Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Leonurine Alleviates Cognitive Dysfunction and Reduces Oxidative Stress by Activating Nrf-2 Pathway in Alzheimer’s Disease Mouse Model
Authors Xie Y, Jin Y, Li S, Shen B, Ma L, Zuo L, Gao Y, Yang G
Received 26 January 2023
Accepted for publication 22 May 2023
Published 1 June 2023 Volume 2023:19 Pages 1347—1357
DOI https://doi.org/10.2147/NDT.S404798
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yue Xie,1,* Yaning Jin,2,* Shuyue Li,3 Baoxi Shen,4 Liping Ma,5 Lujie Zuo,6 Ya Gao,3 Guofeng Yang3
1Department of Neurology, the Second Medical Center and National Clinical Research Center for Geriatric Disease, Chinese PLA General Hospital, Beijing, 100853, People’s Republic of China; 2Chaoyang Second Retired Cadre Rest Center of Beijing Garrison, Beijing, 100853, People’s Republic of China; 3Department of Geriatrics, the Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050000, People’s Republic of China; 4Department of Neurosurgery, the First Medical Centre, Chinese PLA General Hospital, Medical School of Chinese PLA, Beijing, 100853, People’s Republic of China; 5Department of Neurology, Xinzhou People’s Hospital, Shanxi, 034000, People’s Republic of China; 6Department of Otolaryngology, Head and Neck Surgery, Children’s Hospital of Hebei Province, Shijiazhuang, Hebei, 050000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ya Gao; Guofeng Yang, Department of Geriatrics, the Second Hospital of Hebei Medical University, Shijiazhuang, Hebei, 050000, People’s Republic of China, Email [email protected]; [email protected]
Introduction: Alzheimer’s disease (AD) is the most common type of dementia, impacting approximately 50 million individuals globally. However, the current treatments available for AD are only symptomatic and have limited efficacy. This study aimed to investigate whether Leonurine could alleviate cognitive dysfunction in a mouse model of AD and explore its underlying molecular mechanisms.
Methods: In this study, male APP/PS1 mice were orally administered Leonurine for two consecutive months. The cognitive functions of the mice were then evaluated using novel object recognition (NOR) and Morris water maze (MWM) tests. Hippocampal neuronal damage was observed through Nissl staining, Aβ levels were determined through ELISA, oxidative stress activity was detected through biochemical methods, and the nuclear factor erythroid-2-related factor 2 (Nrf-2) pathway was analyzed using western blot and real-time quantitative polymerase chain reaction analysis.
Results: Our results demonstrated that Leonurine treatment markedly improved cognitive functions, as indicated by the improved performance in the model. Additionally, histopathology showed a reduction in hippocampal neuronal damage. This can be attributed to the potential of Leonurine to reduce Aβ 1-40 and Aβ 1-42 levels and alleviate oxidative stress. Its antioxidant effect is linked to the activation of the Nrf-2 signaling pathway in APP/PS1 mice, which promotes Nrf-2 nuclear translocation and expression of HO-1 and NQO-1.
Conclusion: These findings suggest that Leonurine could be explored further as it could emerge as a promising drug for AD treatment.
Keywords: Alzheimer’s disease, cognitive deficit, Leonurine, Nrf-2, oxidative stress
Introduction
Alzheimer’s disease (AD) is a neurodegenerative disorder with insidious deterioration and gradual progression in older adults.1 In clinical settings, AD is strongly associated with irreversible loss of memory, neurodegeneration, progressive cognitive decline, and personality changes. Currently available drugs for AD such as donepezil, memantine, and rivastigmine only provide symptomatic relief and are not capable of preventing or curing the disease. Moreover, these drugs may cause undesirable gastrointestinal and cardiovascular adverse effects in AD patients.2 Several clinical trials of immunotherapy targeting Aβ (Crenezumab, Gentenerumab, and Solanezumab) have also been unsuccessful.3 It is an urgent task for researchers to find an effective and safe treatment to improve AD symptoms. While the etiology and pathogenesis of AD remains unclear, studies have identified oxidative stress and Aβ as the primary pathways associated with the onset and progression of the disease.4
Excessive production of reactive oxygen species (ROS) in the brain can lead to oxidative stress and damage to antioxidant enzyme systems, thereby accelerating the pathological progression of AD including Aβ deposition. The accumulation of abnormal Aβ can further decrease mitochondrial redox activity and worsen ROS aggregation. Recent studies have shown that antioxidants and free radical scavengers could protect neurons in vivo and in vitro by preventing Aβ-related oxidative stress and lipid peroxidation.5,6 Antioxidant therapy may be a potential treatment for AD.
The nuclear factor erythroid-2-related factor 2 (Nrf-2) pathway is a multi-organ protective pathway against stressful environments, which exerts antioxidant effects by regulating the expression level of antioxidants and epigenetics, ultimately delaying the progression of the disease.7 It has been found that the Nrf-2 signaling pathway is closely associated with AD. The expression of Nrf-2 and its downstream genes heme oxygenase-1 (HO-1) and NADPH quinone oxidoreductase-1 (NQO1) were reduced in both elderly and AD brains.8 Further research showed that Nrf-2 deficiency exacerbated learning and memory deficits in mouse models of AD and increased amyloidosis and tau-like pathology in the brain.9,10 However, activating Nrf-2 had a positive effect on cognitive defects in AD model mice by inhibiting oxidative stress and neuroinflammation.11 Numerous Nrf-2 activators have demonstrated significant efficacy in halting the progression of AD by targeting key pathogenic factors such as Aβ and p-tau, as well as regulating mitochondrial function and reducing neuroinflammation.12–14 As a result, targeting the Nrf-2 pathway may represent a promising strategy for the treatment of AD. To this end, natural antioxidants have been extensively investigated to identify safe and effective therapeutic agents targeting Nrf-2.
Leonurine (Leo, C14H21N3O5, 4‐guanidino‐n‐butyl syringate) (Figure 1A), also known as SCM-198, is a bioactive agent derived from Herba leonuri (a traditional Chinese herb). It has received much attention for its extensive spectrum of biological activities, including antioxidant, anti-inflammatory, anti-tumor, and cardioprotective properties.15 As a safe and promising new drug, Leo is currently undergoing clinical trials in China.16 Besides, Leo has been shown to provide benefits for various central nervous system diseases such as ischemic stroke, Parkinson’s disease (PD), depression, and multiple sclerosis.17–19 Moreover, Leo treatment effectively inhibited the overactivation of microglia and reduced the neuroinflammatory responses in the Aβ1-40-induced rat model.20 Recent studies have demonstrated that Leo could ameliorate oxidative damage in aging mice and mice with ischemic stroke by activating the Nrf2 signaling pathway.17,21 However, to date, the neuroprotective effect and antioxidant mechanism of Leo in the APP/PS1 mouse model of AD have not yet been reported. Based on these findings, we hypothesize that Leo could potentially serve as a viable treatment option for AD due to the activating effect of Nrf-2.
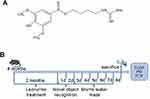 |
Figure 1 (A) Chemical structure of Leo. (B) The timeline for the experimental procedure. |
Here, we investigated the potential neuroprotective effects of Leo against oxidative stress in APP/PS1 mice of AD. Additionally, we explored whether the observed effects were mediated through Nrf-2 signaling pathways.
Materials and Methods
Animals and Drug Administration
Twenty male APP/PS1 (4-month-old) double transgenic AD mice and 20 C57BL/6J mice of the same age and sex were acquired from Beijing HFK Bioscience Co., LTD in China. Before the experiment, all mice were placed in a relative humidity of 50 ± 5%, with a constant temperature of (23 ± 1°C) and a 12-hour light-dark cycle for at least 1 week with free access to water and food. All experimental protocols were authorized by the Animal Experimentation Ethics Committee of the Second Hospital of Hebei Medical University (approval number: 2022-AE027) and followed the Guidelines of the Animal Care and Use Committee.22
Leo was obtained from MedChemExpress (NJ, USA), and the purity is above 98%. Mice were classified into 4 groups of 10 mice each: wild-type C57BL/6J group (WT, intragastric administration of normal saline); WT + Leo group (WT + Leo, intragastric administration of 150 mg/kg/d Leo); APP/PS1 transgenic group (Tg, intragastric administration of normal saline); Tg + Leo group (Tg + Leo, intragastric administration of 150 mg/kg/d Leo). The dosage of Leo was determined according to a previous study.21 The mice’s general health and body weight were monitored daily. After 2 months of the administration, behavioral assays were conducted based on the progress of the experiment, as shown in Figure 1B.
Novel Object Recognition (NOR) Test
The NOR test was conducted as previously described with a slight modification.23 In brief, the procedure included a training phase and a testing phase on two consecutive days. On the first day, mice (n = 10) were placed in an open-field arena (29 cm × 29 cm × 29 cm) and allowed to freely explore two identical objects with the same color, shape and size for 10 minutes. The next day, one of the same objects was exchanged for a new and unfamiliar one. The mice were released back into the arena to explore freely for 10 minutes. The location preference (the ratio of the time mice spent exploring one object to the time they spent exploring two objects) in the training phase and the discrimination index (the ratio of the time mice spent exploring a novel object to the time they spent exploring two objects) in the testing phase were analyzed.
Morris Water Maze (MWM) Test
The MWM test was performed as described previously with modifications.24 Briefly, the test consisted of a training phase of 4 trials per day for 5 consecutive days and a probe trial for 1 day. Mice (n = 10) were tested in a circular water tank with a diameter of 120 cm, which was divided into four quadrants. The water in the tank was opaque and maintained at a temperature of 21 ± 0.5°C. In the middle of one quadrant, a 9-cm-diameter hidden platform was positioned 1 cm under the water surface. In each trial of the training sessions, the time it took for the mice to find the hidden platform within 60 seconds was determined as the escape latency. Probe trial was conducted on day 6, with the platform removed. Mice were permitted to explore freely for 60 seconds. The number of crossing platforms and the time spent in the target quadrant by mice were recorded as measures of retrieval memory.
Brain-Tissue Preparation
After the behavioral tests, all mice were anesthetized with sodium pentobarbital intraperitoneally at a dose of 50 mg/kg. The mice were sacrificed by decapitation following transcardial perfusion with ice‐cold 0.9% saline. The brains of three mice from each group were sagittally sectioned on the surface of the ice. The left hemisphere was preserved in 4% paraformaldehyde for 48 hours, and then dehydrated and embedded in paraffin for histological analysis. From the right hemisphere and the remaining mouse brain of each group, hippocampal tissue was extracted for biochemical measurements.
Nissl Staining
Nissl staining was carried out by immersing brain sections in Cresyl violet (Beyotime, China) for 30 minutes after deparaffinization and rehydration. The sections were then differentiated using 1% glacial acetic acid. The morphology of Nissl-positive cells was observed using an optical microscope (Olympus BX60, Japan).
ROS Detection
Intracellular ROS was detected with a DCFH-DA probe (Nanjing Jiancheng Bioengineering Institute, China) following the supplier’s protocol. Fresh hippocampal tissue (n = 6) was cut and rinsed in pre-cooled PBS. After 30 minutes of digestion, the cells were filtered and collected. Then, the cells were resuspended in 10 μM DCFH-DA and cultured at 37°C for 20 minutes in the dark. Cell precipitates were gathered after centrifugation for 10 minutes at 1000 g, and washed twice with PBS to fully remove the DCFH-DA that did not enter the cells. Finally, the fluorescence intensity was determined at the excitation/emission wavelength of 488 nm/525 nm.
ELISA for Aβ Level
In this study, hippocampal tissue samples (n = 6) were analyzed for Aβ1-42 and Aβ1-40 levels using ELISA kits from Invitrogen, Carlsbad, CA, USA, following the previous description.25 Absorbance values were determined at 450 nm with a multifunctional microplate reader, and results were expressed as ng/ml.
Measurement of SOD, GSH-Px and MDA
The activities of SOD and GSH-Px and the content of MDA in brains (n = 6) were detected with the corresponding kits (Nanjing Jiancheng Bioengineering Institute, China) following the supplier’s directions. All protein concentrations were determined with the BCA kit (Beyotime Biotechnology, Shanghai, China). SOD and GSH-Px activity were assayed from the absorbance at 450 nm and 412 nm, respectively, and MDA were assayed from the absorbance at 532 nm.
Western Blot Analysis
Western blot analysis was conducted according to previously mentioned methods.26 Cytoplasmic and nuclear proteins were isolated from the hippocampal tissues (n = 3) with a Nuclear and Cytoplasmic Protein Extraction Kit (P0028, Beyotime Biotechnology, Shanghai, China). Protein concentration was determined by a BCA method. 20 μg protein was separated from each sample by polyacrylamide gel electrophoresis utilizing 10% sodium dodecyl sulfate, and then transferred to a polyvinylidene fluoride (PVDF) membrane. The PVDF membranes were blocked in 5% BSA-PBST buffer for 1 hour at room temperature. Next, the membranes were incubated with primary antibodies overnight at 4°C. The primary antibodies included: Nrf-2 (1:1000), HO-1 (1:1000) were obtained from Cell Signaling Technology (MA, USA), NQO1 (1:1000), Histone H3 (1:2000) and GAPDH (1:2000) were obtained from Abcam (MA, USA). Then, the membranes were incubated with a specific horseradish peroxidase-conjugated secondary antibody at room temperature for 1 hour. The relative density of target bands was normalized to Histone H3 or GAPDH levels.
Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
RT-qPCR was performed as previously described.27 Total RNA was extracted from the hippocampal tissues (n = 3) with RNA Rapid Extraction Kit (Takara, Japan). For subsequent experiments, the OD260/280 ratio of total RNA was maintained between 2.1 and 2.2. A total of 2 μg of RNA was reverse-transcribed into complementary DNAs with a First-Strand cDNA Synthesis kit (GeneCopoeia, Guangzhou, China). The levels of Nrf-2, HO-1, NQO1, and GAPDH mRNA were quantitatively analyzed using an RT-qPCR system (Bio-Rad, USA). The primer sequences used for RT-qPCR were listed in Table 1. Gene expressions of Nrf-2, HO-1 and NQO1 were analyzed using the Ct (2−ΔΔCt) method. The expression levels were presented as the ratio of threshold cycle (Ct) values to GAPDH.
 |
Table 1 RT-qPCR Primer Sequences |
Statistical Analysis
All data were graphed as mean ± SEM. The escape latency in the MWM test was examined by repeated-measures one-way analysis of variance (ANOVA), and the other data were examined by ANOVA using SPSS 23.0 software (SPSS, Inc) followed by Tukey’s test. Significance was presented as P < 0.05.
Results
Leo Improved Cognitive Impairment of APP/PS1 Mice
Cognitive function of mice in each group was assessed using the NOR and MWM tests. During the NOR test (Figure 2A and B), all groups showed similar preference scores of around 50% in the learning trial. In the subsequent testing trial, Leo did not significantly affect the discrimination index of WT mice, but APP/PS1 mice had a significantly reduced discrimination index compared to WT controls (Figure 2B, P < 0.01). However, when Leo was administered to APP/PS1 mice, they exhibited a higher discrimination index in the testing trial compared to APP/PS1 mice without Leo treatment (P < 0.05).
Similarly, no significant differences were observed in the MWM test between Leo-treated WT mice and WT mice. In contrast, APP/PS1 mice took more time to find a platform than WT mice, indicated by a prolonged escape latency (Figure 3A, P < 0.01), and this result was reversed by Leo treatment in APP/PS1 mice (P < 0.05). Additionally, in the probe test, we found a significant increase in the number of crossing platforms and target quadrant residence time in Leo-treated Tg mice compared to Tg mice (Figure 3B and C, P < 0.05 and P < 0.05, respectively). Furthermore, there was no notable difference in swimming speed among the groups (data not shown), suggesting that the enhancement of cognitive ability in the Leo-treated Tg group was not attributed to the movement speed of mice.
Leo Prevented the Neuronal Damage in the Hippocampus of APP/PS1 Mice
The effect of Leo treatment on neuronal damage in the hippocampus of APP/PS1 mice was investigated using Nissl staining. Surviving cells were identified as those with pale stained and round nuclei. As shown in Figure 4, Tg mice exhibited neuronal injury in the hippocampal CA1 region, characterized by evident nuclear rupture and less intact Nissl material, indicating neuronal degeneration, when compared to WT mice. However, treatment with Leo significantly reduced neuronal damage in the CA1 region of the hippocampus of APP/PS1 mice.
 |
Figure 4 Leo reduced neuronal damage in the hippocampal CA1 region of APP/PS1 mice, as evidenced by representative Nissl staining sections of the same region. Scale bar: 100 μm. |
Leo Reduced Aβ Levels in the Hippocampus of APP/PS1 Mice
We quantified the levels of Aβ1-42 and Aβ1-40 in the hippocampus of mice using an ELISA assay. As illustrated in Figure 5A, the Tg mice exhibited significantly higher Aβ1-42 and Aβ1-40 levels compared to WT mice (P < 0.0001 and P < 0.0001, respectively). However, treatment with Leo significantly reduced the levels of Aβ1-42 and Aβ1-40 in Tg mice (P < 0.001 and P < 0.001, respectively). These results indicated that Leo treatment effectively decreased Aβ levels in the hippocampus of APP/PS1 mice.
Leo Suppressed Oxidative Stress in APP/PS1 Mice
To investigate the impact of Leo on the level of oxidative stress in the hippocampus of APP/PS1 mice, we analyzed the contents of ROS, MDA, SOD and GSH-Px. Our results showed that the level of ROS in the hippocampus of Tg mice was significantly higher than in WT mice (Figure 5B, P < 0.01). However, treatment with Leo significantly reduced the ROS levels in Tg mice (P < 0.05). Furthermore, Leo treatment increased the activities of SOD (Figure 5C, P < 0.05) and GSH-Px (Figure 5D, P < 0.01) while decreasing the amount of MDA (Figure 5E, P < 0.01) in the hippocampus of APP/PS1 mice.
Leo Evoked the Activation of Nrf-2 Pathway in APP/PS1 Mice
To evaluate the expression of Nrf-2 pathway proteins, we detected the protein levels of nuclear-Nrf-2 (N-Nrf-2), cytoplasmic-Nrf-2 (C-Nrf-2), HO-1 and NQO1 using western blot. Our findings indicated that in Tg mice, the protein levels of N-Nrf-2 (Figure 6A and B, P < 0.05), C-Nrf-2 (Figure 6C and D, P < 0.05), HO-1 (Figure 6C and E, P < 0.05) and NQO1 (Figure 6C and F, P < 0.01) protein levels were lower compared to WT mice, suggesting that the Nrf-2 pathway was blocked. However, after Leo treatment in Tg mice, we observed an increase in N-Nrf-2 (P < 0.05), C-Nrf-2 (P < 0.05), HO-1 (P < 0.05) and NQO1 (P < 0.01) protein levels compared to Tg mice, suggesting that Leo treatment may have a positive effect on the Nrf-2 pathway.
Similar results were found in the gene expression of Nrf-2 pathway. As illustrated in Figure 7A-C, it is evident that the Tg mice treated with Leo had a significant increase in mRNA expression of Nrf-2, HO-1, and NQO1 compared to Tg mice (P < 0.01, P < 0.01 and P < 0.05, respectively). These results suggested that Leo evoked the activation of Nrf-2 pathway in APP/PS1 mice.
Discussion
AD is a prevalent neurodegenerative disease that causes a gradual decline in memory and cognitive function in older individuals. Unfortunately, current treatment options for AD are limited, resulting in a significant economic and social burden on public health systems worldwide. However, our research has shown that Leo may be a potential drug for the treatment of AD. In particular, Leo has been found to alleviate cognitive deficits, prevent neuronal damage, and reduce the levels of Aβ1-42 and Aβ1-40 in the hippocampus of the brain in APP/PS1 mice.
Leo, derived from the traditional Chinese medicine Herba leonuri, has the potential to exhibit antioxidant, anti-inflammatory, and anti-apoptotic effects on both the cardiovascular and central nervous systems. Studies have shown that Leo could protect against LPS-induced myocarditis by inhibiting the NF-кB signaling pathway.28 Additionally, Leo has been found to have an antidepressant-like effect in a mouse model of chronic mild stress depression by inhibiting neuroinflammation.19 Moreover, Leo attenuated cognitive impairment induced by Aβ1-40 in rats by inhibiting microglial hyperactivation through JNK and NF-кB pathways.20 Furthermore, Leo improved cardiac function in rats with myocardial infarction by initiating an anti-apoptosis role through PI3K/AKT/GSK3β pathway.29 In addition, Leo has been shown to exhibit antioxidant and anti-apoptotic effects in PD rats and cell models induced by 6-OHDA through increasing SOD, decreasing ROS and maintaining mitochondrial function.18 Based on the multiple biological roles of Leo in neurological disorders, it may be used in the treatment of AD. In our study, we demonstrated for the first time that Leo improved cognitive function in APP/PS1 mice through NOR and MWM tests and reduced hippocampal neuronal damage through Nissl staining. Therefore, we proposed that Leo could be a potential alternative drug for AD treatment and further explored the mechanism underlying the therapeutic effect of Leo on AD.
Although the etiology and pathological mechanism of AD are still unclear, toxic Aβ-related oxidative stress is one of the central hypotheses. Studies have shown that there is an increase in oxidative stress in areas of the brain that contain high levels of Aβ1-42 in both AD patients and mouse models.30 This increase in oxidative stress is believed to be caused by Aβ, which in turn leads to the production of cytotoxicity by boosting the production of mitochondrial ROS.31 Oxidative stress can destroy the antioxidant activity of cells and lead to neuronal damage, which is a key “bridge” connecting multiple pathways and pathogenesis of AD.32 Drugs targeting oxidative stress have great application prospects in treating of AD. In the study conducted, it was observed that Leo decreased the levels of Aβ1-42 and Aβ1-40 and exhibited remarkable antioxidant ability in APP/PS1 mice. This was evident from the increase in antioxidant enzyme SOD and GSH-Px activities and the decrease in ROS and MDA levels. The findings suggest that Leo has a neuroprotective effect by inhibiting Aβ-related oxidative stress. It was found that Nrf-2 plays a crucial role in maintaining cellular redox homeostasis and regulating inflammatory responses. We then validated whether the antioxidant effect of Leo was achieved through Nrf-2.
Recent studies have shown that Nrf-2 is involved in several critical pathological processes of Aβ and p-tau pathways in AD.33 Nrf-2 deficiency worsens spatial learning and memory abilities, as well as AD-like pathology in APP/PS1 mice.34 On the other hand, Nrf-2 activation has been found to improve cognitive deficits in an AD mouse model by inhibiting oxidative stress and neuroinflammation.11 Upon activation, Nrf-2 is translocated from the cytoplasm to the nucleus, where it triggers the transcription of downstream target genes such as HO-1 and NQO1, ultimately enhancing the antioxidant capacity of cells.35 Leo was observed to promote Nrf-2 nuclear translocation in the kidney tissues of rats with ischemic acute kidney injury and in the liver tissues of aging mice, resulting in increased expression of HO-1 and NQO1, which helped to counteract oxidative stress damage.17,36 Besides, Leo upregulated N-Nrf‐2 protein and increased total Nrf‐2 protein expression and mRNA levels in brain tissues of mice with ischemic stroke. Similar to these findings, our study discovered that Leo increased levels of N-Nrf-2 and C-Nrf-2 protein, as well as Nrf-2 mRNA levels, indicating that Leo promoted Nrf-2 activation and nuclear translocation. Furthermore, Leo elevated the protein and mRNA expression of HO-1 and NQO1, suggesting that Leo acts as an antioxidant through the Nrf-2 pathway.
Conclusion
Our findings of the current study in vivo show that Leo could ameliorate cognitive deficits and hippocampal neuronal damage in APP/PS1 mice by reducing Aβ levels and inhibiting oxidative stress. This is achieved by increasing antioxidant enzyme activity and decreasing ROS levels. In addition, the antioxidant effect of Leo may be attributed to the activation of the Nrf-2 signaling pathway (Figure 8). These findings provide new insights into the beneficial role of Leo in the treatment of AD.
Data Sharing Statement
The datasets used in this study may be obtained from the corresponding author upon reasonable request.
Funding
This work was supported by the Scientific Research Project of Hebei Administration of Traditional Chinese Medicine (Grant No. 2023325) and the Medical Science Research Project of Hebei Provincial Health Commission (Grant No. 20220997).
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590.
2. Campbell NL, Perkins AJ, Gao S, et al. Adherence and Tolerability of Alzheimer’s Disease Medications: a Pragmatic Randomized Trial. J Am Geriatr Soc. 2017;65(7):1497–1504.
3. Pleen J, Townley R. Alzheimer’s disease clinical trial update 2019-2021. J Neurol. 2022;269(2):1038–1051.
4. Tonnies E, Trushina E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J Alzheimers Dis. 2017;57(4):1105–1121.
5. Liu Y, Chen Z, Li B, et al. Supplementation with gamma-glutamylcysteine (gamma-GC) lessens oxidative stress, brain inflammation and amyloid pathology and improves spatial memory in a murine model of AD. Neurochem Int. 2021;144:104931.
6. Gray NE, Zweig JA, Matthews DG, Caruso M, Quinn JF, Soumyanath A. Centella asiatica Attenuates Mitochondrial Dysfunction and Oxidative Stress in Abeta-Exposed Hippocampal Neurons. Oxid Med Cell Longev. 2017;2017:7023091.
7. Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism. Cell Mol Life Sci. 2016;73(17):3221–3247.
8. Silva-Palacios A, Ostolga-Chavarria M, Zazueta C, Konigsberg M. Nrf2: molecular and epigenetic regulation during aging. Ageing Res Rev. 2018;47:31–40.
9. Branca C, Ferreira E, Nguyen TV, Doyle K, Caccamo A, Oddo S. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum Mol Genet. 2017;26(24):4823–4835.
10. Rojo AI, Pajares M, Rada P, et al. NRF2 deficiency replicates transcriptomic changes in Alzheimer’s patients and worsens APP and TAU pathology. Redox Biol. 2017;13:444–451.
11. Uruno A, Matsumaru D, Ryoke R, et al. Nrf2 Suppresses Oxidative Stress and Inflammation in App Knock-In Alzheimer’s Disease Model Mice. Mol Cell Biol. 2020;40(6):46.
12. Wang C, Chen S, Guo H, et al. Forsythoside A Mitigates Alzheimer’s-like Pathology by Inhibiting Ferroptosis-mediated Neuroinflammation via Nrf2/GPX4 Axis Activation. Int J Biol Sci. 2022;18(5):2075–2090.
13. Ikram M, Muhammad T, Rehman SU, et al. Hesperetin Confers Neuroprotection by Regulating Nrf2/TLR4/NF-kappaB Signaling in an Abeta Mouse Model. Mol Neurobiol. 2019;56(9):6293–6309.
14. Yang Q, Lin J, Zhang H, et al. Ginsenoside Compound K Regulates Amyloid beta via the Nrf2/Keap1 Signaling Pathway in Mice with Scopolamine Hydrobromide-Induced Memory Impairments. J Mol Neurosci. 2019;67(1):62–71.
15. Huang L, Xu DQ, Chen YY, Yue SJ, Tang YP. Leonurine, a potential drug for the treatment of cardiovascular system and central nervous system diseases. Brain Behav. 2021;11(2):e01995.
16. Liao J, Suguro R, Zhao X, Yu Y, Cui Y, Zhu YZ. Leonurine affected homocysteine-methionine metabolism based on metabolomics and gut microbiota studies of clinical trial samples. Clin Transl Med. 2021;11(10):e535.
17. Xie YZ, Zhang XJ, Zhang C, Yang Y, He JN, Chen YX. Protective effects of leonurine against ischemic stroke in mice by activating nuclear factor erythroid 2-related factor 2 pathway. CNS Neurosci Ther. 2019;25(9):1006–1017.
18. Shi XR, Hong ZY, Liu HR, Zhang YC, Zhu YZ. Neuroprotective effects of SCM198 on 6-hydroxydopamine-induced behavioral deficit in rats and cytotoxicity in neuronal SH-SY5Y cells. Neurochem Int. 2011;58(8):851–860.
19. Jia M, Li C, Zheng Y, et al. Leonurine Exerts Antidepressant-Like Effects in the Chronic Mild Stress-Induced Depression Model in Mice by Inhibiting Neuroinflammation. Int J Neuropsychopharmacol. 2017;20(11):886–895.
20. Hong ZY, Shi XR, Zhu K, Wu TT, Zhu YZ. SCM-198 inhibits microglial overactivation and attenuates Abeta(1-40)-induced cognitive impairments in rats via JNK and NF-small ka, CyrillicB pathways. J Neuroinflammation. 2014;11:147.
21. Chen P, Chen F, Zhou BH. Leonurine ameliorates D-galactose-induced aging in mice through activation of the Nrf2 signalling pathway. Aging. 2019;11(18):7339–7356.
22. National Research Council. Guide for the Care and Use of Laboratory Animals.
23. El-Sahar AE, Shiha NA, El Sayed NS, Ahmed LA. Alogliptin Attenuates Lipopolysaccharide-Induced Neuroinflammation in Mice Through Modulation of TLR4/MYD88/NF-kappaB and miRNA-155/SOCS-1 Signaling Pathways. Int J Neuropsychopharmacol. 2021;24(2):158–169.
24. Assaf N, El-Shamarka ME, Salem NA, Khadrawy YA, El Sayed NS. Neuroprotective effect of PPAR alpha and gamma agonists in a mouse model of amyloidogenesis through modulation of the Wnt/beta catenin pathway via targeting alpha- and beta-secretases. Prog Neuropsychopharmacol Biol Psychiatry. 2020;97:109793.
25. Zhang Y, Hu Y, Han Z, et al. Cattle Encephalon Glycoside and Ignotin Ameliorate Palmitoylation of PSD-95 and Enhance Expression of Synaptic Proteins in the Frontal Cortex of a APPswe/PS1dE9 Mouse Model of Alzheimer’s Disease. J Alzheimers Dis. 2022;88(1):141–154.
26. Zhang C, Li S, Sun C, et al. Vitexin ameliorates glycochenodeoxycholate-induced hepatocyte injury through SIRT6 and JAK2/STAT3 pathways. Iran J Basic Med Sci. 2021;24(12):1717–1725.
27. Wang CY, Zhang Q, Xun Z, et al. Increases of iASPP-Keap1 interaction mediated by syringin enhance synaptic plasticity and rescue cognitive impairments via stabilizing Nrf2 in Alzheimer’s models. Redox Biol. 2020;36:101672.
28. Wang R, Li D, Ouyang J, et al. Leonurine alleviates LPS-induced myocarditis through suppressing the NF-small ka, CyrillicB signaling pathway. Toxicology. 2019;422:1–13.
29. Xu L, Jiang X, Wei F, Zhu H. Leonurine protects cardiac function following acute myocardial infarction through antiapoptosis by the PI3K/AKT/GSK3beta signaling pathway. Mol Med Rep. 2018;18(2):1582–1590.
30. Butterfield DA, Boyd-Kimball D. Oxidative Stress, Amyloid-beta Peptide, and Altered Key Molecular Pathways in the Pathogenesis and Progression of Alzheimer’s Disease. J Alzheimers Dis. 2018;62(3):1345–1367.
31. Franca MB, Lima KC, Eleutherio EC. Oxidative Stress and Amyloid Toxicity: insights From Yeast. J Cell Biochem. 2017;118(6):1442–1452.
32. Bai R, Guo J, Ye XY, Xie Y, Xie T. Oxidative stress: the core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res Rev. 2022;77:101619.
33. Osama A, Zhang J, Yao J, Yao X, Fang J. Nrf2: a dark horse in Alzheimer’s disease treatment. Ageing Res Rev. 2020;64:101206.
34. Ren P, Chen J, Li B, et al. Nrf2 Ablation Promotes Alzheimer’s Disease-Like Pathology in APP/PS1 Transgenic Mice: the Role of Neuroinflammation and Oxidative Stress. Oxid Med Cell Longev. 2020;2020:3050971.
35. Zgorzynska E, Dziedzic B, Walczewska A. An Overview of the Nrf2/ARE Pathway and Its Role in Neurodegenerative Diseases. Int J Mol Sci. 2021;22(17):54.
36. Han L, Chen A, Liu L, Wang F. Leonurine Preconditioning Attenuates Ischemic Acute Kidney Injury in Rats by Promoting Nrf2 Nuclear Translocation and Suppressing TLR4/NF-kappaB Pathway. Chem Pharm Bull (Tokyo). 2022;70(1):66–73.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.