Back to Journals » Drug Design, Development and Therapy » Volume 14
Leonurine Promotes Cisplatin Sensitivity in Human Cervical Cancer Cells Through Increasing Apoptosis and Inhibiting Drug-Resistant Proteins
Authors Lin M , Pan C , Xu W , Li J , Zhu X
Received 2 March 2020
Accepted for publication 27 April 2020
Published 15 May 2020 Volume 2020:14 Pages 1885—1895
DOI https://doi.org/10.2147/DDDT.S252112
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Min Lin, Chunyu Pan, Wenbin Xu, Jingwei Li, Xueqiong Zhu
Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325027, People’s Republic of China
Correspondence: Xueqiong Zhu
Department of Obstetrics and Gynecology, The Second Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang 325027, People’s Republic of China
Tel +86 577 8800 2796
Fax +86 577 88002796
Email [email protected]
Background: Cisplatin-based neoadjuvant chemotherapy and concurrent radiotherapy and chemotherapy are the main treatment for advanced cervical cancer. However, the development of multidrug resistance (MDR) leads to chemotherapy failure, tumor recurrence and poor survival. In this research, we investigated the effect and corresponding mechanism of leonurine on cisplatin sensitivity of cervical cancer cells.
Methods: Anti-cervical cancer efficacy of leonurine and leonurine combined with cisplatin was examined in C33A and Ms751 cells. The cell counting kit-8 assay and bromodeoxyuridine assay were applied for measuring cell proliferation. CompuSyn software was used to calculate the combination index and assess the synergistic effect of leonurine and cisplatin on cell proliferation. The cell cycle distribution and cell apoptosis were analyzed by flow cytometry. The expression of cleaved caspase-3, poly ADP-ribose polymerase (PARP), B-cell lymphoma-2 associated X (BAX), B-cell lymphoma-2 (BCL-2), P glycoprotein (P-Gp) protein and multiple drug resistance protein 1 (MRP1) was analyzed by Western blotting.
Results: Leonurine had time- and dose-dependent anti-proliferative effects on C33A and MS751 cells. Leonurine and cisplatin combination was more efficacious in inhibiting the growth of cervical cancer cells than either of the two drugs. The combined application has shown that the cervical cancer cells were arrested at G1 phase after treatments. Moreover, flow cytometry analysis indicated that the combined treatment could cause more cell apoptosis than the single drug treatment. Consistently, combined treatment elevated BAX/BCL-2 ratio, and the expression of BAX, PARP and cleaved caspase-3 proteins. Mechanistic investigations uncovered that the tumor-inhibiting effects of the co-treatment were mediated by repressing MDR, including MRP1 and P-Gp protein, thereby enhancing the efficiency of cisplatin.
Conclusion: Leonurine and cisplatin have synergistic antitumorigenic effects on cervical cancer. Combination with leonurine may serve as a novel strategy for enhancing cisplatin sensitivity via the inhibition of the expression of MRP1 and P-Gp.
Keywords: cervical cancer, leonurine, cisplatin, multidrug resistance
Introduction
Cervical cancer is one of the main causes of cancer-related deaths in women worldwide, which seriously threatens women’s health. According to the World Health Organization, there are approximately 570,000 new cases annually, and about 311,000 women’s deaths from cervical cancer, 80% of which are in developing countries.1 In China, there are approximately 106,430 new cases of cervical cancer being diagnosed in women annually, of these around 47,739 will die.2 The traditional treatment of cervical cancer is mainly surgery and radiotherapy, while chemotherapy is mainly utilized to treat patients with metastasis and recurrence of cervical cancer.3 Cisplatin-based neoadjuvant chemotherapy and concurrent chemoradiotherapy (CCRT) have been used to treat advanced cervical cancer, which has effectively improved the quality of life and prognosis of these patients.4,5 However, about 20% of patients have progressed to chemoresistance, resulting in chemotherapy failure, tumor recurrence and worse prognosis.6 Therefore, searching for specific and sensitive chemotherapeutic drugs for cervical cancer and further improving the sensitivity of cisplatin are bound to be the new focus in the field of cervical cancer chemotherapy.
Leonurus heterophyllus, also known as Motherwort, is a fresh and dry aerial part of Leonurus.7 With a long history, it has been commonly used in traditional medicine in Asia, America and Europe.7 In China, it is generally used to treat gynecological diseases, including dysmenorrhea, irregular menstruation, amenorrhea and abnormal uterine bleeding.8,9 The pharmacological effects of Leonurus heterophyllus are closely related to the kinds of alkaloids, which include Leonurine, Stachydrine, Leonurinine and Leonurdine.7 Leonurine is one of the main components of Leonurus heterophyllus, and it is also considered to be one of the primary effective components. A wealth of studies has verified that leonurine is beneficial to many systemic diseases of the human body, such as protecting cardiovascular, antioxidant stress, and inhibiting inflammation of the nervous system. 10,12 In the research of malignant tumors, Mao et al illustrated that leonurine could up-regulate the expression of phosphorylated p38 and down-regulate phosphorylated Akt expression through inhibiting mitochondrial pathway, thus suppressing the proliferation and promoting the lung cancer cell apoptosis.13 Sitarek et al analyzed that the extract of Leonurus sibiricus transgenic roots could induce in different grades of human glioma cell apoptosis.14,15 These data unveiled the inhibitory role of leonurine in cancer cells. However, the effect of leonurine on chemosensitivity, thus far, has not been reported yet. In this article, we first evaluated the effect of leonurine on cisplatin chemosensitivity in cervical cancer cells and explored the underlying molecular mechanism.
Materials and Methods
Cell Lines and Cell Cultures
Human cervical cancer C33A and MS751 cells derived from the human epithelial cervix carcinoma were originally purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). The C33A cells and the MS751 cells were cultured in the RPMI 1640 medium (Invitrogen, Carlsbad, USA) and Dulbecco’s Modified Eagle Medium (DMEM, Gibco, USA), respectively, containing with 10% fetal bovine serum (Gibco, USA), 100U/mL penicillin and 100µg/mL streptomycin in 5% CO2 humidified incubator at 37°C.
Reagents and Antibodies
Leonurine was bought from MedChemExpress (Monmouth Junction, NJ, USA). It was dissolved in Dimethyl Sulfoxide (DMSO) and diluted with indicated medium to different concentrations (0, 200, 400, 800, 1200, 1600, 2000µM). Cisplatin dissolved by phosphate buffer saline (PBS) was obtained from Sigma (St. Louis, MO) and diluted to various concentrations (0, 3, 5, 10, 15µM) in the medium. Antibodies for PARP, cleaved caspase-3, BAX, BCL-2, and BrdU were procured from Cell Signaling Technology (Boston, MA, USA). Antibodies for P-Gp and MRP1 were procured from Abcam (Cambridge, Massachusetts, USA).
Cell Viability Assay: Cell Counting Kit-8 (CCK-8) Assay
Briefly, 5000 C33A cells/well or 3000 MS751 cells/well were seeded in a 96-well plate at in quadruplicate and located in a 5% CO2 humidified incubator until they reached between 70% and 80% confluence. Cells were then treated with the leonurine at the following concentration (0, 200, 400, 800, 1200, 1600, 2000µM) for 24 h and 48 h, respectively. For cisplatin treatment, cells were treated with the increasing concentration of cisplatin (0, 3, 5, 10, 15µM) for 24 h and 48 h. Subsequently, cells were co-treated with the following concentrations of leonurine (200, 400, 800µM) and cisplatin (3, 5, 10, 15µM) for 48 h, respectively. Then, C33A cells were incubated with 10 µL CCK-8 solutions (Dojindo, Tokyo, Japan) in a 5% CO2 incubator of 37°C for 4 h, while MS751 cells were incubated for 2 h. Ultimately, the absorbance was evaluated at 480 nm wavelength on a Microplate Reader (Bio Tek Instruments, Winooski, VT, USA). Three independent experiments were performed for the final analyses.
Drug Combination Index
A combination index (CI) values were obtained to analyze the efficacy in inhibiting cell proliferation between leonurine and cisplatin via using Compusyn software.16,17 Synergism is described when the scale of CI is 0 ~ 1, while antagonism is determined when CI is more than 1. The smaller the value, the stronger the synergy (CI value: very strong synergism 0 ~ 0.1, strong synergism 0.1 ~ 0.3, synergism 0.3 ~ 0.7, moderate synergism 0.7 ~ 0.85, slight synergism 0.85 ~ 0.9, nearly additive 0.9 ~ 1.10, and antagonism more than 1.10).18 According to the combination index, the ultimate co-treatment concentrations of leonurine and cisplatin were determined. Hence, the cells were further separated into four different groups: control group (treatment with DMSO), leonurine group, cisplatin group and co-treatment group (combined treatment with leonurine and cisplatin). Cell proliferation after 48 h treatment was analyzed.
Cell Proliferation Assay: Bromodeoxyuridine (BrdU) Assay
Cells were seeded in a 6-well plate with a grass slide at 2.5 × 105 cells/well (C33A) or 1.2 × 105 cells/well (MS751) and then placed in a 5% CO2 incubator. After the cells were incubated for 12 h and attached to plate, cells were treated with the control group, leonurine group, cisplatin group and co-treatment group for 48 h, respectively. Then a concentration of 1 mg/mL BrdU solution was supplemented to cells for co-cultivating 12 h before measuring the proliferating cells. The concentrations of antibody were as follows: the primary anti-BrdU (mouse monoclonal antibody: 1:200) and the second antibody (IgG/Alexa fluor ® 594: 1:100). Finally, the nuclei were stained with 1µg/mL 4ʹ, 6-diamidino-2-phenylindole (DAPI). The marked cells were detected and calculated by a microscope (Olympus, Tokyo, Japan) in randomly selected 5 microscopic fields at 400 × magnification. Three independent experiments were performed for each treatment condition.
Cell Cycle Analysis
Cells (control group, leonurine group, cisplatin group and co-treatment group) were digested by trypsin with Ethylenediaminetetraacetic Acid (EDTA) and centrifuged at 1000 rpm for 5 min. Then, Cells were resuspended in the precooled phosphate buffer saline (PBS) (adjust concentration of 1 × 106 cells/mL) and fixed in 2 mL cold 70% ethanol at 4°C for overnight. The next day, after washing twice with PBS, cells were added with 500 µL RNAase at a concentration of 20 µg/mL for 30 min. Finally, it was incubated with a concentration of 50 µg/mL propidium iodide (PI) 500 µL at room temperature without light exposure for 30 min. Subsequently, cell cycle analysis was completed by CytoFLEX flow cytometry purchased from Beckman Coulter (Brea, CA, USA). The ModFit LT4.1 software was used to analyze the data obtained from the flow cytometry.
Annexin V-FITC/PI Dual Staining
Cells were seeded in 60mm dishes (C33A: 6 × 105 or MS751: 3 × 105), cultured until 50–60% confluence and then divided into the above four groups (control group, leonurine group, cisplatin group and co-treatment group) for 48 h treatment, respectively. Cells were then performed to an Annexin V-FITC/PI dual staining kit procured from BD Biosciences. According to the manufacturer’s protocol, cells were stained with 5 µL of recombinant Annexin V-fluorescein isothiocyanate (FITC) and 10 µL of propidium iodide (PI) solution for 15 min at room temperature in the dark. The percentage of apoptotic cells were measured using the CytoFLEX flow cytometry (Beckman Coulter, Fullerton, CA, USA). The percentage of cells with FITC+/PI- and FITC+/PI+ were determined as the early apoptosis and late apoptosis, respectively. The sum of them is considered to be the final cell apoptosis ratio.
Western Blot Analysis
Cells in the above four different groups were directly extracted from the RIPA lysis buffer (Beyotime Biotechnology) containing protease and phosphatase inhibitors (Calbiochem, Billerica, MA, USA), respectively. The protein concentration of cell lysates was quantified utilizing BCA™ Protein Assay Kit (Pierce, Appleton, WI, USA). The total protein samples were electrophoresed by sodium dodecyl sulfate- polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred onto a polyvinylidene fluoride (PVDF) transfer membrane. Subsequently, the PVDF membranes were blocked with 5% milk fat for 1 h. After incubating with corresponding primary antibodies overnight at 4 °C, the PVDF membranes were co-cultured with horseradish peroxidase-conjugated rabbit anti-mouse IgG (1:4000, Biosharp) or goat anti-rabbit IgG secondary antibody (1:3000, Biosharp) for 1 h at the room temperature. The expression of protein was visualized by enhanced chemiluminescence (ECL) reagents (Millipore, USA), meanwhile the internal parameters of these bands were quantified using the Image J software (Bethesda, MA, USA). The concentrations of primary antibodies were as follows: anti-cleaved caspase-3 (1:500), anti-PARP (1:2000), anti-BAX (1:1000), anti-BCL-2 (1:1000), anti-P-Gp (1:1000), anti-MRP1 (1:1000), and anti-β-Tubulin (1:4000). The experiments were performed in triplicate.
Statistical Analysis
Data were statistically analyzed using the SPSS25.0 software program (IBM Corporation, Armonk, NY, USA). Results are expressed as mean ± SD. Two-tailed Student’s t-tests were used to compare two groups. One-way ANOVA was used to compare multiple groups, following by least significant difference (LSD) method. The figure generation was performed by Prism 6.0 (Graph Pad software) (Inc., La Jolla, CA, USA). Two-tailed P < 0.05 was considered as statistically significant.
Results
Leonurine Increases the Antiproliferative Effect of Cisplatin in Cervical Cancer Cells
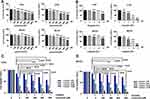
To explore the biological function of Leonurine, CCK-8 assay was used to estimate the effect of leonurine on the viability of C33A and MS751 cells. Compared to the control group, leonurine inhibited the C33A and MS751 cell viability in dose- and time-dependent manners, respectively (Figure 1A). Furthermore, cisplatin noticeably suppressed the cellular viability, suggesting the antiproliferative effects of cisplatin on cervical cancer cells (Figure 1B). The half maximum inhibitory concentration (IC50) of cisplatin was 7.8μmol/l for C33A cells and 9.3μmol/l for MS751 cells for 48 h (Figure 1B). Subsequently, in the presence of cisplatin, application of leonurine could further increase the cellular damage as illustrated by decreasing cell viability after 48 h (Figure 1C and D). Moreover, compared with the 5µM cisplatin group, 5 µM cisplatin plus 400 µM leonurine or plus 800 µM leonurine had the obviously synergistic antiproliferative function in cervical cancer cells (CI, 0.69, 0.67, respectively). According to the combination index, 5μM cisplatin and 800μM leonurine were determined as the concentration of the combination therapy (CI =0.67) (Table 1).
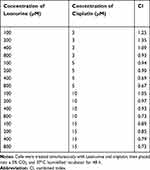 |
Table 1 Combined Index Data on Combination Treatment of Leonurine and Cisplatin |
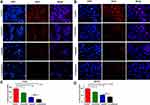
To further acquaint the effect of 48 h co-treatment on cell proliferation, the BrdU assay was used next. After comparing with the control group, leonurine group, cisplatin group, and co-treatment group could dramatically repress cervical cancer cell proliferation, respectively (Figure 2). Moreover, compared with cisplatin group, the proliferation of C33A and MS751 cells in the co-treatment group was lower. These results revealed that leonurine not only repressed cervical cancer cell proliferation, but also promoted the inhibition of cisplatin on the cell proliferation.
Leonurine Enhances the Inhibited Effect of Cisplatin on the Cell Cycle of Cervical Cancer
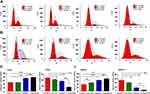
To further investigate whether co-treatment affects the cell cycle, flow cytometry was performed. Compared with either of the two single drug groups, the co-treatment group significantly elevated the frequency of above both cell lines at the G1 phase of cell cycle, but decreased the S phase. It revealed that leonurine could enhance the antiproliferative effect of cisplatin, which inhibited cervical cancer cell proliferation partly by inducing cell cycle arrest at the G1 phase (Figure 3).
Leonurine Promotes the Apoptosis Effect of Cisplatin on Cervical Cancer Cells
To observe the role of leonurine on cisplatin-mediated cell apoptosis, the flow cytometry based Annexin-V/PI double staining analysis was utilized. Compared to the control group, leonurine increased the apoptosis in both C33A and MS751 cells (Figure 4). Furthermore, co-treatment with cisplatin and leonurine could further augment the percentage of cell apoptosis compared to the cisplatin group (Figure 4). These results suggested that leonurine was not only induced cell apoptosis but also promoted the apoptosis effect of cisplatin on cervical cancer cells.
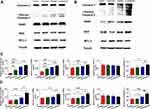
To further confirm whether leonurine improves the apoptosis effect of cisplatin, the expression of quite a lot apoptosis related proteins were detected after different treatments. Compared with the cisplatin group, it is verified that the co - treatment group significantly induced the expression of cleaved caspase-3 and PARP proteins in both C33A and MS751 cells (Figure 5). Although no significant difference was found in the BCL-2 protein expression between the cisplatin group and co-treatment group, there was a markedly higher expression of BAX protein and the BAX/BCL-2 ratio in co-treatment group (Figure 5).
Leonurine Significantly Reduces the Drug-Resistant Proteins in Cervical Cancer Cells
To further demonstrate the role of leonurine on cisplatin chemosensitivity, Western blotting was utilized to measure the MDR related protein, including P-Gp and MRP1. Compared with the cisplatin group, the expression of drug-resistant proteins P-Gp and MRP1 was downregulated in the co-treatment group both in C33A and MS751 cell (Figure 6). It may predict that leonurine improves the cisplatin chemosensitivity through declining the expression of certain drug-resistant proteins.
Discussion
Cisplatin, a small-molecule platinum compound, is initially discovered to inhibit bacterial growth.19 Subsequently, it is identified as a chemotherapeutic agent, severing as to most effectually treat advanced or recurrent cervical cancer.20 Specifically, increasing evidence has implicated that targeting mitochondria, inducing DNA damage and later resulting in apoptosis of cells are the primary effect of cisplatin-mediated cervical cancer chemotherapy.6,21 However, increasing intrinsic and/or acquired resistance to cisplatin-based chemotherapy has become the challenge in the treatment of many cancer patients.22 The underlying molecular mechanisms of cisplatin resistance are complex, including decreased intracellular drug concentration and activity, enhanced DNA damage repair, inactivated the apoptosis, activated the epithelial mesenchymal transition (EMT) and cancer stem cells characteristics, and so on.6 Especially, emerging evidence has also demonstrated that chemotherapy and/or radiotherapy can induce the formation of MDR in malignant tumors.23–25 Hence, cisplatin resistance may develop, reducing the efficiency of cisplatin chemotherapy. Additionally, increased expression of drug-resistant proteins, elevated drug outflow and decreased intracellular drug concentration are contributed to the chemoresistance.26,27 These factors ultimately lead to an elevated cellular survival rate.
It has been revealed that leonurine suppressed cell proliferation and promoted cell apoptosis in lung cancer cells by inhibiting mitochondrial pathway.13 Considering that mitochondrion is also the main target of the cisplatin-based chemotherapy, we hypothesized that leonurine could improve the vulnerability of cervical cancer cells to cisplatin by inducing apoptosis. Consistent with this hypothesis, our results showed that leonurine did not only particularly inhibit the cervical cancer cell viability in dose- and time-dependent manner, but also increased the antiproliferative effect of cisplatin in cervical cancer cells. In this study, cisplatin plus leonurine had a synergistic antiproliferative function in cervical cancer cells. Furthermore, leonurine combined with cisplatin could further increase cell cycle arrest at G1 phase, but having opposite effects of S phase, which played an anti-tumor role through regulation of cell cycle arrest.
In our study, compared with both single groups, co-treatment could significantly induce cervical cancer cell apoptosis. The above results were further established by analysis of cleaved caspase-3 apoptosis protein using Western blot. It suggested that the synergistic effect of leonurine on cisplatin may be related to its promotion of apoptosis. Likewise, poly ADP-ribose polymerase (PARP), a multifunctional post-translational protein modifying enzyme, exists in most eukaryotic cells.28 It is activated by recognizing DNA fragments with damaged structure and is considered as DNA damage receptor.29 Wu et al identified that PARP inhibitor induces leukemia cell apoptosis via DNA damage and reverses leukemia cell adriamycin resistance.30 Our data have shown that combined therapy with leonurine and cisplatin elevated the PARP protein expression and accelerated the DNA damage. BAX protein, an apoptotic protein, is proven to enhance the mitochondrial membrane permeability in vitro and induce the release of cytochrome.31 The role of BAX in apoptosis can be neutralized by BCL-2. In our data, it was found that combined therapy resulted in an obvious increase the Bax protein and the ratio of BAX/BCL-2 in cervical cancer cell lines, suggesting that leonurine mainly affects cervical cancer cell apoptosis through repressing the mitochondria of cervical cancer cells through modifying the expression of Bax but not BCL-2.
It has been validated that intrinsic or acquired multidrug resistance (MDR) is one of the major roots of cancer chemotherapy failure. Multidrug resistance is a phenomenon that cancer cells exert cross-resistance to various functional and structural unconcerned with their chemotherapeutic drugs after exposure to certain chemotherapeutic drugs.32 The mechanism of multidrug resistance in tumors is very extensive. P-glycoprotein (P-Gp), a product of MDR, is one of the earliest and most significant pathways of multidrug resistance.33 It is a member of ATP-binding cassette transporters and has ATP-dependent drug efflux pump function which could pump drugs out of cell membrane and reduce intracellular drug concentration, leading to MDR.34 Accumulated evidence has identified that declining the expression of P-Gp may sever as increasing chemosensitivity in tumor cells.35,36 Multidrug resistance-associated protein 1 (MRP1), another ATP-binding cassette transporter protein, is associated with MDR in cancer.37 As a membrane transporter, MRP1 could exclude cytotoxic drugs directly in many cells by transport, causing drugs not binding to target sites.38 One elegant research has validated that natural products, such as Alkaloids, Flavonoids, Paeonol, and Quercetin, play positive effects on tumor treatment, including reducing chemotherapy and radiotherapy side effects, and reversing multidrug resistance, as well as improving prognosis.23 Our data unveiled that co-treatment with leonurine and cisplatin could strikingly reduce the expression of MRP1 and P-Gp, which indicates that leonurine could reduce ATP-binding cassette transporters protein expression, enhance the intracellular cisplatin concentration and inverse the MDR. Nevertheless, the underlying pathway of the reverse MDR process is still indistinct and needs to be sightseen in further researches. Furthermore, whether leonurine has similar sensitization effects on other kinds of chemotherapeutic drugs, such as paclitaxel, deserves further evaluation.
Conclusion
Conclusively, our research revealed that leonurine significantly promotes cisplatin sensitivity in cervical cancer cells via repressing cell proliferation, improving intrinsic cell apoptosis and inhibiting the expression of P-Gp and MRP1 protein, which has potential therapeutic value in cervical cancer treatment. Correspondingly, the sighting of leonurine in the treatment of cervical cancer provides a novel clue that develops the anti-cancer reagents derived from natural herbal products, and also provides the possibility for the inhibitor of multidrug resistance in the future.
Acknowledgments
This work was sponsored by Science and Technology Plan of Traditional Chinese Medicine of Zhejiang Province (No. 2020ZQ034), Science and Technology Planning Project of Wenzhou city (No. Y20180013) and Gynecology and Obstetrics of Combine Traditional Chinese and Western Medicine of Zhejiang Province (2017-XK-A42).
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi:10.3322/caac.21492
2. China: Human Papillomavirus and Related Cancers, Fact Sheet 2019. ICO/IARC Information Centre on HPV and Cancer; 2019.
3. Zhu H, Chen A, Li S, et al. Predictive role of galectin-1 and integrin alpha5beta1 in cisplatin-based neoadjuvant chemotherapy of bulky squamous cervical cancer. Biosci Rep. 2017;37:5. doi:10.1042/BSR20170958
4. Chen X, Zou H, Li H, et al. Weekly versus triweekly cisplatin-based chemotherapy concurrent with radiotherapy in the treatment of cervical cancer: a meta-analysis. Int J Gynecol Cancer. 2017;27(2):344–349.
5. Petrelli F, De Stefani A, Raspagliesi F, Lorusso D, Barni S. Radiotherapy with concurrent cisplatin-based doublet or weekly cisplatin for cervical cancer: a systematic review and meta-analysis. Gynecol Oncol. 2014;134(1):166–171.
6. Zhu H, Luo H, Zhang W, Shen Z, Hu X, Zhu X. Molecular mechanisms of cisplatin resistance in cervical cancer. Drug Des Devel Ther. 2016;10:1885–1895.
7. Zhang RH, Liu ZK, Yang DS, Zhang XJ, Sun HD, Xiao WL. Phytochemistry and pharmacology of the genus Leonurus: the herb to benefit the mothers and more. Phytochemistry. 2018;147:167–183.
8. Zhu YZ, Wu W, Zhu Q, Liu X. Discovery of Leonuri and therapeutical applications: from bench to bedside. Pharmacol Ther. 2018;188:26–35.
9. Miao LL, Zhou QM, Peng C, Liu ZH, Xiong L. Leonurus japonicus (Chinese motherwort), an excellent traditional medicine for obstetrical and gynecological diseases: a comprehensive overview. Biomed Pharmacother. 2019;117:109060.
10. Sitarek P, Rijo P, Garcia C, et al. Antibacterial, anti-inflammatory, antioxidant, and antiproliferative properties of essential oils from hairy and normal roots of Leonurus sibiricus L. and their chemical composition. Oxid Med Cell Longev. 2017;2017:7384061. doi:10.1155/2017/7384061
11. Sermukhamedova OV, Sakipova ZB, Ternynko II, Gemedzhieva NG. Representatives of Motherwort Genus (Leonurus Spp.): aspects of pharmacognostic features and relevance of new species application. Acta Pol Pharm. 2017;74(1):31–40.
12. Chen C, Zhu Z, Hu N, Liang X, Huang W. Leonurine hydrochloride suppresses inflammatory responses and ameliorates cartilage degradation in osteoarthritis via NF-kappaB signaling pathway. Inflammation. 2019.
13. Mao F, Zhang L, Cai MH, Guo H, Yuan HH. Leonurine hydrochloride induces apoptosis of H292 lung cancer cell by a mitochondria-dependent pathway. Pharm Biol. 2015;53(11):1684–1690. doi:10.3109/13880209.2014.1001406
14. Sitarek P, Skala E, Toma M, et al. Transformed root extract of leonurus sibiricus induces apoptosis through intrinsic and extrinsic pathways in various grades of human glioma cells. Pathol Oncol Res. 2017;23(3):679–687. doi:10.1007/s12253-016-0170-6
15. Sitarek P, Skala E, Toma M, et al. A preliminary study of apoptosis induction in glioma cells via alteration of the Bax/Bcl-2-p53 axis by transformed and non-transformed root extracts of Leonurus sibiricus L. Tumour Biol. 2016;37(7):8753–8764. doi:10.1007/s13277-015-4714-2
16. Chou TC. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58(3):621–681. doi:10.1124/pr.58.3.10
17. Chou TC. Drug combination studies and their synergy quantification using the Chou-Talalay method. Cancer Res. 2010;70(2):440–446. doi:10.1158/0008-5472.CAN-09-1947
18. Tong Z, Mejia A, Veeranki O, et al. Targeting CDK9 and MCL-1 by a new CDK9/p-TEFb inhibitor with and without 5-fluorouracil in esophageal adenocarcinoma. Ther Adv Med Oncol. 2019;11:1758835919864850. doi:10.1177/1758835919864850
19. Cullen KJ, Yang Z, Schumaker L, Guo Z. Mitochondria as a critical target of the chemotheraputic agent cisplatin in head and neck cancer. J Bioenerg Biomembr. 2007;39(1):43–50. doi:10.1007/s10863-006-9059-5
20. Wei X, Zhou Y, Qiu J, Wang X, Xia Y, Sui L. Low expression of TUG1 promotes cisplatin sensitivity in cervical cancer by activating the MAPK pathway. J BUON. 2019;24(3):1020–1026.
21. Pariente R, Pariente JA, Rodriguez AB, Espino J. Melatonin sensitizes human cervical cancer HeLa cells to cisplatin-induced cytotoxicity and apoptosis: effects on oxidative stress and DNA fragmentation. J Pineal Res. 2016;60(1):55–64. doi:10.1111/jpi.12288
22. Chen J, Solomides C, Parekh H, Simpkins F, Simpkins H. Cisplatin resistance in human cervical, ovarian and lung cancer cells. Cancer Chemother Pharmacol. 2015;75(6):1217–1227. doi:10.1007/s00280-015-2739-2
23. Guo Q, Cao H, Qi X, et al. Research progress in reversal of tumor multi-drug resistance via natural products. Anticancer Agents Med Chem. 2017;17(11):1466–1476. doi:10.2174/1871520617666171016105704
24. Hill BT, Moran E, Etievant C, et al. Low-dose twice-daily fractionated X-irradiation of ovarian tumor cells in vitro generates drug-resistant cells overexpressing two multidrug resistance-associated proteins, P-glycoprotein and MRP1. Anticancer Drugs. 2000;11(3):193–200. doi:10.1097/00001813-200003000-00007
25. Tada Y, Wada M, Migita T, et al. Increased expression of multidrug resistance-associated proteins in bladder cancer during clinical course and drug resistance to doxorubicin. Int J Cancer. 2002;98(4):630–635. doi:10.1002/ijc.10246
26. Sedlakova I, Laco J, Caltova K, et al. Clinical significance of the resistance proteins LRP, Pgp, MRP1, MRP3, and MRP5 in epithelial ovarian cancer. Int J Gynecol Cancer. 2015;25(2):236–243.
27. Sedlakova I, Laco J, Tosner J, Spacek J. [Prognostic significance of Pgp, MRP1, and MRP3 in ovarian cancer patients]. Ceska gynekologie. 2015;80(6):405–413.
28. Smulson ME, Simbulan-Rosenthal CM, Boulares AH, et al. Roles of poly(ADP-ribosyl)ation and PARP in apoptosis, DNA repair, genomic stability and functions of p53 and E2F-1. Adv Enzyme Regul. 2000;40:183–215. doi:10.1016/S0065-2571(99)00024-2
29. Almahli H, Hadchity E, Jaballah MY, et al. Development of novel synthesized phthalazinone-based PARP-1 inhibitors with apoptosis inducing mechanism in lung cancer. Bioorg Chem. 2018;77:443–456. doi:10.1016/j.bioorg.2018.01.034
30. Wu J, Xiao S, Yuan M, et al. PARP inhibitor resensitizes Adriamycin resistant leukemia cells through DNA damage and apoptosis. Mol Med Rep. 2019;19(1):75–84. doi:10.3892/mmr.2018.9628
31. Pena-Blanco A, Garcia-Saez AJ. Bax, Bak and beyond - mitochondrial performance in apoptosis. FEBS J. 2018;285(3):416–431. doi:10.1111/febs.14186
32. Szakacs G, Paterson JK, Ludwig JA, Booth-Genthe C, Gottesman MM. Targeting multidrug resistance in cancer. Nat Rev Drug Discov. 2006;5(3):219–234. doi:10.1038/nrd1984
33. Xue C, Wang C, Liu Q, et al. Targeting P-glycoprotein expression and cancer cell energy metabolism: combination of metformin and 2-deoxyglucose reverses the multidrug resistance of K562/Dox cells to doxorubicin. Tumour Biol. 2016;37(7):8587–8597. doi:10.1007/s13277-015-4478-8
34. Kathawala RJ, Gupta P, Ashby CR
35. Abdallah HM, Al-Abd AM, El-Dine RS, El-Halawany AM. P-glycoprotein inhibitors of natural origin as potential tumor chemo-sensitizers: a review. J Adv Res. 2015;6(1):45–62. doi:10.1016/j.jare.2014.11.008
36. Kobori T, Harada S, Nakamoto K, Tokuyama S. Mechanisms of P-glycoprotein alteration during anticancer treatment: role in the pharmacokinetic and pharmacological effects of various substrate drugs. J Pharmacol Sci. 2014;125(3):242–254. doi:10.1254/jphs.14R01CR
37. Lu JF, Pokharel D, Bebawy M. MRP1 and its role in anticancer drug resistance. Drug Metab Rev. 2015;47(4):406–419.
38. Hu P, Wong PT, Zhou Q, et al. Clinical relevance of the multidrug resistance-associated protein 1 gene in nonsmall cell lung cancer: A systematic review and metaanalysis. Oncol Rep. 2018;40(5):3078–3091.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.