Back to Journals » Vascular Health and Risk Management » Volume 16
Lesion Type Analysis of Hemodialysis Patients Who Underwent Endovascular Management for Symptomatic Central Venous Disease
Authors Aljarrah Q , Allouh M , Hallak AH , Alghezawi SE , Al-Omari M , Elheis M , Al-Jarrah M , Bakkar S , Aleshawi AJ, Al-Jarrah H, Ibrahim KS , Al Shishani JM, Almukhtar A
Received 28 July 2020
Accepted for publication 23 September 2020
Published 9 October 2020 Volume 2020:16 Pages 419—427
DOI https://doi.org/10.2147/VHRM.S273450
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Qusai Aljarrah,1 Mohammed Allouh,2 Amer H Hallak,3 Shamikh E Alghezawi,3 Mamoon Al-Omari,4 Mwaffaq Elheis,4 Mooath Al-Jarrah,4 Sohail Bakkar,5 Abdelwahab J Aleshawi,6 Hussam Al-Jarrah,1 Khalid S Ibrahim,7 Jan Mohammed Al Shishani,8 Aws Almukhtar9
1Department of General & Vascular Surgery, Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan; 2Department of Anatomy, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain 17666, United Arab Emirates; 3Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan; 4Department of Diagnostic and Interventional Radiology, Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan; 5Department of Surgery, Faculty of Medicine, The Hashemite University, Zarqa 13133, Jordan; 6Department of Ophthalmology, Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan; 7Department of General & Cardiovascular Surgery, Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan; 8Department of Vascular Surgery, King Hussein Medical Center, Amman 11733, Jordan; 9Department of Surgery and Cancer, Imperial College London, London SW7 2BU, UK
Correspondence: Qusai Aljarrah
Department of General & Vascular Surgery, Faculty of Medicine, Jordan University of Science and Technology, Irbid 22110, Jordan
Tel +962 775593131
Email [email protected]
Mohammed Allouh
Department of Anatomy, College of Medicine and Health Sciences, United Arab Emirates University, Al Ain, United Arab Emirates
Tel +971 37 137 551
Email [email protected]
Purpose: Central venous lesions (CVLs) can adversely affect hemodialysis access maturation and maintenance, which in turn worsen patient morbidity and access circuit patency. In this study, we assessed several clinical variables, patient characteristics, and clinical consequences of symptomatic central vein stenosis and obstruction in patients who underwent renal replacement therapy in the form of hemodialysis.
Patients and Methods: The medical records of all hemodialysis patients with clinically symptomatic CVLs who underwent digital subtraction angiography treatment at King Abdullah University Hospital between January 2017 and December 2019 were retrieved. Patient characteristics and the clinical and anatomical features of CVLs were analyzed retrospectively. Pearson’s chi-square tests of association were used to identify and assess relationships between patient characteristics and CVLs.
Results: The study cohort comprised 66 patients with end-stage renal disease who developed symptomatic central vein stenosis. Of the 66 patients, 56.1% were men, and their mean age was approximately 52 years. Most (62.1%) of the patients were determined to have a history of central catheter insertion into the jugular vein. Hypertension was the most common comorbidity (78.8%, p< 0.001), followed by type 2 diabetes mellitus (47.0 %, p< 0.01). The incidence of stenosis was found to be significantly higher in the brachiocephalic vein than in other central veins (43.9%, p< 0.001). A repeated central catheter insertion in a patient was predictive of central venous occlusion (p< 0.05). Stenotic lesions were found to be associated with a significantly higher success rate than occlusive lesions (91.2%, p< 0.01).
Conclusion: Multiple central venous catheters (CVCs) are found to be associated with occlusive CVLs and unfavorable recanalization outcomes. Multiple CVC should be avoided by creating a permanent vascular access in a timely fashion for patients with chronic kidney disease and by avoiding the ipsilateral insertion of CVC and AVF.
Keywords: central venous lesions, brachiocephalic vein, central line catheterization, percutaneous transluminal angioplasty, fistula, hemodialysis
Introduction
Central venous lesions (CVLs) are somewhat clinically overlooked, and their true incidence is likely underestimated1–3 In developing countries such as Jordan, a lack of access monitoring protocols and surveillance of access-related issues serves as a challenge to access circuit patency. Consequently, diagnosis is limited to symptomatic patients with intractable complications that are mostly related to upper limb edema and inadequate dialysis. The clinical manifestations of CVLs can be subtle and covert, but they may become clinically cumbersome when a clinically silent CVL is uncovered during vascular access creation.1,4,5 The symptoms observed may include arm swelling, ipsilateral breast and neck swelling, visible venous collaterals, and loss of access circuit patency.1,5,6 The severity of symptoms remains to be unpredictable and poorly understood.2 Studies on various patient-, intervention-, and access-related features such as previous central catheterizations, venous collaterals, lesion-related characteristics (eg, lesion location including stenotic and obstructive lesions), access flow, and type and site of vascular access have been published.2,4,7–10 Furthermore, the etiopathogenesis of CVLs was determined to be multifactorial and controversial. Proposed mechanisms of CVL development include endothelial trauma due to repeat catheterizations, uremic milieu, flow dynamics with increased shear stress, platelet dysfunction, and intimal hyperplasia with fibrotic response, and these mechanisms may act synergistically.1,11
Access circuit complications (mostly due to sepsis and stenosis of access outflow) account for 20–30% of dialysis patient hospitalizations.12 Pre-emptive treatment of clinically silent CVLs is not recommended, and there is a paradigm shift toward treatment using current modalities of only symptomatic lesions that result from rapid progression of stenotic segments following intervention and from disappointing long-term intervention-free period.13,14
The aim of this study was to retrospectively assess hemodialysis patients with symptomatic CVLs. A digital subtraction angiography was used to assess and treat symptomatic CVLs. Cohort demographics, lesion type, catheter- and non-catheter-related lesions, and procedural outcomes were analyzed to identify correlations between CVLs and these variables.
Materials and Methods
Patients
We retrospectively assessed the demographic characteristics of 66 hemodialysis patients who underwent endovascular treatment for CVLs between January 2017 and December 2019. The following data are extracted from our university hospital electronic medical records: demographics, type and site of vascular access, location and nature of CVL, history of central venous catheterization, indications for central venous interventions, and the outcomes of each intervention. Patient consent was waived as data was used in aggregate with no personal identifiers.
Lesion Characteristics
According to the Society of Interventional Radiology classification, central veins include intrathoracic segments of internal jugular veins (IJVs), subclavian veins (SCVs), brachiocephalic veins (BCVs), and superior vena cava (SVC).15 CVL diagnosis was made based on clinical and radiological data. Patients with debilitating symptoms of edema in the affiliated limb, breast, and face and with inadequate dialysis were included in our analysis. CVLs were then diagnosed using digital subtraction angiography. Radiologically, all selected CVLs were referenced to the adjacent upstream normal vein to assess the degree of stenosis. A stenosis of greater than 50% in a central intrathoracic vein was considered an indication for treatment. In this study, patients with thrombosed access or <50% stenosis were excluded, and compression by extrinsic structures was not examined. Additionally, patients with pacemaker interventions were excluded to avoid the confounding effect of the pacemaker.
Procedural Success
Percutaneous endovascular management was initiated by placing a vascular sheath under ultrasound guidance into the main draining vein of the fistula or via the venous side of the graft in the symptomatic arm. Right common femoral vein approaches were used in cases of complete central venous occlusion that cannot be crossed using the venous outflow approach. Our standard approach to central vein stenosis in hemodialysis patients was high-pressure plain balloon angioplasty (PBA) as first-line therapy, with reference to adjacent normal-looking veins in order to accurately assess balloon size. Additionally, the balloon was carefully inflated with concern to the patient’s pain complaint during dilatation. Intravenous heparin was administered at a range of 3000–5000 IU with most patients received 3000 IU. Certain patients received 5000 IU of heparin as those were patients with AVG or obese patients with high body mass index. Angioplasty of the CVL was initially performed using high-pressure non-compliant 10–16 mm angioplasty balloons (Atlas or Conquest; Bard Peripheral Vascular Inc., Tempe, Arizona, USA). These balloons had a burst pressure of 1600–2000 kPa. Repeat balloon dilatation was performed for 3 minutes if the initial dilatation did not render stent placement unnecessary. Stents were inserted if the CVL was due to refractory angioplasty with immediate flow-limiting elastic recoil, residual stenosis >30% with persistent and significant collaterals, or early recurrent symptomatic stenosis within 4 weeks after PBA. Self-expanding nitinol bare-metal stents (Sinus-Venous stent, OptiMed GmbH, Ettlingen, Germany) 12–18 mm in diameter and 40–80 mm in length were used. To ensure adequate stent–vessel contact, the stents were dilated using balloons of appropriate size following deployment.
Successful treatment was administered in accordance with radiological and clinical criteria. In radiological terms, success was considered as anatomical luminal gain with <30% residual stenosis and resolution of most collateral vessels. Clinical success was determined using symptom resolution and adequate dialysis. We did not use SCV dialysis catheters as per hospital policy.
Statistical Analysis
The factors that were investigated in relation to CVLs were described using frequency distribution for categorical variables and mean ± standard deviation for continuous variables. Pearson’s chi-square (χ2) tests were used to analyze the associations between categorical variables, and Student’s t-tests were used for continuous variables. In addition, logistic regression analysis was used to determine the main predictors of CVL in the study model, and P<0.05 was considered statistically significant. If a substantial association was found between categorical variables, a post-hoc residual analysis was then conducted to determine the exact significance in the contingency table.
Results
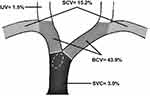
The study cohort consisted of 66 hemodialysis patients with CVLs (stenotic or occlusive). All patient characteristics and clinical presentations are summarized in Table 1. The mean age of patients was 51.9 ± 14.9 years, almost 50% of the patients were 41–60 years old, and approximately 56% of the patients were men. Sixty patients (90.9%) were determined to have arteriovenous fistula (AVF), but six of these patients switched to arteriovenous graft (AVG) after failed AVF. Another six patients had AVG from the beginning without AVF creation. With regard to comorbidities, there was a significantly high prevalence of hypertension (78.8%, P<0.001) and type 2 diabetes mellitus (47.0%, P<0.01). Interestingly, 41 of the 66 patients (62.1%) had a history of central venous catheterization. The duration from initiation of hemodialysis until symptomatic CVL was widely ranged among the patients from 1 to 36 months, with a median duration of 6 months. The most commonly affected solitary vein was the BCV (43.9%, P<0.001), and more than a third of the cases (36.4%) involved a combination of more than one central vein. Nineteen out of 24 combined cases (28.8% of total patients) had contiguous CVLs that spanning two or more veins as follows: 9 cases involved contiguous stenosis for both SCV and BCV, 9 cases BCV and SVC, and 1 case involved the three veins IJV, SCV, and BCV at their junction. The anatomical distribution of the CVLs is described in Figure 1.
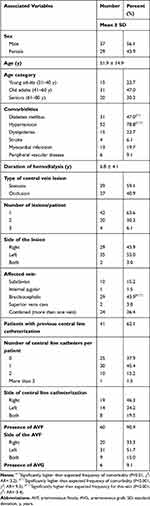 |
Table 1 Characteristics and Clinical Presentations of Hemodialysis Patients with Central Vein Disease |
Factors Affecting Lesion Type
There was no significant difference in the number between patients with partial stenosis (39/66, 59.1%) and patients with total occlusion (27/66, 40.9%) (Table 1). Further, no significant differences were determined in terms of sex, age, comorbidities, and affected central vein between these two patient groups (Table 2). However, patients with two central vein dialysis catheters were more likely to have an occlusive lesion than a stenotic lesion (P<0.05). The technical outcomes after the intervention were documented for 56 of the 66 patients. The rate of successful recanalization was significantly (P<0.01) higher in patients with stenosis (31/34, 91.2%) than in patients with occlusion (12/22, 54.5%). The results of the comparison between patients with central venous stenosis and patients with central venous occlusion are summarized in Table 2.
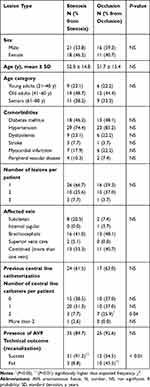 |
Table 2 Factors Associated with the Type of Central Vein Lesion (Stenosis Vs Occlusion) in Hemodialysis Patients |
In addition, a binary logistic regression model that included all the variables in Table 2 was also performed. The regression analysis revealed that patients with central venous occlusion had a significantly (6.35 times) higher risk of recanalization failure than patients with central venous stenosis did (P<0.05).
Factors That Determine the Side of CVLs
A summary of the factors that determine the side of a CVL is provided in Table 3. No significant associations were determined in terms of sex, age, or affected vein with relation to the lesion side. However, we found a significant correlation between the number of CVLs and the anatomical distribution of these lesions, ie, if a patient is suffering from three synchronous lesions both ipsilateral and contralateral central veins will be affected compared to patients having two lesions where synchronous lesions tend to lateralize to one body side (P<0.05). Furthermore, there were significant associations between the side of the lesion and the sides of the catheter and fistula as CVLs tend to develop on the ipsilateral side of the catheter and fistula (P<0.01).
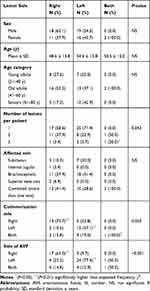 |
Table 3 Factors Associated with the Side of the Central Vein Lesion in Hemodialysis Patients |
In addition, a multinomial logistic regression model that included all the variables in Table 3 was used. The regression analysis revealed that the side of the AVF was the only significant predictor of the side of the lesion (P<0.05). For example, patients with left-sided fistulae were found to have a 16.67 times higher risk of developing a CVL on the left-hand side of the body than on the right-hand side of the body.
Central Catheter-Related Factors
No significant association was found between CVC placement and sex or affected vein. However, a significant association was found between catheter installation and age category (P<0.05). The number of young adult patients aged 21–40 who needed a CVC was less than expected (Table 4). Patients with dyslipidemia were found to have a significantly higher association with CVC placement than patients without dyslipidemia (P<0.05). Finally, all patients with a CVC (n = 41) also had an AVF (P<0.01). A summary of the variables affecting central vein catheterization is presented in Table 4.
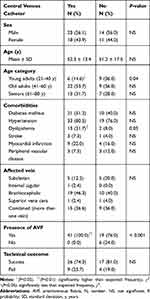 |
Table 4 Factors Associated with Installation of a Central Venous Catheter in Hemodialysis Patients with Central Vein Disease |
Discussion
To the best of our knowledge, this is the most comprehensive study of its kind in the Middle East and North Africa region that analyzed the contemporary management of CVLs. The association between patient demographics and the onset of central vein disease has not been well substantiated in literature, and sex, age, and comorbidities are possible prognostic variables that may predict disease progression.16 Sex distribution was comparable in our study, whereas other earlier studies reported that women were more susceptible to CVL development than men.16–18 Further, age at hemodialysis initiation was reported to provide a more comprehensive picture of the distribution of lesion onset.2 The mean age of patients at hemodialysis initiation in our study was 51.9 ± 14.9 years, which was found consistent with the age ranges reported in other studies.10,12,19 Renaud et al13 concluded that older populations (age: 75 ± 10 years) are more likely to have symptomatic CVLs than younger populations. Older patients usually have more comorbidities and longer catheter dwell times than younger patients, and these factors increase the duration of injury to vessel walls. In our study, more central lines were inserted into older patients than into younger patients. Our data showed that, compared to older patients (patients more than 40 years old), the number of central lines inserted into younger patients (patients less than 40 years old) was far less than expected (p= 0.04).
Hemodialysis patients with CVL are more likely to have multiple comorbidities.20 Although the results of the study by MacRae et al21 suggest that there are null associations between diabetes and central venous stenosis, in a later study by Wang et al22 it was reported that the risk of central venous stenosis is determined to be higher in patients with diabetes than in patients with no primary disease. However, we reckon that there is no correlation between these two comorbidities and CVL, and we suppose that the association is a coincidence as the two conditions are the most common comorbidities in the Jordanian population.
Data on the most common sites of CVLs are inconsistent.7 Although several studies reported that the SCV is the most frequent site of CVLs,16 others identified the BCV as the most common site of CVLs.6 In a recent study, it was reported that most CVLs occurred in the BCV, and stenotic lesions were four times more common than occlusive lesions.17 Oguzkurt et al8 have identified important correlations between previous central vein catheterizations and SCV stenosis and between concomitant extrinsic compression and BCV lesions. We reckon that CVLs are more likely to occur in the BCV in patients who had IJV instrumentation, while SCV is the most common site of CVLs in patients who had SCV catheterization. Moreover, CVLs associated with previous central vein catheterization usually develop faster than CVLs not associated with previous central vein catheterization.23
In this study, we compared the types of CVLs (stenotic versus obstructive). Increased frequency of central vein catheterization using >2 catheters were found to be significantly associated with occlusive lesions. Our results are in concordance with the reports in the literature. Adwaney et al19 identified patients with multiple CVCs and reported an increased risk of CVL with an increased number of previous catheter exposures in these patients. Endothelial injury, hemodynamic turbulence with stasis, and prothrombotic status are fundamental components of vascular occlusion identified by Rudolf Virchow over a century ago.1 Multiple central vein catheterizations were performed due to catheter malfunction, infection, or access dysfunction. The aforementioned indications are associated with exaggerated uremic environment, provoked inflammatory response, and access circuit complications in addition to direct endothelial injury from multiple instrumentations. Hernandez et al24 reported a threefold increase in the incidence of CVLs in patients with documented catheter infections, which may predispose patients to stagnation and infection. Furthermore, peri-catheter sleeve and thrombus formation are suggestive of a prothrombotic environment in such patients.1 Regardless of the inciting factor, a challenging CVL remains the ultimate result. Individual data on the indications for multiple central vein catheterizations in our cohort remain to be lacking; however, they are likely related to catheter dysfunction or infection. To avoid multiple CVC insertions, it is important to adopt reliable measures that maintain CVCs until a permanent vascular access is created in a timely fashion.
In renal replacement therapy, hemodialysis catheters play a vital role as a bridging solution or occasionally as a permanent resort.22,23 The inclusion of right IJV catheterization in our analysis is in accordance with our hospital policy. Published literature identified the right IJV as the best access site for CVCs as it presents the shortest traversed vessel pathway with the least vessel–catheter interaction. A CVC advanced via the left IJV passes across the mediastinum for it to reach the SVC with greater vessel–catheter interaction and extra turbulence due to complex angulated paths, thereby increasing the risk of catheter-related complications. Furthermore, the left IJV usually has a smaller cross-sectional area than the right IJV, and intimate endothelial contact is inevitable.16 Laterality of insertion has consistently been reported to be a predictor of CVLs.2,25 In our study, 26 of the 41 patients (about two-thirds) who underwent central vein catheterization developed an ipsilateral CVL.
However, there is a disparity in published literature regarding the effect of previous central vein catheterizations on the development of CVLs. Some authors consider previous central vein catheterization as the main cause of CVLs, while others suppose that the majority of CVLs occur in the absence of previous central vein catheterizations.7 Thus, our understanding of the etiopathogenesis of CVLs continues to evolve, and a lot remains unknown. Despite being associated with approximately 50% of non-catheter-related CVLs, compression by extrinsic thoracic structures (ie, innominate vein compression syndrome) and access flow rates were not examined in this study.7,8,10 The influence of non-catheter-related factors may explain the 15.2% incidence of SCV lesions in our study despite a lack of direct-vein instrumentation at this site. Additionally, from 31 patients who received AVF on the left side there were 9 patients with initial ipsilateral CVCs and 4 patients with repeated bilateral catheterization. In these 13 patients, it is challenging to predict whether the stenosis occurred due to the catheter and tended to uncover later by the flow from the fistula or it was actually initiated by the increased flow rate from the fistula itself.
Despite the adoption of fistula-first policy for renal replacement therapy in clinical practice guidelines,26 our analysis revealed that dialysis was initiated in 62.1% of patients via central vein catheterization, and this is a considerably higher percentage than that in developed countries.27 It is noteworthy that young age at dialysis is protective against catheterization. Younger patients are more likely to accept fistula-first policy than elderly patients who are medically depleted and therefore more likely to refuse fistula creation. Further, surgeons are more likely to turn down or defer operations on elderly patients. However, central vein catheterization was found not to be predictive of treatment outcomes of CVLs as the technical success rate of recanalization does not differ significantly between patients with previous central vein catheterization and patients without previous central vein catheterization.
The limitations of our study are mainly reflected in the data limitations encountered during retrospective analysis. One limitation of this study is that this was a single-center study. As such, the patient population in this tertiary center may be more complex and have advanced comorbidities; therefore, this is not representative of the broader population. Another limitation of this study is its lacking data on the confounding variables (access flow rates and extrinsic compression) that were not assessed but can predict CVLs in patients with non-catheter-related CVLs. Additionally, the diagnosis of CVLs was confined to digital subtraction angiography without using CT-scan. It is agreed that the vein diameter can be better studied with CT-scan. Lastly, the technical success rate was reported to 56 patients as there were ten patients without post-interventional outcome documentation. These were elderly patients with multiple comorbidities, and living in far rural areas. It was very difficult to communicate with them as they did not attend their assigned follow-up visits and did not respond to our phone calls.
Conclusion
The BCV was the most affected vein in our study cohort. Multiple central vein catheterizations were associated with occlusive-type CVLs, which result in unfavorable recanalization outcomes. Therefore, multiple CVC should be avoided by creating a permanent vascular access in a timely fashion for patients with chronic kidney disease and by avoiding the ipsilateral insertion of CVC and AVF. In addition, the side of AVF was found to be the main predictor of lesion side as CVLs were observed to develop more often on the ipsilateral side of the fistula. Lastly, young age was found to be protective against the initial use of CVCs. However, central vein catheterization did not affect recanalization outcomes.
Abbreviations
CVL, central venous lesion; CVC, central venous catheter; SVC, superior vena cava; SCV, subclavian vein; BCV, brachiocephalic vein; IJV, internal jugular vein; AVF, arteriovenous fistula; AVG, arteriovenous graft.
Consent for Publication
Not applicable. Data was used in aggregate with no personal identifiers.
Ethics Approval and Consent to Participate
This is a retrospective cohort study in which we utilized existing data from electronic medical records. Institutional Review Board approval was obtained from Jordan University of Science and Technology. For this type of study, formal and informed consent were waived since data was used in aggregate with no personal identifiers. The study was conducted with assurance of patient data confidentiality and in accordance to the Declaration of Helsinki and its later amendments for ethical research performance.
Disclosure
The authors declare that they have no competing interests.
References
1. Agarwal AK, Patel BM, Haddad NJ, Agarwal AK, Patel BM, Haddad NJ, eds Central vein stenosis: a nephrologist’s perspective. Semin Dial. 2007;20(1):53–62. doi:10.1111/j.1525-139X.2007.00242.x
2. Prasad V, Baghai S, Gandhi D, Moeslein F, Jindal G. Cerebral infarction due to central vein occlusion in a hemodialysis patient. J Neuroimaging. 2015;25(3):494–496. doi:10.1111/jon.12152
3. Molina JC, Martinez-Vea A, Riu S, et al. Pseudotumor cerebri: an unusual complication of brachiocephalic vein thrombosis associated with hemodialysis catheters. Am J Kidney Dis. 1998;31(5):E3. doi:10.1111/j.1525-139X.2007.00242.x
4. Mickley V. Central vein obstruction in vascular access. Eur J Vasc Endovasc Surg. 2006;32(4):439–444. doi:10.1016/j.ejvs.2006.04.011
5. Kundu S. Review of central venous disease in hemodialysis patients. J Vasc Interv Rad. 2010;21(7):963–968. doi:10.1540/jsmr.39.95
6. Park HS, Choi J, Baik JH. Central venous disease in hemodialysis patients. Kidney Res Clin Pract. 2019;38(3):309–317. doi:10.4103/ejs.ejs_143_19
7. Kotoda A, Akimoto T, Kato M, et al. Central venous stenosis among hemodialysis patients is often not associated with previous central venous catheters. ASAIO J. 2011;57(5):439–443. doi:10.1097/MAT.0b013e3182246bf8
8. Oguzkurt L, Tercan F, Yıldırım S, Torun D. Central venous stenosis in haemodialysis patients without a previous history of catheter placement. Eur J Rad. 2005;55(2):237–242. doi:10.1016/S0272-6386(98)70065-4
9. Wu TY, Wu CK, Chen YY, Lin CH. Comparison of percutaneous transluminal angioplasty with stenting for treatment of central venous stenosis or occlusion in hemodialysis patients: a systematic review and meta-analysis. Cardiovasc Intervent Rad. 2020;43(4):525–540. doi:10.1007/s00270-019-02383-7
10. Shi Y, Zhu M, Cheng J, Zhang J, Ni Z. Venous stenosis in chronic dialysis patients with a well-functioning arteriovenous fistula. Vascular. 2016;24(1):25–30. doi:10.1177/1708538115575649
11. Wali MA, Eid RA, Dewan M, Al-Homrany MA. Intimal changes in the cephalic vein of renal failure patients before arterio-venous fistula (AVF) construction. J Smooth Muscle Res. 2003;39(4):95–105. doi:10.1540/jsmr.39.95
12. Gonsalves CF, Eschelman DJ, Sullivan KL, DuBois N, Bonn J. Incidence of central vein stenosis and occlusion following upper extremity PICC and port placement. Cardiovasc Intervent Rad. 2003;26(2):123–127. doi:10.1007/s00270-002-2628-z
13. Renaud CJ, Francois M, Nony A, Fodil-Cherif M, Turmel-Rodrigues L. Comparative outcomes of treated symptomatic versus non-treated asymptomatic high-grade central vein stenoses in the outflow of predominantly dialysis fistulas. Nephrol Dial Transplant. 2012;27(4):1631–1638. doi:10.1093/ndt/gfr506
14. Yevzlin AS, Yevzlin AS, eds Hemodialysis catheter‐associated central venous stenosis. Semin Dial. 2008;21(6):522–527. doi:10.1111/j.1525-139X.2008.00496.x
15. Dolmatch BL, Gurley JC, Baskin KM, et al. Society of interventional radiology reporting standards for thoracic central vein obstruction: endorsed by the American Society of Diagnostic and Interventional Nephrology (ASDIN), British Society of Interventional Radiology (BSIR), Canadian Interventional Radiology Association (CIRA), Heart Rhythm Society (HRS), Indian Society of Vascular and Interventional Radiology (ISVIR), Vascular Access Society of the Americas (VASA), and Vascular Access Society of Britain and Ireland (VASBI). J Vasc Access. 2019;20(2):114–122. doi:10.1177/1129729818791409
16. Narendra JB, Sreenivas J, Karthikeyan VS, Nagaraja NH. Innominate vein stenosis in association with ipsilateral hyperdynamic brachiobasilic fistula causing ipsilateral limb and hemifacial swelling. Indian J Nephrol. 2017;27(6):452–455. doi:10.4103/0971-4065.194393
17. Ibrahim Ahmed MI, Atalla K, Taha MH. Treatment of hemodialysis related-central venous stenosis: 1-year results of venoplasty and follow-up in 50 patients. Egypt J Surg. 2020;39(1):105. doi:10.4103/ejs.ejs_143_19
18. Young EW, Dykstra DM, Goodkin DA, Mapes DL, Wolfe RA, Held PJ. Hemodialysis vascular access preferences and outcomes in the Dialysis Outcomes and Practice Patterns Study (DOPPS). Kidney Int. 2002;61(6):2266–2271. doi:10.1046/j.1523-1755.2002.00387.x
19. Adwaney A, Lim C, Blakey S, Duncan N, Ashby DR. Central venous stenosis, access outcome and survival in patients undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. 2019;14(3):378–384. doi:10.2215/CJN.07010618
20. Aitken E, Honour P, Johnson N, Kingsmore D. Is there an association between central vein stenosis and line infection in patients with tunnelled central venous catheters (TCVCs)? J Vasc Access. 2015;16(suppl 9_suppl):S42–S47. doi:10.5301/jva.5000335
21. MacRae JM, Ahmed A, Johnson N, Levin A, Kiaii M. Central vein stenosis: a common problem in patients on hemodialysis. ASAIO J. 2005;51(1):77–81. doi:10.1097/01.mat.0000151921.95165.1e
22. Wang K, Wang P, Liang X, Lu X, Liu Z. Epidemiology of haemodialysis catheter complications: a survey of 865 dialysis patients from 14 haemodialysis centres in Henan Province in China. BMJ Open. 2015;5(11):e007136. doi:10.1136/bmjopen-2014-007136
23. Teruya TH, Abou-Zamzam Jr AM, Limm W, Wong L, Wong L. Symptomatic subclavian vein stenosis and occlusion in hemodialysis patients with transvenous pacemakers. Ann Vasc Surg. 2003;17(5):526–529. doi:10.1007/s10016-003-0048-4
24. Hernández D, Díaz F, Rufino M, et al. Subclavian vascular access stenosis in dialysis patients: natural history and risk factors. J Am Soc Nephrol. 1998;9(8):1507–1510.
25. Salgado OJ, Urdaneta B, Colmenares B, García R, Flores C. Right versus left internal jugular vein catheterization for hemodialysis: complications and impact on ipsilateral access creation. Artif Organs. 2004;28(8):728–733. doi:10.1111/j.1525-1594.2004.07316.x
26. Daugirdas JT, Depner TA, Inrig J; National Kidney Foundation. KDOQI clinical practice guideline for hemodialysis adequacy: 2015 update. Am J Kidney Dis. 2015;66(5):884–930. doi:10.1053/j.ajkd.2015.07.015
27. Pisoni RL, Young EW, Dykstra DM, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002;61(1):305–316. doi:10.1046/j.1523-1755.2002.00117.x
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.