Back to Journals » OncoTargets and Therapy » Volume 13
Lentivirus-Mediated VEGF Knockdown Suppresses Gastric Cancer Cell Proliferation and Tumor Growth in vitro and in vivo
Authors Park JH, Seo JH, Jeon HY, Seo SM, Lee HK, Park JI, Kim JY , Choi YK
Received 11 October 2019
Accepted for publication 2 February 2020
Published 13 February 2020 Volume 2020:13 Pages 1331—1341
DOI https://doi.org/10.2147/OTT.S234344
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Jong-Hyung Park,1,2 Jin-Hee Seo,3 Hee-Yeon Jeon,1,4 Sun-Min Seo,1 Han-Kyul Lee,1 Jin-Il Park,1,2 Jun-Young Kim,1 Yang-Kyu Choi1
1Department of Laboratory Animal Medicine, College of Veterinary Medicine, Konkuk University, Seoul 05029, Republic of Korea; 2Helixmith Co. Ltd., Seoul 08826, Republic of Korea; 3Laboratory Animal Center, Korea Institute of Radiological and Medical Sciences, Seoul 01812, Republic of Korea; 4Department of Core Research Laboratory, Clinical Research Institute, Kyung Hee University Hospital at Gangdong, Seoul 05278, Republic of Korea
Correspondence: Yang-Kyu Choi
Department of Laboratory Animal Medicine, College of Veterinary Medicine, Konkuk University, 120 Neungdong-ro, Gwangjin-gu, Seoul 05029, Republic of Korea
Tel +82-2-2049-6113
Fax +82-2-450-3037
Email [email protected]
Purpose: Gastric cancer has a high mortality rate worldwide. Although treatments, such as molecular-targeted therapy, have been introduced, the resulting long-term survival and prognosis remain unsatisfactory. Downregulation of the target genes using lentivirus-mediated short hairpin RNA (shRNA) can be an effective therapeutic strategy for patients with gastric cancer. Overexpressed vascular endothelial growth factor A (VEGF) in human gastric cancer cells can be an effective novel therapeutic target for human gastric cancer. Thus, this study aimed to evaluate the therapeutic effects of lentivirus-mediated knockdown of VEGF gene expression in human gastric cancer growth.
Materials and Methods: Specific shRNA sequences targeting VEGF were designed to construct a lentiviral expression vector. After human gastric carcinoma cells (cell line NCI-N87) were infected with the lentiviral vector, the therapeutic effects of the lentivirus-mediated shRNA targeting VEGF were analyzed both in vitro and in vivo.
Results: Stable suppression of VEGF gene expression in NCI-N87 cells using shRNA (ShVEGF) showed significant inhibition of cell proliferation, clonogenicity, and cell motility. ShVEGF also showed increased G0/G1 cell cycle arrest and apoptosis. In addition, in vivo results from nude mice xenografted ShVEGF showed significant inhibition of tumor growth. Assessing the therapeutic effects of intratumoral injection of lentivirus-targeting VEGF (Virus_VEGF) revealed that it significantly inhibited tumor growth compared to that in the Virus_Scramble or saline injection control groups.
Conclusion: The constructed ShVEGF showed significant inhibition of NCI-N87 gastric cancer cell growth both in vitro and in vivo. These experimental results suggest a novel therapeutic strategy for patients with gastric cancer using lentivirus-mediated shRNA targeting VEGF.
Keywords: gastric carcinoma, NCI-N87, ShRNA, VEGF
Introduction
Gastric cancer, one of the most common malignant tumors, is the third leading cause of cancer-related deaths worldwide.1,2 Chemotherapy is the main approach used for treating patients with gastric cancer. Currently, many new anticancer drugs, surgical technique improvements, and immunotherapies, including molecularly targeted therapy, are in use. However, long-term survival and prognosis remain unsatisfactory because most gastric cancers are diagnosed at an advanced stage.3,4 Therefore, improved understanding of tumorigenesis is needed to elucidate a novel treatment that will overcome gastric cancer.
Expanding new blood vessels from the pre-existing vessels is closely related to tumor growth and tumor pathogenesis.5 Vascular endothelial growth factor (VEGF), one of the most studied markers in cancer is a major mediator of tumor angiogenesis.6,7 VEGF also plays important roles in the proliferation, migration, and invasion of vascular endothelial cells.8–13 Accordingly, the inhibition of VEGF signaling could be an innovative strategy for cancer treatment.
RNA interference (RNAi), involving short interfering RNA (siRNA) and short hairpin RNA (shRNA), is an effective gene silencing method and has been introduced as a novel treatment for gastric cancer to inhibit tumor growth and metastasis.14,15 Both siRNA and shRNA may induce sequence-target gene silencing in mammalian cells by transfecting exogenously synthesized hairpins into cells. However, shRNA may suppress gene expression stably in mammalian cells by both exogenous and endogenous expression of shRNA.16 Lentiviral vectors have been widely used due to their ability to infect mammalian cell types and their safety, which has been supported by many clinical results in animal experiments. The first US Food and Drug Administration-approved lentiviral vector-mediated therapy is tisagenlecleucel.17,18 Although there is a theoretical potential for insertional oncogenesis being caused by lentiviral vectors, the lentiviral vector-mediated shRNA system may be effective for cancer treatment.
In this study, we examined the effect of lentivirus-mediated knockdown of VEGF gene expression in human gastric cancer growth in vitro and in vivo. To explore the effect of VEGF gene silencing in cancer progression, third-generation lentiviral vectors were used to integrate the shRNA targeting VEGF (ShRNA_VEGF) or scramble sequence (ShRNA_Scramble) in the NCI-N87 human gastric cancer cell line. We examined the effects of silencing VEGF on gastric cancer cell proliferation, cell cycle, apoptosis, and cell motility in vitro. ShRNA_VEGF-induced NCI-N87 (ShVEGF) xenografts were used to compare tumor growth inhibition with ShRNA_Scramble-induced NCI-N87 (ShScramble) xenografts in vivo. In addition, the therapeutic effects of the intratumoral injection of lentivirus-targeting VEGF (Virus_VEGF) on established tumors were compared to those of the intratumoral injection of saline or lentivirus-targeting scramble (Virus_Scramble).
Materials and Methods
Cell Line and Chemicals
NCI-N87 and MKN-45 gastric carcinoma cell lines were obtained from the Korean Cell Line Bank (Seoul, Korea). Cells were maintained in RPMI 1640 containing 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. The human embryonic kidney cell line 293TN cell line, obtained from System Biosciences (SBI, Mountain View, CA, USA), was maintained in high-glucose Dulbecco’s modified Eagle’s medium containing 10% FBS, GlutaMAX™, and 1% penicillin/streptomycin.
ShRNA-Expressing Plasmid DNA
Four targeted sequences—homologous to VEGFA (GenBank: AY047581.1)—were synthesized, annealed, and cloned in the lentiviral expression vector (pGreenPuro™ Vector, SBI). Target sites in human genes encoding VEGFA were as follows: VEGF_1 sense strand, 5′-AAGATCCGCAGACGTGTAAATGTTC-3′; VEGF_1 antisense strand, 5′-GAACATTTACACGTCTGCGGATCTT-3′; VEGF_2 sense strand, 5′-AAACGAACGTACTTGCAGATGTGAC-3′; VEGF_2 antisense strand, 5′-GTCACATCTGCAAGTACGTTCGTTT-3′; VEGF_3 sense strand, 5′-GGGCAGAATCATCACGAAGT-3′; VEGF_3 antisense strand, 5′-ACTTCGTGATGATTCTGCCC-3′; VEGF_4 sense strand, 5ʹ-ACGAACGTACTTGCAGATGTGA-3ʹ; and VEGF_4 antisense strand, 5ʹ-TCACATCTGCAAGTACGTTCGT-3ʹ. These four plasmid DNAs transcribed shRNA with 12-bp loop sequences of 5′-CTTCCTGTCAGA-3′. The non-targeting sequences (scramble sense strand, 5′-AATTCTCCGAACGTGTCACGT-3′ and scramble antisense strand, 5′-ACGTGACACGTTCGGAGAATT′), which had no homology with the human gene, were also designed using the same process. Each DNA was used to transform E. coli DH5α using the heat shock method, and the transformed E. coli cells were inoculated on an LB-ampicillin agar plate. Bacterial colonies containing the target sequences were isolated and used for plasmid purification. The recombinant vector was extracted using a plasmid purification kit (Qiagen, Valencia, CA, USA). In addition, plasmid extraction was confirmed using polymerase chain reaction (PCR) and sequencing.
Lentivirus Generation and Development of Stably Expressing shRNA in NCI-N87 Cell Line
Lentivirus production and the establishment of a stable cancer cell line expressing shRNA were performed as previously described, with some modification.19 Briefly, cells were seeded at a density of 3 × 106 cells/10 cm dish; 293TN cells were co-transfected with the lentiviral expression vector containing ShRNA_VEGF or ShRNA_Scramble using lipofectamine. After 48 h, supernatants containing lentivirus were collected and mixed with PEG-it™ virus precipitation solution. The mixture containing the supernatants and solution was centrifuged at 1500 × g for 30 min at 4°C, and the pellet was then resuspended in ice-cold RPMI medium. One day prior to cell transduction, the cells were seeded at 105 cells/well in 12-well plates. On the day of transduction, lentiviral particles were added to NCI-N87 cells with 5 ug/mL polybrene. The next day, stable clones were selected by replacing the culture medium with that containing puromycin (3 ug/mL). Transduction efficiency of ShVEGFs or ShScramble with green fluorescent protein signals was determined using flow cytometry.
Relative Quantitative Real-Time PCR
Total RNA extraction of ShVEGFs and ShScramble cells was performed using the TRIzol reagent, according to the manufacturer’s instructions. cDNA was synthesized using Moloney murine leukemia virus (M-MLV) reverse transcriptase (Invitrogen Life Technologies, Merelbeke, Belgium). Quantitative real-time (RT) PCR was performed using a Bio-Rad CFX96 RT-PCR detection system (Bio-Rad, Mississauga, ON, Canada). The sequences of PCR primers used were as follows: for VEGF, forward 5ʹ-AGG GCA GAA TCA CGA AGT-3ʹ and reverse 5ʹ-AGG GTC TCG ATT GGA TGG CA-3ʹ and for GAPDH, forward 5ʹ-GGA GCG AGA TCC CTC CAA AAT-3ʹ and reverse 5ʹ-GGC TGT TGT CAT ACT TCT CAT GG-3ʹ. The relative VEGF mRNA levels were normalized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mRNA levels in the same samples.
Western Blotting
VEGF protein expression was determined using Western blotting. Protein lysates of cell samples (NCI-N87, ShScramble, and ShVEGFs) were separated using 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Proteins were then transferred onto 0.45-µm polyvinylidene fluoride membranes (Millipore, USA). The membrane was incubated with an anti-rabbit anti-VEGF (1:1000, Abcam) or mouse anti-GAPDH (1:1000, Santa-Cruz Biotechnology) antibodies at 4°C overnight. After washing, horseradish peroxidase-conjugated secondary antibodies (anti-rabbit or anti-mouse according to the primary antibody) were used for visualization by chemiluminescence.
Cell Proliferation
The proliferation of cells was determined using Cell Counting Kit-8 (CCK-8, Dojindo Laboratories, Kumamoto, Japan), according to the manufacturer’s protocols. Cells were plated at 5 × 103 cells/well in 96-well plates and treated 24 h after seeding with 10 µL of CCK-8 solution into each well. After 2 h of incubation, the absorbances of the wells were observed daily for 4 days using an enzyme-linked immunosorbent assay (Tecan Sunrise, USA) reader.
Colony Formation Assay
NCI-N87 cells expressing shRNA were plated at a density of 103 cells/well in 6-well plates. Following a 3-week cell culture, formed colonies were fixed with 100% methanol and stained with 1% crystal violet solution. The colonies were counted and then photographed under a microscope. The relative number of colonies in ShVEGF_3 was adjusted to the number of colonies in ShScramble.
Scratch-Wound Healing Assay
Confluent monolayers of ShScramble and ShVEGF_3 cells in 6-well plates were wounded using a pipette tip to determine the role of VEGF in cell migration.20 The cells were cultured for 48 h, and the images of cells were obtained at 0 and 48 h after wounding using the Zeiss Axiovert 200 inverted microscope (Carl Zeiss MicroImaging, Thornwood, NY, USA).
Cell Cycle Analysis and Apoptosis Analysis
Cells were harvested using trypsin-ethylenediaminetetraacetic acid and washed with ice-cold phosphate-buffered saline (PBS). The cell pellet was resuspended in PBS containing 5 µL of 10 mg/mL RNase A and 10 µL of 1 mg/mL propidium iodide (PI). After 1 h of incubation at 37°C in the dark, the percentage of cell populations in different phases of the cell cycle were analyzed using FACSCalibur and CELLQUEST software. For apoptosis analysis, cells from ShVEGF_3 and ShScramble were collected and incubated for 15 min with Annexin-V-APC (Allophycocyanin, BD Biosciences Pharmingen, San Diego, USA) and PI (BD Biosciences Pharmingen) in the dark. After incubation, 400 µL of ice-cold binding buffer was added. The apoptotic cells were quantified using flow cytometry.
Tumorigenicity in Nude Mouse
Animal experiments were approved (KU15139-1) and conducted in accordance with the guidelines of Konkuk University Animal Care and Use Committee (IACUC). Male athymic BALB/c nude mice (5-week-old) were purchased from the Nara Bio animal center (NARA Biotech, Seoul, Korea). All mice were housed in ventilated cages under specific pathogen-free conditions at the laboratory facility of Konkuk University (Seoul, Korea). Mice were fed sterilized food and autoclaved distilled water ad libitum. Tumorigenicity in nude mice was determined as described previously.21 The mice were randomly divided into two groups with 10 mice per group. NCI-N87 cells stably expressing ShScramble or ShVEGF_3 (5 × 106 cells) were subcutaneously injected into the posterior right thigh of mice. Tumor sizes were measured with a Vernier caliper from 9 days to 35 days after tumor injection. Tumor volumes (mm3) were calculated using the following formula: volume (mm3) = (length × width2)/2. On day 35, the mice were euthanized by cervical dislocation.
Intratumoral Injection
To assess whether the generated lentivirus containing the VEGF_3 sequence (Virus_VEGF_3) could efficiently inhibit tumor growth, NCI-N87 cells (3 × 106 cells) in 100 µL saline were subcutaneously injected into the posterior right thighs of 5-week-old male athymic BALB/c male nude mice divided into three groups (n=10 per group). Upon reaching an average volume of 80–100 mm3, mice of three groups were received an intratumoral injection of 50 µL of saline, 5 × 107 of Virus_Scramble, or Virus_VEGF_3. After 1 week, saline, Virus_Scramble, or Virus_VEGF_3 was reinjected in the tumor mass. The in vivo therapeutic experiment was approved by the IACUC of Konkuk University (KU16170).
H&E Staining and TUNEL Staining
Formalin-fixed subcutaneous tumors were processed routinely for paraffin sectioning and cut into 4-µm-thick serial sections. The sections were stained with hematoxylin-eosin (H&E) for histopathological examination. The numbers of mitotic figures were counted in five random high-power fields (×400) per slide. TUNEL staining of the subcutaneous tumors was performed using the In Situ Cell Death Detection Kit (Roche, Basel, Switzerland), according to the manufacturer’s protocol. Deparaffinized 4-µm-thick tumor tissue sections were stained with DAB and counterstained with hematoxylin. Cells stained with brown were considered apoptotic. Images were captured using a DP71 digital camera (Olympus, Tokyo, Japan) using a high-power microscopic field.
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism 5 Software (GraphPad Software, San Diego, USA). Both in vitro and in vivo data are presented as mean ± standard error of the mean (SEM). Student’s t-test was used to assess differences between two groups, and one-way analysis of variance (ANOVA) was used to compare more than two groups. Tumor growth profiles were compared using two-way ANOVA. Differences were considered statistically significant at P < 0.05.
Results
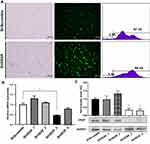
ShRNA-Mediated VEGF Knockdown Led to Reduced mRNA and Protein Levels in NCI-N87 Cells
To achieve a stable reduction in VEGF expression, we transfected NCI-N87 cells with four recombinant shRNA expression plasmids targeting VEGF. Stable NCI-N87 cells were prepared via the infection of lentivirus-carrying ShScramble or ShVEGFs, followed by puromycin selection (3 µg/mL). After continuous culture for 7 days, the transduction efficiency of the established stable transduced NCI-N87 cells was measured using flow cytometry and fluorescence microscopy. The transduction efficiencies of ShScramble and ShVEGF cell lines were 97.15% and 98.45%, respectively, as measured using flow cytometric analysis (Figure 1A). Quantitative RT-PCR and Western blotting were conducted to assess the ability of four different ShVEGFs to silence VEGF expression in vitro. ShVEGF_3 showed significant silencing ability of VEGF mRNA using qRT-PCR (Figure 1B). VEGF protein levels were markedly downregulated by ShVEGF_3 and ShVEGF_4, as determined using Western blotting (Figure 1C). Results of mRNA and protein expression revealed that ShVEGF_3 was the most effective in silencing VEGF. Thus, VEGF_3 was chosen in the following experiments, including in vivo studies. Results revealed no difference in the VEGF mRNA or protein expression level between NCI-N87 cells transfected with ShScramble and NCI-N87 cells.
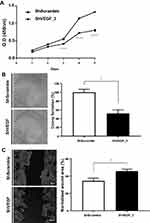
VEGF Downregulation Led to Reduced Proliferation, Colony Formation, and Motility in vitro
To evaluate the inhibitory effect of VEGF downregulation on cancer cell proliferation, the number of ShScramble and ShVEGF_3 cells was measured using the CCK-8 assay. Viability assessment of ShVEGF_3 showed marked inhibition of cell proliferation compared to that of ShScramble cells. Suppression of proliferation by ShVEGF_3 was more dramatic from day 3 (Figure 2A).
To test whether VEGF silencing in NCI-N87 cells affected clonogenic potential—an important characteristic of in vivo tumor formation—we performed a colony formation assay. As shown in Figure 2B, ShVEGF_3 showed decreased colony numbers compared to ShScramble. Compared with ShScramble, the focus number of ShVEGF_3 dropped significantly (48.3%, P < 0.05). These results demonstrated that the knockdown of VEGF expression may prevent cell proliferation and suppress the colony formation ability of NCI-N87 cells.
To examine the invasion ability for ShVEGF_3 compared to that for ShScramble, the scratch-wound healing assay was used. A scratch wound was created across the cell monolayer, and the cellular migration was monitored for 48 h after scratching. ShVEGF_3 showed a larger wound area than ShScramble. The scratch closure areas were 45.7% and 34.5% in ShVEGF_3 and ShScramble, respectively (Figure 2C). The wound area of ShVEGF_3 significantly decreased compared to that of ShScramble (P < 0.05), indicating that ShVEGF_3 cells have impairment in their migration ability.
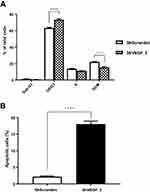
VEGF Silencing Affected Cell Cycle Distribution and Apoptosis in vitro
To identify mechanism responsible for the reduced proliferation of ShVEGF cells, cell cycle distribution of ShVEGF was evaluated using flow cytometry. The distribution of ShVEGF_3 cells in G0/G1 increased by 9.9% compared to that of ShScramble (Figure 3A). This result was accompanied by a reduction of the G2/M phase cells. These results suggested that ShVEGF_3 inhibits cell proliferation by inducing G0/G1 phase arrest. As shown in Figure 3B, the rate of apoptosis in ShVEGF_3 cells increased by 13.7% compared to that in ShScramble cells, indicating that VEGF knockdown reduced apoptotic cell death in NCI-N87 cells.
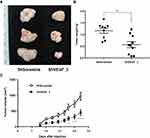
ShVEGF_3 Inhibited Xenograft Tumor Growth in vivo
ShRNA directed against VEGF downregulated VEGF expression and inhibited tumor cell growth in vitro. To further examine the inhibitory effect of tumor growth for ShVEGF_3 in vivo, a mouse xenograft model was used. Since the first tumor appeared on day 11, growth inhibitory effects were observed in the ShVEGF_3 xenografts throughout the entire experiment (Figure 4C). We terminated the tumor growth experiment as soon as the difference in the tumor size between ShVEGF_3 and ShScramble xenografts became evident (P < 0.001; Figure 4A). On the last day, tumor volume was 971.6 ± 393.9 mm3 in the ShVEGF_3 group, whereas the tumor volume in the ShVEGF_3 group was 449.5 ± 299.9 mm3. The average tumor weight was significantly (P < 0.01) higher in the ShVEGF_3 group than in the ShScramble group (Figure 4B; ShVEGF_3, 0.6 ± 0.4 g; ShScramble, 1.1 ± 0.4 g). These data showed the antitumor effect of ShVEGF_3 in NCI-N87 gastric cancer cells in nude mouse xenografts.
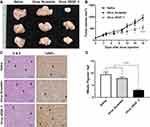
Intratumoral Injection of Virus_VEGF Attenuated the Established Tumor Growth
To assess the therapeutic effect of lentivirus-targeting VEGF, saline, Virus_Scramble, or Virus_VEGF_3 was injected into NCI-N87-derived tumors. The injection of Virus_VEGF_3 subsequently attenuated tumor growth from day 12 compared to the injection of Virus_Scramble (Figure 5B). At the endpoint of the experiment, the tumor in the Virus_VEGF_3 group showed decreased size compared to that in the Virus_Scramble group (Figure 5A). H&E and TUNEL staining of tumor sections excised from the mice was conducted to determine the biological effects of Virus_VEGF_3. To assess cell proliferation and apoptosis, tumor sections excised from the mice were stained with H&E and TUNEL, respectively. Intratumoral injection of Virus_VEGF_3 showed a significant (P < 0.001) decrease in the mitotic figure number compared to the injection of saline or Virus_Scramble and showed an increased number of apoptotic cells compared to the saline and Virus_Scramble injection control groups (Figure 5C and D). Together with the results for the ShVEGF_3 xenograft mice, these results indicated that Virus_VEGF_3 attenuated cancer cell proliferation and increased apoptotic effect, thereby implying its therapeutic effect on human gastric cancer.
Discussion
VEGF is a well-known angiogenetic factor that helps tumor growth and metastasis.22 In gastric and all the other cancers, VEGF overexpression is closely related to the clinical stage, local tumor extent, metastasis to other organs, and recurrence of gastric carcinoma.23–25 Many studies have indicated that VEGF downregulation causes cancer cell apoptosis and inhibits cancer growth and angiogenesis.5,26
Since the RNAi mechanism was discovered, RNAi technology has been used both in vitro and in vivo because of its diverse advantages, such as gene targeting, potencies for mRNA inhibition, chemical modifications, modifying target-specific sequence design without off-target effects, and compromising potency.27–30 siRNAs, which have a length of ~21 nucleotides, served as effector molecules of sequence-specific gene knockdown.31 An effective delivery strategy of siRNA is critical for successful RNAi application.32 In this study, lentiviral vectors for the delivery of shRNA were used to suppress human gastric cancer. Because lentiviral vectors can transduce not only dividing cells but also non-dividing cells and are less likely to cause transgene silencing, they offer advantages for gene therapeutic applications, pharmaceutical target validation, and functional genomics.33,34
Here, candidate sequences were generated for silencing the protein expression of VEGF; however, no changes in the cell proliferation of MKN-45 cells were observed in our experiment (data not shown). The reason why knockdown of target protein expression by shRNA did not occur in transduced cells has remained unclear.35 Presumably, differences in protein expression may due to several reasons such as the promoter activity differences, regions of integration site, or multiple numbers of integrations in the clone.35–37 This is because these factors influence the expression levels of shRNA; therefore, multiple sequences of shRNA should be designed for an effective gene downregulation. Thus, lentivirus-mediated shRNA targeting VEGF, which showed efficient silencing of both mRNA and protein expression, was chosen to study in vivo tumorigenicity.
In this study, ShVEGF_3 showed stable integration into the cellular genome via a lentivirus vector and showed significant inhibition of VEGF protein expression. VEGF downregulation inhibits NCI-N87 cell proliferation, colony formation, and cell motility. In addition, VEGF silencing affects the cell cycle and apoptosis; ShVEGF_3 significantly induced apoptosis and G0/G1 phase arrest. As our previous study indicated, G0/G1 arrest induced DNA repair or apoptosis in cancer cells; the G0/G1 arrest maintained homeostasis of the normal tissue stages and removed mutated or hyperproliferating neoplastic cells.19 These in vitro results showed that the anticancer effect of ShVEGF_3 was due to the induction of cell cycle arrest and apoptosis. Subsequently, in vivo experiments were completed to analyze lentivirus-mediated ShVEGF_3 as a potential candidate for gastric cancer. Lentivirus expressing ShVEGF_3 was intratumorally injected into established tumors. The growth of established tumors from NCI-N87 cells was effectively suppressed by the intratumoral injection of the recombinant lentivirus. These data demonstrate that VEGF downregulation using lentivirus-mediated shRNA can be an effective therapeutic strategy for gastric cancer.
These data were also supported by mitotic figure count in the H&E slides of mouse xenograft. Mitotic figure is a marker of cell proliferation. The observed mitotic figure count thus suggested that VEGF silencing inhibited cancer cell growth in vivo. The mitotic figure count is associated with VEGF, and the results are consistent with in vitro results performed in this study.38 There are several reasons why the intratumoral injection of Virus_VEGF may have diverse advantageous effects. Targeting VEGF, a key regulator of angiogenesis, may induce apoptosis in several cancer cells.39,40 Cancer-stromal cell interaction affects tumor growth and metastasis in most solid tumors, including gastric cancer. The stroma constitutes a large portion of the gastric cancer, and together with tumor cells, stromal cells produce various angiogenic factors, such as VEGF, interleukin-8, and platelet-derived endothelial cell growth factor.41,42
The intratumoral injection of Virus_VEGF to established gastric tumors would result in VEGF downregulation not only in cancer cells but also in stromal cells. Thus, the intratumoral injection of Virus_VEGF effectively suppressed gastric cancer growth by simultaneously blocking cancer cell and non-cancer cell growth. These results suggest that the lentivirus-mediated intratumoral injection of ShVEGF_3 can be an efficient treatment for gastric cancer. But some limitations including off-target effect or side-effect of lentivirus-mediated shRNA in nude mouse exist in this study.
Conclusion
Lentivirus-mediated shRNA targeting VEGF efficiently inhibited gastric cancer growth both in vitro and in vivo. In addition, the intratumoral injection of Virus_VEGF exhibited an inhibitory effect on tumor growth. Although there are some limitations to the suggested therapy, such as high immunogenicity, lack of tumor-specific targeting, and potential of proto-oncogenic activation, the results of this study support the safety and therapeutic potency of lentivirus-mediated shRNA targeting VEGF and its applicability in treating patients with gastric cancer.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning (2013R1A2A2A01067900).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi:10.3322/caac.21332
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi:10.3322/caac.v68.6
3. Qing Y, Li Q, Ren T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther. 2015;9:901–909. doi:10.2147/DDDT.S75152
4. Xu Y, Yu X, Wei C, Nie F, Huang M, Sun M. Over-expression of oncigenic pseudogene DUXAP10 promotes cell proliferation and invasion by regulating LATS1 and β-catenin in gastric cancer. J Exp Clin Cancer Res. 2018;37(1):13. doi:10.1186/s13046-018-0684-8
5. Chen H, Li L, Wang S, et al. Reduced miR-126 expression facilitates angiogenesis of gastric cancer through its regulation on VEGF-A. Oncotarget. 2014;5(23):11873–11875. doi:10.18632/oncotarget.2662
6. Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049.
7. McMahon G. VEGF receptor signaling in tumor angiogenesis. Oncologist. 2000;5(Suppl 1):3–10. doi:10.1634/theoncologist.5-suppl_1-3
8. Yu P, Liu Q, Liu K, Yagasaki K, Wu E, Zhang G. Matrine suppresses breast cancer cell proliferation and invasion via VEGF-Akt-NF-κB signaling. Cytotechnology. 2009;59:219–229. doi:10.1007/s10616-009-9225-9
9. Lin -S-S, Lai K-C, Hsu S-C, et al. Curcumin inhibits the migration and invasion of human A549 lung cancer cells through the inhibition of matrix metalloproteinase-2 and-9 and Vascular Endothelial Growth Factor (VEGF). Cancer Lett. 2009;285:127–133. doi:10.1016/j.canlet.2009.04.037
10. Zhu B-H, Zhan W-H, Li Z-R, et al. (-)-Epigallocatechin-3-gallate inhibits growth of gastric cancer by reducing VEGF production and angiogenesis. World J Gastroenterol. 2007;13:1162–1169. doi:10.3748/wjg.v13.i8.1162
11. Kim K, Song H, Kim TS, et al. Interleukin-18 is a critical factor for vascular endothelial growth factor-enhanced migration in human gastric cancer cell lines. Oncogene. 2007;26:1468–1476. doi:10.1038/sj.onc.1209926
12. Zhao R, Liu X-Q, Wu X-P, et al. Vascular endothelial growth factor (VEGF) enhances gastric carcinoma invasiveness via integrin alpha (v) beta6. Cancer Lett. 2010;287:150–156. doi:10.1016/j.canlet.2009.06.006
13. Suboj P, Babykutty S, Gopi DRV, Nair RS, Srinivas P, Gopala S. Aloe emodin inhibits colon cancer cell migration/angiogenesis by downregulating MMP-2/9, RhoB and VEGF via reduced DNA binding activity of NF-κB. Eur J Pharm Sci. 2012;45:581–591. doi:10.1016/j.ejps.2011.12.012
14. Gong M, Lu Z, Fang G, Bi J, Xue X. A small interfering RNA targeting osteopontin as gastric cancer therapeutics. Cancer Lett. 2008;272:148–159. doi:10.1016/j.canlet.2008.07.004
15. Tu SP, Chi AL, Ai W, et al. p53 inhibition of AP1-dependent TFF2 expression induces apoptosis and inhibits cell migration in gastric cancer cells. Am J Physiol Gastrointest Liver Physiol. 2009;297:G385–396. doi:10.1152/ajpgi.90620.2008
16. Paddison PJ, Caudy AA, Bernstein E, Hannon GJ, Conklin DS. Short hairpin RNAs (shRNAs) induce sequence-specific silencing in mammalian cells. Genes Dev. 2002;16:948–958. doi:10.1101/gad.981002
17. Milone MC, O’Doherty U. Clinical use of lentiviral vectors. Leukemia. 2018;32:1529–1541. doi:10.1038/s41375-018-0106-0
18. Vairy S, Garcia JL, Teira P, Bittencourt H. CTL019 (tisagenlecleucel): CAR-T therapy for relapsed and refractory B-cell acute lymphoblastic leukemia. Drug Des Devel Ther. 2018;12:3885–3898. doi:10.2147/DDDT
19. Seo JH, Jeong ES, Choi YK. Therapeutic effects of lentivirus-mediated shRNA targeting of cyclin D1 in human gastric cancer. BMC Cancer. 2014;14:175. doi:10.1186/1471-2407-14-175
20. Li C-L, Nie H, Wang M, et al. microRNA-155 is downregulated in gastric cancer cells and involved in cell metastasis. Oncol Rep. 2012;27:1960–1966. doi:10.3892/or.2012.1719
21. Singh SV, Mohan RR, Agarwal R, et al. Novel anti-carcinogenic activity of an organosulfide from garlic: inhibition of H-RAS oncogene transformed tumor growth in vivo by diallyl disulfide is associated with inhibition of p21H-ras processing. Biochem Biophys Res Commun. 1996;225:660–665. doi:10.1006/bbrc.1996.1226
22. Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9(6):653–660. doi:10.1038/nm0603-653
23. Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. Circulating VEGF levels in the serum of gastric cancer patients: correlation with pathological variables, patient survival, and tumor surgery. Ann Surg. 2002;236:37–42. doi:10.1097/00000658-200207000-00007
24. Tao H-Q, Lin Y-Z, Wang R-N. Significance of vascular endothelial growth factor messenger RNA expression in gastric cancer. World J Gastroenterol. 1998;4:10–13. doi:10.3748/wjg.v4.i1.10
25. Konno H, Baba M, Tanaka T, et al. Overexpression of vascular endothelial growth factor is responsible for the hematogenous recurrence of early-stage gastric carcinoma. Eur Surg Res. 2000;32:177–181. doi:10.1159/000008760
26. Kasahara Y, Tuder RM, Taraseviciene-Stewart L, et al. Inhibition of VEGF receptors causes lung cell apoptosis and emphysema. J Clin Invest. 2000;106:1311–1319. doi:10.1172/JCI10259
27. Sledz CA, Williams BR. RNA interference in biology and disease. Blood. 2005;106:787–794. doi:10.1182/blood-2004-12-4643
28. Behlke MA. Chemical modification of siRNAs for in vivo use. Oligonucleotides. 2008;18:305–319. doi:10.1089/oli.2008.0164
29. Rettig GR, Behlke MA. Progress toward in vivo use of siRNAs-II. Mol Ther. 2012;20(3):483–512. doi:10.1038/mt.2011.263
30. Zuckerman JE, Davis ME. Clinical experiences with systemically administered siRNA-based therapeutics in cancer. Nat Rev Drug Discov. 2015;14(12):843–856. doi:10.1038/nrd4685
31. Kim DH, Rossi JJ. Strategies for silencing human disease using RNA interference. Nat Rev Genet. 2007;8:173–184. doi:10.1038/nrg2006
32. Janas J, Skowronski J, Van Aelst L. Lentiviral delivery of RNAi in hippocampal neurons. Methods Enzymol. 2006;406:593–605.
33. Van den Haute C, Eggermont K, Nuttin B, Debyser Z, Baekelandt V. Lentiviral vector-mediated delivery of short hairpin RNA results in persistent knockdown of gene expression in mouse brain. Hum Gene Ther. 2003;14:1799–1807. doi:10.1089/104303403322611809
34. Anderson J, Akkina R. HIV-1 resistance conferred by siRNA cosuppression of CXCR4 and CCR5 coreceptors by a bispecific lentiviral vector. AIDS Res Ther. 2005;2(1):1. doi:10.1186/1742-6405-2-1
35. An DS, Qin -FX-F, Auyeung VC, et al. Optimization and functional effects of stable short hairpin RNA expression in primary human lymphocytes via lentiviral vectors. Mol Ther. 2006;14(4):494–504. doi:10.1016/j.ymthe.2006.05.015
36. Qin X-F, An DS, Chen IS, Baltimore D. Inhibiting HIV-1 infection in human T cells by lentiviral-mediated delivery of small interfering RNA against CCR5. Proc Natl Acad Sci USA. 2003;100:183–188. doi:10.1073/pnas.232688199
37. Mittal V. Improving the efficiency of RNA interference in mammals. Nat Rev Genet. 2004;5:355–365. doi:10.1038/nrg1323
38. Li X, Gao Y, Li J, et al. FOXP3 inhibits angiogenesis by downregulating VEGF in breast cancer. Cell Death Dis. 2018;9:744. doi:10.1038/s41419-018-0790-8
39. Chung DC, Le Thanh Long HNS, Le Tri Bao DMS. Downregulation of vascular endothelial growth factor enhanced chemosensitivity by induction of apoptosis in hepatocellular carcinoma cells. Cell J. 2015;17:273–287. doi:10.22074/cellj.2016.3730
40. Khromova N, Kopnin P, Rybko V, Kopnin B. Downregulation of VEGF-C expression in lung and colon cancer cells decelerates tumor growth and inhibits metastasis via multiple mechanisms. Oncogene. 2012;31:1389–1397. doi:10.1038/onc.2011.330
41. Ferrara N, Hillan KJ, Gerber H-P, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi:10.1038/nrd1381
42. Kitadai Y. Cancer-stromal cell interaction and tumor angiogenesis in gastric cancer. Cancer Microenviron. 2010;3:109–116. doi:10.1007/s12307-009-0032-9
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.