Back to Journals » International Medical Case Reports Journal » Volume 12
Lesser Sac Glomangiosarcoma With Simultaneous Liver And Lymph Nodes Metastases Mimicking Small Bowel Gastrointestinal Stromal Tumor; Immunohistochemistry And Empirical Chemotherapy
Authors Negahi A , Jahanshahi F , Shahriari-Ahmadi A, Sadeghipour A
Received 22 June 2019
Accepted for publication 22 October 2019
Published 12 November 2019 Volume 2019:12 Pages 339—344
DOI https://doi.org/10.2147/IMCRJ.S220455
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ronald Prineas
Alireza Negahi,1 Fatemeh Jahanshahi,2 Ali Shahriari-Ahmadi,3 Alireza Sadeghipour4
1Department of General Surgery, Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran; 2Student Research Committee, Faculty of Medicine, Iran University of Medical Science, Tehran, Iran; 3Department of Oncology, Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran; 4Department of Pathology, Rasoul Akram Hospital, Iran University of Medical Sciences, Tehran, Iran
Correspondence: Fatemeh Jahanshahi
Rasoul Akram Hospital, Niayesh Avenue, Sattarkhan Street, Tehran, Iran
Tel +98 9124801231
Fax +98 21 64352353
Email [email protected]
Abstract: Glomangiosarcoma is a rare malignant mesenchymal tumor. Despite malignant histopathological feature of glomangiosarcoma, metastasis was observed extremely rare in these tumors. Moreover, malignant glomus tumor with stomach origination and simultaneous metastasis to liver and lymph nodes were not reported so far. This report presented a 57-year-old male patient with an exophytic gastric glomangiosarcoma in lesser sac and simultaneous liver and lymph node metastasis.
Keywords: glomangiosarcoma, malignant, glomus tumor
Introduction
Glomus tumors compose approximately 2% of soft tissue tumors and often their manifestation is benign and solitary. They arise from glomus bodies which are modified smooth muscle cells and exist arteriovenous anastomosis without the involvement of capillaries and participate in thermoregulation. Therefore, their common location is where glomus bodies are abundant such as subungual of finger and wrist in upper extremities and less frequently in toes. Another infrequent place is visceral organs such as mediastinum, stomach, lungs, kidney, and ileum. As it is mentioned above glomus tumors are usually benign but malignancy incidence in these tumors either represents clinically metastasis malignancy or simply histopathologically malignant. No specific pattern is recognized for metastasis of malignant glomus tumor and metastasis via both the lymphatic system and blood is reported in the literature.1–3 The present report is the first report of an exophytic gastric glomangiosarcoma with simultaneous liver and lymph nodes metastases that can help to recognize different features of this type of tumor.
Case Report
A fifty-seven-year-old male patient was presented to the general surgery clinic with a complaint of pain at the right lower quadrant of the abdomen, stating the pain was unendurably increasing since 1 month ago.
The general condition of the patient was good and he was not cachectic and anemic.
In the first examination, there was no abdominal distention. Tenderness was observed only at the right lower quadrant of the abdomen without rebound tenderness. In deep palpation, a firm mass was felt at the tender region of abdomen.
Based on the examination, abdominopelvic ultrasonography was requested for further investigations.
The report demonstrated a mass adjacent to the transverse colon and several heterogeneous hypoechoic lesions in the liver. Therefore, a hypothesis was formed suggesting transverse colon as the origin of the mass.
For acquiring precise information about the origin of the mass and liver lesions, the abdominopelvic spiral computed tomography scan with and without intravenous contrast was suggested. The radiological report revealed a perijejunal mass sized 141*85 mm consist of a necrotic and calcific center that proposed a suspicious diagnosis of the gastrointestinal stromal tumor (GIST) and adenocarcinoma (Figure 1A and B).
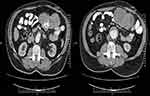 |
Figure 1 (A, B) Computed tomography scan depicted perijejunal mass sized 141*85 mm with central necrosis and calcification. |
Additionally, several scattered metastatic lesions were reported in liver with the largest lesion sized 38 mm in the liver segment 6 (Figure 2A and B).
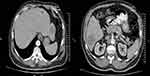 |
Figure 2 (A, B) Scattered metastatic hypodense lesions in liver. |
The patient’s liver function test reported AST=19, ALT=23, ALKP=178, direct bilirubin=0/8, total bilirubin=0/2, and albumin=3/8 that varied in the normal range.
Despite the normal result of the respiratory examination, metastatic workup for lung was performed by requesting a chest spiral computed tomography scan without injection. However, the report depicted no metastatic lesions in the lungs.
Due to indecisive results obtained from previous investigations, it was necessary to conduct an ultrasound-guided needle biopsy of liver lesions. The biopsy report surprisingly demonstrated a fatty liver. Moreover, it was reported that definite discrimination between reactive and neoplastic infiltrative cells was impossible. Furthermore, there is a possibility of admitting a sample from an inappropriate region and therefore it was not a true representative of the lesions tissue. With that being said, further investigation was suggested in the report.
Accordingly, diagnostic laparoscopy and repeating appropriate biopsy were conducted.
Contrary to the radiologic findings, in laparoscopic exploration, it was observed that the location of mass was at lesser sac. As a result, the lesser sac was cut open and due to the mass mobility and no attachment to the adjacent tissue, an upper midline incision was performed and the 20*30 cm mass was excised completely from transverse mesocolon while retaining midcolic vein and artery. It was observed that the mass was originated from greater curvature approximately 18 cm from incisures as well, and it was separated with a stapler.
Despite the suspicious diagnosis of GIST with metastasis, the surgeon’s discretion was that, according to several metastatic lesions in the liver, excision of the mass could improve the prognosis and reduce the progressive process of metastasis. Therefore, the excised mass and liver biopsy samples were sent to pathology.
The created defect in transverse mesocolon was reformed, and after correction of hemostasis, the incision was closed.
Grossly, the huge abdominal mass consisted of a solid mass sized 16*11*4 cm. The external surface was grayish and bosselated.
Cut sections were heterogeneous and had yellowish-brown discoloration. Moreover, areas of calcification were noted.
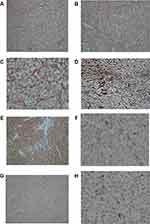
Histopathologically, the abdominal mass composed of cellular tissue formed by branching vascular channels separated by stroma containing, nests and sheets of polyhedral to spindle cells with uniform round to oval nuclei, focal pleomorphism, some distinct nucleoli and pale to eosinophilic cytoplasm which in some foci entrapped in a fibrous stroma showing marked nuclear atypia (Figure 3A–C). Reticulin staining showed positive intratumoral reticulin deposition with a cobwebby appearance (Figure 3D).
By immunohistochemistry, the tumoral cells were positive for vimentin, scattered positive for Sm-action and S100 with negative reactivity for Pan-CK, EMA, CD117, CD56, CD34, Desmin, Dog1, HMB 45, chromogranin, synaptophysin, and CD10. Ki67 were positive in up to 10% of tumoral cells (Figure 3E–H).
Additionally, liver samples showed the infiltration of sheets and nests of the tumoral cells with the same histology.
Histomorphology and immunohistochemical studies were more compatible with malignant glomus tumor (glomangiosarcoma).
The post-operative course was uneventful and the patient was in good general condition with no complaint and was discharged from general surgery service and referred to the oncology clinic for continuing further treatment.
Therefore, due to the good general condition of the patient and chemotherapy tolerating, the patient underwent a six-course treatment with three-week intervals using 60 mg/m2 of liposomal doxorubicin, 5 mg/kg of bevacizumab and 175 mg/m2 of paclitaxel, based on the vascular nature of the tumor and the decision of tumor board. Afterward, the maintenance treatment was 5 mg/kg of bevacizumab for every 3 weeks and propranolol 20 mg three times a day.
During a 4-year follow-up period, the patient’s general condition was good and there was no evidence of mass recurrence. The progression of liver and lung metastases was halted.
The last abdominopelvic spiral computed tomography scan depicted the involvement of pancreaticoduodenal and hepatoduodenal lymph nodes with a short-axis diameter of 17 mm and paraaortic and aortocaval lymph nodes with a short-axis diameter of 10 mm (Figure 4A and B).
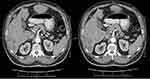 |
Figure 4 Enhanced abdominopelvic computed tomography demonstrated lymph nodes involvement (A) Pancreaticoduodenal lymph node metastasis; (B) Paraaortocaval lymph node metastasis. |
However, the patient was in good general condition and received periodic maintenance chemotherapy regimen and follow-up with serial computed tomography every 3 months.
Discussion
Glomus tumors are often benign neoplasm that originates from glomus bodies that participate in thermoregulation. Thus, they are mostly found in areas such as dermis, subdermal of distal extremities in which glomus bodies are abundant. They are rarely observed in deep locations such as the stomach, mediastinum, trachea, lungs, and kidneys. Glomus tumors are normally benign and solitary, however, in 1% of cases, they could be malignant, and they represent pathologic characteristics and very rarely they appear with clinical metastasis. Clinical metastasis or malignancy increases its fatality and leaves a poor survival rate of patients.
Malignant glomus tumors are categorized into two groups. First, tumors that are malignant from the very beginning (de novo) and second, tumors that are created through malignancy transformation in benign tumors.1 In 2001, Folpe et al defined three criteria for the identification of malignant and benign glomus tumors. These criteria consisted of 1) tumor sizes larger than 2 cm, 2) visceral location or deep location, and 3) mitotic activity of 5 per 50 high power fields or atypical mitotic figures.4 Among visceral organs, the stomach is the most prevalent location for glomus tumors; however, malignancy is very rare in this area with only seven metastatic clinical malignant glomus tumors being reported in the literature. Manifestations of gastric glomus tumors could be bleeding in the form of hematemesis or melena, dyspepsia, and epigastric discomfort.3
Although glomus tumors are basically benign tumors with a good prognosis, low recurrence, low local invasion and rarely with metastasis, they could be potentially very malignant and they can lead to the death of the patient after causing widespread metastasis.1 In the gastrointestinal tract, the stomach is the prevalent place for glomus tumors; however, there are only seven cases of malignant glomus tumors with metastasis in the stomach reported in the literature (Table 1).1,4–9 The first case was reported in 2001 by Folpe et al in which the patient was a sixty-nine-year-old man with a 9.5-cm mass and liver metastasis.4 In 2002, Miettinen et al reported another patient who was also 69 years old with a 6.5-cm mass and liver metastasis.5 In 2009, two cases were reported by Lee et al.6 One of them was a female patient, aged 65 years old, with a 3-cm mass and widespread metastasis in humerus, brain, and kidney who was the first case of malignant glomus tumor with multiorgan metastasis. The second case was a 9-cm mass in a 63 years old male patient with liver metastasis.6 In the same year, Bray et al reported another case as a 58 years old man who had a 17-cm mass with multiple metastases in the scalp, brain, lung, and liver.7 A 3-cm mass in a sixty-five-year-old woman with metastasis to the brain and kidney was another case published by Song et al in 2010.8 The next published case was introduced in 2018 by Bodolan et al. In this case, the patient was an eighty-year-old woman with a mass sized 7.1 cm and liver metastasis.1 And the last published case was a seventy-two-year-old male with a mass sized 6 cm that was introduced by Toti et al’s in 2019.9 Among the aforementioned patients, only the two last cases were alive currently, and Bray et al’s patient had the most survival rate which was 72 months.7 Often in these kinds of cases, due to the poor general condition of patient, chemotherapy is not possible, and regarding the 2018 case in which chemotherapy plan was mentioned, there were no further details.1 Besides, cases in which radiotherapy was conducted, there were no satisfactory results. However, there is another case introduced by Milia et al as a forty-year-old patient with a mass in the upper cervical region. In this case, promising outcome was reported as the result of a combination of chemotherapy with cisplatin and radiotherapy.10
 |
Table 1 Review Of Published Case Reports Of Gastric Malignant Glomus Tumors With Metastases |
In the present case, due to the proper condition of the patient, empirical chemotherapy with drugs mentioned above was possible. In the four-year follow-up, there was no sign of recurrence. In addition, the speed of progression was reduced and the general condition of the patient was good.
Although the pattern of glomus tumor metastasis is not determined due to the rarity of the tumor, in malignant glomus tumors in the visceral organs, metastasis through both lymph and blood, either individually or simultaneously, were observed so far. However, regarding the gastric cases, lymph node metastasis is not reported in the literatures and the present case is the first one as simultaneous lymph nodes involvement and liver metastasis.
Moreover, other reported cases are in the stomach and often in its prevalent location, the antrum, which makes them usually simple to identify and biopsy using endoscopy. While still originated from stomach, the uncommon location of the tumor in this case, which is in lesser sac, made the possibility of identification limited. It is safe to say that this is the first case of the uncommon place of this tumor. It is believed that since this case is very rare and therefore the behavior and features of the masses are unidentified, reporting it could be beneficial in differentiating it from other differential diagnoses such as gastrointestinal stromal tumor, leiomyoma, carcinoid tumor, and paraganglioma, and also a proper guide for the excision of tumors with or without metastasis. Obviously, in most cases, it was believed that it could hugely affect the survival rate.
To the best of our knowledge, the elected treatment in solitary histopathologically malignant glomus tumor is a wide excision of the mass, while in clinically malignant glomus tumor (with metastasis) chemotherapy and radiotherapy were tried, as well. However, significant effect of the treatments in the latter case on glomangiosarcoma progressive process remained to be determined due to the rare occurrence of malignancy in the glomus tumors and extremely rare occurrence of the metastasis and consequently poor possibility of establishing an appropriate treatment plan.
According to the high mortality rate of patients with multiple metastatic glomangiosarcoma and the fact that most of the reported cases in the literature led to death, this patient’s 4 years of survival is acceptable and it is believed that the result of the excision of the mass and empirical chemotherapy was effective.
Conclusion
In most cases, malignant glomus tumor with multiple metastases has been fatal and led to the death of patient. On the other hand, since the limited numbers of survived patients were in poor general condition, there is no definitive chemotherapy approach for these patients. As a result of reporting these cases, the features and behavior of these tumors could be identified and investigated and therefore differentiated from other stomach tumors so that choosing the proper treatment will become easier. Regarding the present case, although the tumor was in stage IV, it is perceived that debulking the tumor and empirical chemotherapy positively affected the patient’s survival.
Ethics Approval
Institutional review board approval for the case report is not required at our institution. To keeping ethical principles, names of the patients were not pointed in the paper and the rights of the subject were protected.
Consent For Publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Acknowledgments
The authors thank the Rasoul Akram Hospital Clinical Research Development Center (RCRDC) for its technical and editorial assists.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bodolan AA, Wilcox R, Yang MX. Malignant glomus tumor of the gastric antrum with hepatic metastases: a case report and literature review. Hum Pathol Case Rep. 2018;14(Cancer 50 8 1982):81–84. doi:10.1016/j.ehpc.2018.09.004
2. Wang S, Ding C, Tu J. Malignant glomus tumor of the lung with multiple metastasis: a rare case report. World J Surg Oncol. 2015;13:22. doi:10.1186/s12957-014-0423-3
3. Masouminia M, Ghani HA, Foote D, Hari D, French S. Rare presentation of the glomus tumor in the stomach. Exp Mol Pathol. 2018;104(1):9–11. doi:10.1016/j.yexmp.2017.11.016
4. Folpe AL. Glomus tumors. In: Fletcher CDM, Unni KK, Mertens F, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002:136–137.
5. Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinalglomus tumors: a clinicopathologic, immunohistochemical,and molecular genetics study of 32 cases. Am J Surg Pathol. 2002;26(3):301–311. doi:10.1097/00000478-200203000-00003
6. Lee H, Choi YS, Oh SC, et al. Malignant glomus tumors of the stomach - a report of 2 cases with multiple metastases. Korean J Pathol. 2009;43(4):358–563. doi:10.4132/KoreanJPathol.2009.43.4.358
7. Bray AP, Wong NA, Narayan S. Cutaneous metastasis from gastric glomus tumour. Clin Exp Dermatol. 2009;34(8):e719–721. doi:10.1111/j.1365-2230.2009.03445.x
8. Song SE, Lee CH, Kim KA, Lee HJ, Park CM. Malignant glomus tumor of the stomach with multiorgan metastases: report of a case. Surg Today. 2010;40(7):662–667. doi:10.1007/s00595-008-4113-z
9. Toti L, Manzia TM, Roma S, et al. Rare malignant glomus tumor of the stomach with liver metastases. Radiol Case Rep. 2019;14(4):463–467. doi:10.1016/j.radcr.2019.01.012
10. Milia ME, Turri L, Beldi D, Deantonio L, Pareschi R, Krengli M. Multidisciplinary approach in the treatment of malignant paraganglioma of the glomusvagale: a case report. Tumori. 2011;97(2):225–228. doi:10.1700/667.7788
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.