and Identification of Escherichia coli
Fish samples were processed in a complete aseptic condition. A total of 25 g of raw or cooked fish samples were homogenized for 2 min in a sterile bag containing 225 mL of buffered peptone water (0.1%) (Lab M, London; United Kingdom) using a stomacher (Seward Stomacher 400 circulator, London, United Kingdom). All samples were inoculated onto EMB agar and incubated at 37°C for 24–48 h. Suspected colonies on EMB agar were sub-cultured on MacConkey agar medium and nutrient agar and were incubated at 37°C for 24–48 h.22 Colonies of Escherichia coli on eosin methylene blue agar (EMB) show green metallic sheen. Colonies suspected to be Escherichia coli were subjected to biochemical identification.23
Identification and Characterization of Escherichia coli
The suspected result from the abovementioned media was inoculated into nutrient agar and tested by different biochemical tests: the Indole test, Methyl red test, Simon citrate test, Triple sugar iron agar (TSI) test, and Urease test.
Enumeration of Escherichia coli Count (Aerobic Plate Count)
The enumeration of E. coli count was done by using a standard plate count method. From the 10-fold dilutions of the homogenates of the original sample, 0.1 mL of 10−2, 10−3, 10−4, 10−5 and 10−6 dilutions of the homogenates were spread-plated on fresh standard plate count agar (HiMedia, Mumbai; India). The plates were then incubated at 37°C for 24 to 48 h. At the end of the incubation period, plates exhibiting 30 to 300 colonies were counted by using a digital colony counter. The counts for each plate were expressed as a colony-forming unit of the suspension (cfu/g).24 Identification of colonies and appropriate biochemical tests were done in accordance with Oyeleke and Manga.25 The isolates were identified by comparing their morphological and biochemical characteristics:
Antimicrobial Susceptibility Test
The antimicrobial susceptibility test was done with the disk diffusion method (Bauer et al, 1966) using Mueller–Hinton agar (Difco). Initially, an emulsion of sample in saline solution was prepared by adjustment to the 0.5 McFarland turbidity standards. The susceptibility of the E. coli strains was tested in relation to several antibiotics, including: chloramphenicol (CAF) (30 μg), ciprofloxacin (5 μg), gentamycin (10 μg), trimethoprim (1.25 μg-sulfamethoxazole 23.75 μg), erythromycin (15 μg), streptomycin (10 μg) and tetracycline (30 μg) (Mast Group Ltd., Merseyside, UK). Using sterile tweezers, commercially available antibiotic disks were placed individually on the surface of Mueller-Hinton agar. After 24 h of incubation at 35°C, the strains were scored as “susceptible”, “intermediate”, or “resistant” to each antibiotic based on the measurement of the inhibition zone, as recommended by clinical laboratory standard institute (CLSI).26
Ethical Review
The study protocol was reviewed and approved by the National Fishery and Aquatic Life Research Center. A letter of support was obtained from the National Fishery and Aquatic Life Research Center and Bahir Dar University and official permission was received from the concerned higher officials of the Amhara Region Public Health Bureau, too. The sampled retailer’s owner permission and interviewee’s willingness to participate in the research were obtained. After thoroughly explaining the objectives and relevance of the study, the procedure, the benefits and their rights, informed consent was obtained from the participants. The participants were informed that their participation was fully voluntary and that they could choose not to answer any question and could stop the discussion at any time. To ensure confidentiality, any personal identifying information on participants was not collected and was maintained using a unique code. An agreement was formed that the information collected for this study was not to be used for any other purpose without the approval of each participant.
Data Analysis
Raw data and laboratory results were recorded into Microsoft Excel and analyzed by using stata software version 12. Descriptive statistics such as percentage and frequency were used for the aerobic plate count (APC) and for positive samples. The degrees of associations were quantified using an odds ratio obtained from univariate logistic regression models. In all the analyses, the confidence level was held at 95% and the p-value was assumed to be less than 5% (p < 0.05).
Results
Occurrence of Escherichia coli
From a total of 180 fish samples taken, 36 (20%) fish samples were positive for Escherichia coli isolates (60 fresh unfilleted fish, 55 filleted and 65 cooked fish samples were sampled). From all fish samples, 17 (23.6%) of Nile tilapia, 15 (20%) of Labeo Barbus and 4 (12.1%) of African catfish were positive for Escherichia coli. Out of 65 ready-to-eat cooked fish samples, 9 (13.8%) Escherichia coli isolates were identified.
Occurrence of Escherichia coli in Raw Fish Foods
From 115 raw fish samples, 27 (23.5%) Escherichia coli isolates were identified at the landing sites. The occurrence of Escherichia coli was higher in filleted fish samples than unfilleted fresh fish samples at the two landing sites. This was due to the contamination of fish by the bare hands of those who did the filleting and the filleting ground during the filleting process (Table 1).
 |
Table 1 Escherichia coli Isolates from Raw Fish Samples in Lake Tana |
Occurrence of Escherichia coli in Cooked Fish Foods
From 65 cooked fish samples, 9 (13.8%) Escherichia coli isolates were identified in hotels and restaurants in Bahir Dar City. The occurrence of Escherichia coli was lower in cooked fish food samples than uncooked fish food samples. This is due to the negative effect of heat during processing of cooked fish foods (Table 2).
 |
Table 2 Escherichia coli Isolates from Cooked Fish Samples in Hotels and Restaurants |
Escherichia coli Count Using Aerobic Plate Count
Bacterial growth is the main cause of fish spoilage and is a public health concern; therefore, the total bacterial count was used as a general index of fish quality. In this study, the mean bacterial count (cfu/g) was found to be 6.13 × 105 cfu/g in raw and 2.81 × 104 cfu/g in cooked fish samples (Table 3).
 |
Table 3 Total Escherichia coli Load Count from Cooked and Raw Fish Samples in Bahir Dar |
Questionnaire Survey Results
Demographic and Operational Characteristics of Fish Handlers
A total of 60 respondents engaged in fishing activity and fish origin food processers were interviewed in the study area (Table 4).
 |
Table 4 Demographic and Operational Characteristics of Fish Handlers (n = 60) |
Hygienic Practices and Knowledge of Fish Cookers in Hotels and Restaurants
All of the respondents had excellent knowledge of the use of hand gloves (100%), the use of sufficient heat and spice (100%) for cooked fish origin foods and the cleaning of contact surfaces (100%) before starting business.
Most of the fish processors had a positive attitude about food safety and hygienic measures. The majority of respondents had good knowledge of and attitudes about using a clean cutting-and-filleting board and fly repellent (83.3%), washing hands before and after handling of fish (73.3%), storing raw and cooked foods separately (76.7%), using an apron (80%) and covering hair (70%) to reduce the risk of fish origin food contamination (Table 5).
 |
Table 5 Knowledge and Attitudes of Fish Cookers in Hotels and Restaurants at Bahir Dar (n = 30) |
Hygienic Practices and Knowledge of Fish Harvesters and Filleters in Fish Landing Sites
All (100%) of the respondents transport fishes without ice by using a plastic bag and most (66.7%) of them harvest Nile tilapia fish. A total of 50% and 30% of raw fish were retailed to hotels and restaurants and consumers, respectively. Eighty percent (80%) of the respondents did not wash or clean their boats before and after starting of fishing activity and 90% of the respondents had little knowledge about proper transportation of fish and the fact that improper use of hooks and filleting boards can be a source of fish food contamination. Most of the fishery men were sold the caught fishes within 2–6 h (Table 6).
 |
Table 6 Food Safety Knowledge of Fish Harvesters and Filleters at Landing Sites in Lake Tana (n = 30) |
Data Obtained by Direct Observation of Fish Handlers
Direct observation was employed to assess the hygienic status and practices of the fish handlers working in the kitchens of different hotels found in Bahir Dar city (Table 7).
 |
Table 7 Food Safety Practice of Fish Handlers in Hotels and Restaurants in Bahir Dar (n = 30) |
Antimicrobial Susceptibility Profile
Isolates of Escherichia coli were tested with seven available antibiotics with a disc diffusion method. All Escherichia coli isolates were 100% susceptible for ciprofloxacin and gentamycin. 58.3% and 75% of Escherichia coli isolates were susceptible for chloramphenicol and trimethoprim-sulfamethoxazole, respectively. Most of the Escherichia coli isolates were resistant for erythromycin (58.3%), streptomycin (88.9%) and tetracycline (55.6%) (Table 8).
 |
Table 8 Susceptibility of E. coli Isolates Against Some Selected Antimicrobials |
A disc diffusion method was applied to determine the susceptibility of Escherichia coli in this study. The main advantages of disc diffusion method are simplicity, reproducibility, ease in modifying antimicrobial discs, the possibility for use as a screening test against numerous isolates, and low cost.
A treatment based on the detection of antimicrobial resistance is usually more effective than empirical treatment. Due to mutation and genetic exchange in bacteria, the effectiveness of antimicrobials may be lowered and treatment failure can occur. This is currently one of the challenges for treating patients in hospitals.
The objective of conducting an antimicrobial susceptibility profile in this study was to select the best drug of choice for treating of patients from locally available and frequently used drugs in the study area.
Discussion
Out of a total of 180 fish samples tested for Escherichia coli, 36 (20%) were positive. This finding indicates that the contamination of fish origin food with Escherichia coli is similar to other researchers’ findings in different areas. Thampuran et al27 have isolated Escherichia coli in finfish samples acquired at the retail market in Cochin, India. The result of this study was higher than the result of Awot et al,28 who reported 9 (9.4%) Escherichia coli isolates were isolated from 96 fish samples in fish meat retailing shops of Mekelle City, Ethiopia. But the result of the current study was lower than the result of Aynadis and Aweke29 who reported that 80 (23.3%) Escherichia coli isolates were isolated from 343 fish samples in Lake Hawassa, Southern Ethiopia.
The occurrence of Escherichia coli in our study might be due to animal dung contamination of the water. The presence of Escherichia coli in aquaculture can be attributed to animal waste pollution of the water bodies.30 The contamination of food and the environment with a bacteriological condition like Escherichia coli originates from human and animal feces.31
Isolation of Escherichia coli was done by taking fresh unfilleted, filleted and cooked (locally called asa tibs, asa dulet and asa wot) fish samples. Isolation of Escherichia coli from raw fish samples and cooked samples did not have a statistically significant difference (p-value 0.10). A total of 27 (23.5%) and 9 (13.8%) Escherichia coli isolates were isolated from the raw and cooked fish samples, respectively. This result was lower than the previous reports of Kumar et al,32 who determined the prevalence of Escherichia coli in tropical seafood and documented a prevalence of 47% for fecal coli forms, including Escherichia coli.
In this study, the occurrence of Escherichia coli was higher in raw fish than cooked fish samples. This is due to the exposure to heat during processing for the cooked fish samples. This work agrees with the work of Gupta et al,22 who found 47 (48.95%) Escherichia coli instances in 96 raw fish samples and 7 (12.96%) Escherichia coli instances in 88 ready-to-eat fish product samples. Regarding the frequency of bacteria isolate from heat-treated fish and other ready-to-eat food stuffs, similar observations have been reported by other researchers.33,34
The findings of this study indicated that 23.5% of raw fish samples carried Escherichia coli isolates. This result was higher than the result of Vieira et al,35 who reported that 12.5% of all samples from Brazilian markets had Escherichia coli, and lower than the result of Wendwesen et al,36 who reported that 42.5% of raw frozen Nile tilapia fish samples had Escherichia coli in Arba Minch town, SNNPR, Ethiopia.
Moreover, this study indicated that 13.8% of cooked (locally called fried fish, asa dulet, asa lebleb, etc.) fish samples had Escherichia coli. Okonko et al37 suggests that improper handling and improper hygiene might lead to the contamination of ready-to-eat foods and this might eventually affect the health of the consumers. The result of this study was higher than the result of Wendwesen et al,36 who reported that 7.5% of Nile tilapia fish (locally called asa lebleb) samples had Escherichia coli from ready-to-eat fish foods in Arba Minch town, SNNPR, Ethiopia and lower than the result of Ohalete et al,38 who reported that 58.3% of fried fish had Escherichia coli in Owerri, Nigeria.
The samples were taken from different fish species, namely, Nile tilapia, African catfish and Labeo Barbus. The highest Escherichia coli isolates were found in Nile tilapia. This agreed with the previous reports of Hanson et al,39 who reported higher infection with E. coli in Plankton feeders (Nile tilapia species) than for Catfish and disagreed with the reports of Aynadis and Aweke,29 who reported that there was no difference in the occurrence of Escherichia coli in three species of fish. This potential disagreement might arise from the difference in the sample size used, the ecosystem of the study area or the sampling methods.
In this study, the aerobic plate count (APC) varied from 3.2×104 cfu/g to 1×107 cfu/g in raw or uncooked fish samples, with a mean value of 6.13×105 cfu/g, and that of ready-to-eat fish meals ranged from 9×102 cfu/g to 6.4×104 cfu/g, with a mean value of 2.81×104 cfu/g. This result indicates that a low mean value of bacterial load was found in ready-to-eat or cooked fish samples. This may be due to the negative effect of heat on the bacteria during fish origin food preparation. This was in agreement with the result of Wendwesen et al,36 who reported 4.63×106 cfu/g Escherichia coli count in frozen raw Nile tilapia fish samples and a 4.92×103 cfu/g Escherichia coli load in asa lebleb (local name) fish origin foods in Arba Minch town, SNNPR, Ethiopia. The Escherichia coli load in raw fish sample was higher than the result of Dhanapal et al,40 who found 4.9×104 cfu/g and lower than the result of Wendwesen et al,36 who reported 4.63×106 cfu/g in frozen raw Nile tilapia fillet samples. This high load in our study might be due to the result of poor handling during the transportation, and/or poor personal hygiene during harvesting and filleting.
The Center for Food Safety organization has set minimum standards for the recovery of microorganisms from foods of various origins. When compared with that standard, the recovery rate in the current study result was higher and this could be due to the absence of hygienic practices and strict follow-up of this sector by the concerned authorities. According to the CFS (Center for Food Safety)15 guidelines, <20 cfu/g is satisfactory, 20–102 intermediate or borderline and >102 unacceptable. None of the fish samples screened in the present study were at the satisfactory level.
Food handlers may be the source of food contamination, either as carriers of pathogen or through poor hygienic practices. Thus, all food handlers have a basic responsibility to maintain a high degree of personal cleanliness and implement hygienic and safe food handling practices. Among the precautions that a food handler must maintain, the major ones are: keeping hands clean, wearing a clean working garment and covering hair.41 However, the result of this study showed that 30% of the fish handlers did not wear an appropriate overcoat. This finding is higher than the result of42 done on food handlers in Hawassa (14%). Moreover, only 23.3% of fish handlers were found with covered hair, which was lower than the finding of Kumie et al43 done in Zeway (40.1%), but better than the result of Teklemariam et al42 assessed in Hawassa (11.8%). It was also observed that 36.7% of fish handlers in this study wore rings on their fingers during food preparations, which was relatively higher than the report of Kumie et al43 done in Zeway (28.7%).
Since food handlers can be the probable sources of contamination for microorganisms, it is important to take all possible measures so that such contaminations can be reduced or eliminated.44 Training of food handlers regarding the basic concepts and requirements of personal hygiene and sanitary handling of food play an integral part in ensuring a safe product to the consumer. However, the result of this study showed that 66.7% of the fish handlers had no taken training concerning sanitary handling of food. This result was inconsistent with the previous result of Mekonnen et al,9 who found that 61.5% of meat handlers do not take training on sanitary handling of food and food hygiene.
The hygienic status of where the fish are found and the manipulation of the fish during processing of fish play significant roles in the hygiene of fish samples.
The current study showed that all 36 Escherichia coli isolates were sensitive to ciprofloxacin and gentamycin. This result was similar to the results of Awot et al28 in fish meat retailing shops of Mekelle City, Ethiopia. For tetracycline, 55.6%, 8.3% and 36.1% of the isolates were resistant, intermediate and susceptible, respectively. Much research indicates that E. coli is not responding to tetracycline treatment,45,46 which corresponds to the results of the current study. However, Mohammed et al47 showed that Escherichia coli was susceptible to tetracycline. Of the isolates, 88.9% were resistant, 2.8% were intermediate and 8.3% were susceptible to streptomycin, and this result was different from Aynadis and Aweke,29 who reported that, of the fish from Lake Hawassa, Southern Ethiopia, 37.5% were resistant, 12.5% were intermediate and 50% were susceptible to streptomycin.
Development of drug resistance/tolerance by Escherichia coli can be achieved via mutation. For example, adaptation to fluoroquinolone has often been due to acquisition of mobile genetic elements. There was evidence supporting the sharing of resistant bacteria among livestock, aquatic animals and humans via food production, which poses a critical threat to public health.48 The World Organization for Animal Health (OIE) suggested that aquatic animal health should rely on constant monitoring and disease surveillance of anti-microbial resistant microbes.49 Generally, fish contamination with Escherichia coli probably results from the environment at harvesting and production process of fish.
Conclusions and Recommendations
Escherichia coli isolates were identified in one-fifth of the fish samples taken from raw and cooked fish samples. This indicates that Escherichia coli is a contaminant of fish in the study area and its occurrence in fish could represent a risk to consumers. Thus, proper attention should be paid to the safety of both raw and cooked fish through proper handling and use of adequate processing procedures. The Escherichia coli count was high in the fish sample and above the recommended level of Center for Food Safety standards. Most fish food handlers and processors had not taken training on the hygienic handling of fish. The filleting ground, fish harvesting materials and fish landing sites in Lake Tana should be kept clean. In this study, all isolates of Escherichia coli were susceptible to ciprofloxacin and gentamycin. However, more than half of Escherichia coli isolates were resistant to tetracycline and erythromycin. In general, the results obtained from this study provide evidence of the unsatisfactory microbiological quality and safety of fish from the local artisanal fish value chain. Further investigations should be conducted to investigate different pathogenic strains of Escherichia coli, such as Escherichia coli O157 H7.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Bahir Dar University (Ref. No: 1/2834/1.3.4 and Date: 22/3/2019).
Data Sharing Statement
The data sets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Informed Consent Statement
Informed verbal consent was obtained from each study participant.
Acknowledgments
We would like to express our sincere gratitude and heartfelt thanks to the Ethiopian National Fishery and Aquatic Life Research Center and Bahir Dar University for their financial, and also laboratory, support, Amhara Regional Veterinary Laboratory and Amhara Public Health Institute workers for their basic support in ideal, technical and for giving of materials, chemicals and reagents.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
This work was supported by Ethiopian Institute of Agricultural Research; National Fishery and Aquatic Life Research Centre; ref. No. 17.2/0409/2019.
Disclosure
This paper was presented as a thesis to Bahir Dar University. The abstract thesis was published in the Bahir Dar University, DSpace Repository (http://dspace.org).The authors declare no conflicts of interest in relation to this work.
References
1. FAO (Food and Agriculture organization); 2009. Available from: https://www.who.int/news-room/fact-sheets/detail/musculoskeletal-conditions.
2. Trana N, Chub L, Yee CC, Genschickc S, Phillipsa MJ, Alexander SK. Fish supply and demand for food security in Sub-Saharan Africa: an analysis of the Zambian fish sector. Mar Policy. 2019;99:343–350. doi:10.1016/j.marpol.2018.11.009
3. Christophe B, Damien G. Baseline report Ethiopia. In: Smart Fish Programme of the Indian Ocean Commission. Ebene, Mauritius: Fisheries Management FAO Component; 2014:24.
4. Rimm EB, Appel LJ, Chiuve SE, et al. A seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American Heart Association. Circulation. 2018;138:e35–e47. doi:10.1161/cir.0000000000000574
5. Zenebe T, Ahlgren G, Boberg M, Gustafsson I-B. Fatty acid and lipid content of Oreochromis niloticus L. in Ethiopian lakes-dietary effects of phytoplankton. Ecol Fresh Fish. 2006;7:146–158. doi:10.1111/j.1600-0633.1998.tb00181.x
6. World Health Organization. Food Safety Issues Associated with Products from Aquaculture. T.R.S. No. 883. Geneva, Switzerland: World Health Organization; 2007.
7. Goja A, Ahmed T, Saeed S, Dirar H. Isolation and identification of Staphylococcus spp. in fresh beef. Pak J Nutr. 2013;12:114–120. doi:10.3923/pjn.2013.114.120
8. Ghaly AE, Dave D, Budge S, Brooks MS. Fish spoilage mechanisms and preservation techniques: review. Am J Appl Sci. 2010;7:859–877. doi:10.3844/ajassp.2010.859.877
9. Haileselassie M, Taddele H, Adhana K, Kalayou S. Food safety knowledge and practices of abattoir and butchery shops and the microbial profile of meat in Mekelle City, Ethiopia. Asian Pac J Trop Biomed. 2013;3:407–412.
10. Fratamico PM, Bhunia AK, Smith JL. Foodborne Pathogens: Microbiology and Molecular Biology. Norwich, England: Caister Academic Press; 2005:454.
11. Dabassa A, Bacha K. The Prevalence and antibiogram of Salmonella and Shigella isolated from abattoir, Jimma Town, South western Ethiopia.
12. Soliman M, Khalil R, Saad T, El-Gamal M, Gebril A. Isolation and identification of E. coli from cultured freshwater fish. J Arab Aqua Sci. 2010;5:8.
13. Kaper JB, Nataro JP, Mobley HLT. Pathogenic Escherichia coli. Nat Rev. 2004;2:123–140.
14. Ishii S, Sadowsky MJ. Escherichia coli in the environmental: implications for water quality and human health. Microbes Environ. 2008;23:101–108. doi:10.1264/jsme2.23.101
15. CFS (Centre for Food Safety). Microbiological guidelines for food (for ready-to-eat food in general and specific food items). In: Food and Environmental Hygiene Department. Hong Kong: 43/F Queens Way Government Offices; 2014:10.
16. Rasheed MU, Thajuddin N, Ahamed P, Teklemariam Z, Jamil K. Antimicrobial drug resistance in strains of Escherichia coli isolated from food sources. Revista Instituto Medicina Tropical São Paulo. 2014;56:341–346. doi:10.1590/s0036-46652014000400012
17. Bell JM, Turnidge JD, Gales A, Pfaller MA, Jones RN. Prevalence of extended spectrum β-lactamase (ESBL)-producing clinical isolates in the Asia-Pacific region and South Africa: regional results from SENTRY Antimicrobial Surveillance Program (1998–99). Diagn Microbiol Infect Dis. 2002;42:193–198. doi:10.1016/s0732-8893(01)00353-4
18. Oluwasinaayomi F, Muluneh W, Truphena E, Bolanle W. Land use and ambient air quality in Bahir Dar and Hawassa, Ethiopia. Air Soil Water Res. 2018;11:1–10. doi:10.1177/1178622117752138
19. Janko AM, Production F. Consumption and management in Ethiopia. Int J Econ Manag Sci. 2014;3:460–466. doi:10.4172/2162-6359.1000183
20. Thrusfield M. Veterinary Epidemiology. Oxford, UK: Blackwell Science Ltd; 2005:233–250.
21. World Health Organization. Food Safety. Geneva, Switzerland: World Health Organization; 2015:1–15.
22. Gupta B, Ghatak S, Gill J. Incidence and virulence properties of E coli isolated from fresh fish and ready-to-eat fish products. Veter World. 2013;6:5–9. doi:10.5455/vetworld.2013.5-9
23. Alexander T, Inglis G, Yanke L, et al. Farm-to-fork characterization of Escherichia coli associated with feedlot cattle with a known history of antimicrobial use. Int J Food Microbiol. 2010;137:40–48. doi:10.1016/j.ijfoodmicro.2009.11.008
24. Fawole MO, Oso BA. Laboratory Manual of Microbiology, Revised Edition. Ibadan, Nigeria: Spectrum Books Ltd; 2001:127.
25. Oyeleke SB, Manga SB. Essentials of Laboratory Practicals in Microbiology. Minna, Nigeria: To Best publisher; 2008:36–75.
26. CLSI. Performance Standards for Antimicrobial Susceptibility Testing.
27. Thampuran N, Surendraraj A, Surendran PK. Prevalence and characterization of typical and atypical Escherichia coli from fish sold at retail in cochin. India J Food Prot. 2005;68:2208–2211. doi:10.4315/0362-028x-68.10.2208
28. Awot T, Tehetna A, Shishay A, et al. Isolation and antimicrobial sensitivity testing of E. coli from fish meat retailing shops of Mekelle City, Ethiopia. Momona Ethiop J Sci. 2019;11:229–238. doi:10.4314/mejs.v11i2.4
29. Tilahun A, Engdawork A. Isolation, identification and antimicrobial susceptibility profile of E.coli (O157: H7)from fish in Lake Hawassa, Southern Ethiopia. Int J Vet Sci Technol. 2019;3:13–19.
30. Abdelhamid A, Gawish M, Sorya K. Comparative study between desert cultivated and natural fisheries of mullet fish in Egypt. Aquac Environ Interact. 2007;31:5681–5687.
31. Carson C, Shear B, Eller Sieck M, Asfaw A. Identification of fecal E. coli from humans and animals by Ribo typing. Appl Environ Microbiol. 2001;67:1503–1507. doi:10.1128/AEM.67.4.1503-1507.2001
32. Kumar HS, Parvathi A, Karunasagar I. Prevalence and antibiotic resistance of Escherichia coli in tropical seafood. World J Microbiol Biotechnol. 2005;21:619–623. doi:10.1007/s11274-004-3555-8
33. Thailambal AS. Effect of processing on bacterial population of cuttle fish and crab and determination of bacterial spoilage and rancidity developing on frozen storage. J Food Process Preserv. 2006;31:13–31.
34. Omenwa VC, Ansa EJ, Agokei OE, Uka A, George OS. Microbiological quality of raw and processed farm reared periwinkles from brackish water earthen pond Buguma, Nigeria. Afr J Food Agric Nutr Dev. 2011;11:4623–4631. doi:10.4314/ajfand.v11i2.65917
35. Vieira RH, Rodrigues DDP, Gonçalves FA, de Menezes FGR, Aragão JS, Sousa OV. Microbicidal effect of medicinal plant extracts (Psidium guajava Linn. and Carica papaya Linn.) upon bacteria isolated from fish muscle and known to induce diarrhea in children. Revista Instituto Medicina Tropical São Paulo. 2001;43:145–148. doi:10.1590/s0036-46652001000300005
36. Wendwesen T, Dagmar N, Yitbarek G, et al. Microbiological quality of frozen raw and undercooked Nile tilapia (Oreochromis niloticus) fillets and food safety practices of fish handlers in Arba Minch town, SNNPR, Ethiopia. J Veter Med Anim Health. 2017;9:55–62. doi:10.5897/JVMAH2015.0424
37. Okonko IO, Donbraye E, Babatunde SOI. Microbiological Quality of Seafood processors and water used in two different sea processing plants in Nigeria. Electron J Environ Agric Food Chem. 2009;8:621–629.
38. Ohalete CN, Obiajuru IOC, Obiukwu CE, Uwaezuoke JC, Nwaehiri UL, Daniel UN. Microbiological quality of fried and smoked fish in Owerri, Imo State Nigeria. WJPPS. 2013;2:1–19.
39. Austin B, Austin DA. Bacterial Fish Pathogens: Disease of Farmed and Wild Fish.
40. Dhanapal KG, Vidya SL, Binay BN, Venkateswarlu G, Devivaraprasad AR, Basu S. Effect of cooking on physical, biochemical, bacteriological characteristics and fatty acid profile of Tilapia (Oreochromis mossambicus) fish steaks. Arch Appl Sci Res. 2012;4:1142–1149.
41. Käferstein FK. Food safety: the fourth pillar in the strategy to prevent infant diarrhoea. Bull World Heal Organ. 2004;81:842–843.
42. Teklemariam S, Roma B, Sorsa S, Worku S, Erosie L. Assessment of sanitary and hygiene status of catering establishments of Hawassa Town. Ethiop J Health Dev. 2000;14:91–98.
43. Kumie A, Genete K, Worku H, Kebede E, Ayele F, Mulugeta H. The sanitary conditions of public food and drink establishments in the district town of Zeway, Southern Ethiopia. Ethiop J Heal Dev. 2002;16:95–103. doi:10.4314/ejhd.v16i1.9831
44. Muinde OK, Kuria E. Hygienic and sanitary practices of vendors of street foods in Nairobi, Kenya. Afr J Food Agric Nutr Dev. 2005;5:1–14. doi:10.18697/ajfand.8.1060
45. Hiko A, Tasisa A, Aga GE. Helminthiasis and gram negative enteric bacteria in fresh water fish from selected lakes of Haramaya district, Ethiopia. Fish Aqua J. 2018;9:242.
46. Mude S, Thomas N, Kemal J, Muktar Y. Cloacael carriage and multidrug resistance Escherichia coli O157: H7 from poultry farms, Eastern Ethiopia. J Vet Med. 2017;1–9. doi:10.1155/2017/8264583
47. Mohammed O, Shimelis D, Admasu P, Feyera T. Prevalence and antimicrobial susceptibility pattern of E. coli isolates from raw meat samples obtained from abattoirs in Dire Dawa city, eastern Ethiopia. Inter J Microbiol. 2014;5:35–39.
48. Hafsat AG, Yaqub AG, Galadima BG, James A, Abubakar S. Methicillin-resistant staphylococcus aureus: a review. Adv Anim Vet Sci. 2015;3:79–98.
49. Smith V, Alday S, Matysczak J, Moulin G, Lavilla CR, Prater D. Monitoring and surveillance of antimicrobial resistance in microorganisms associated with aquatic animals. Rev Sci Tech. 2013;32:583–593.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.