Back to Journals » Journal of Inflammation Research » Volume 15
Lenalidomide Improves Cognitive Function and Reduces Immune Reconstitution Inflammatory Syndrome in HIV-1-Related Cryptococcal Meningitis
Authors Tao R, Peng X, Liu X, Su J, Lang G, Huang Y, Zhang Y, Zhu B
Received 22 December 2021
Accepted for publication 27 April 2022
Published 10 May 2022 Volume 2022:15 Pages 2891—2899
DOI https://doi.org/10.2147/JIR.S353463
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Ning Quan
Ran Tao,1 Xiaorong Peng,1 Xiang Liu,1 Junwei Su,1 Guanjing Lang,1 Ying Huang,1 Yafei Zhang,2 Biao Zhu1
1The Department of Infectious Diseases, State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China; 2PET Center, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China
Correspondence: Biao Zhu, Tel +0086-571-87236417, Fax +0086-571-87236416, Email [email protected]
Abstract: Cryptococcal meningitis (CM) is a common opportunistic infection in patients with acquired immune deficiency syndrome. Although there is a standardized treatment for CM, some patients still have CM-associated immune reconstitution inflammatory syndrome (IRIS) after anti-cryptococcal and antiretroviral therapy, which manifests as cognitive impairment. We report two cases of CM-associated IRIS in human immunodeficiency virus (HIV) patients that were treated with lenalidomide. The treatment yielded a rapid clinical remission and improved cognitive function in both patients; their Montreal Cognitive Assessment (MoCA) and International HIV Dementia Scale (IHDS) scores improved. Furthermore, we evaluated changes in 32 cytokines in the cerebrospinal fluid of two patients and found that both MoCA and IHDS were significantly negatively correlated with inflammation-related factors (growth-related oncogene, interleukin [IL]-10, IL-2, IL-8, macrophage inflammatory protein-1β, tumor necrosis factor [TNF]-α) and significantly positively correlated with dementia-related factors (αβ 42 and total tau). Our study reveals the potential of lenalidomide in treating cognitive impairment caused by immune-mediated inflammation in patients with HIV-CM. Moreover, we speculate that lenalidomide improves cognitive function by regulating intracranial inflammation via multiple pathways, not only by TNF-α blocking.
Keywords: acquired immune deficiency syndrome, Cryptococcus, immunomodulator, cognition, immune reconstitution inflammatory syndrome
Introduction
Cryptococcal meningitis (CM) is a common opportunistic infection in patients with acquired immune deficiency syndrome (AIDS) and is a common cause of death. Although there is a standardized treatment for CM, some patients still have immune reconstitution inflammatory syndrome (IRIS) after anti-cryptococcal and antiretroviral therapy (ART) initiation, which clinically manifests as cognitive impairment.1 The damage from a Cryptococcus infection may come from the pathogen’s toxins or immune-mediated destruction of the invading pathogens by the body. This phenomenon can occur in patients with or without human immunodeficiency virus (HIV).2 The treatment for immune-mediated damage is limited and comprises mainly corticosteroids, whose side effects are not negligible. Lenalidomide is a new immunomodulator, which is reported to be effective in the treatment of HIV-related disease.3–5 Here we report two cases of HIV-associated CM (HIV-CM) who were treated with lenalidomide. The patients experienced rapid clinical remission, and their cognitive function was improved by the treatment.
This study was conducted in compliance with the Declaration of Helsinki and approved by the institutional review board of the First Affiliated Hospital, College of Medicine, Zhejiang University (Reference Number: 2,020,265). The institution had approved to publish the case details. Written informed consent was provided by the patients for the use of their clinical information and the publishing of any accompanying images. This study was registered in the Chinese Clinical Trial Registry (Registration Number: ChiCTR1900023184).
Case Description
Case 1
A 32-year-old male who presented with headache, dizziness, nausea, and vomiting was admitted to local hospital in October 2018. Two days later, he was diagnosed with HIV. He had a history of male sexual partners. His CD4 cell count and plasma HIV load were 15 cells/μL and 14,900 copies/mL, respectively. On physical examination, he had a body temperature (T) of 37.3°C, blood pressure (BP) of 113/50 mmHg, pulse (P) of 105 beats per minute (bpm), and respiration rate (R) of 20 breaths/min. Moreover, he had a stiff neck, positive meningeal irritation sign, and oral candidiasis. A lumbar puncture and cerebrospinal fluid (CSF) analysis was performed; he had an intracranial pressure (ICP) of >400 mmH2O, a CSF white blood cell (WBC) count of 50 cells/μL, neutrophils of 14%, glucose of 1.4 mmol/L, protein of 0.56 g/L, chlorine of 116 mmol/L, and CSF: serum glucose ratio of 0.33. India ink staining of the CSF revealed Cryptococcus, and CSF culture showed Cryptococcus neoformans. Brain imaging of computed tomography (CT) was unremarkable. Based on these findings, he was diagnosed with CM. Ventriculoperitoneal (VP) shunting to release the increased intracranial pressure was performed one week after his admission. He received antifungal therapy for 2 weeks (amphotericin B [0.7 mg/kg/day] and 5-flucytosine [100 mg/kg/day]), followed by consolidation with fluconazole (400 mg/day) for 8 weeks. After 14 days of antifungal therapy, the culture for Cryptococcus was negative. On day 28 of admission, highly active ART (HAART; lamivudine+ Tenofovir disoproxil fumarate +lopinavir/ritonavir) was initiated, and he was discharged from the hospital. After the discharge, the patient was followed up regularly and continued anti-Cryptococcus and ART treatment without obvious symptoms.
From August 2019 to March 2020, the patient was hospitalized in the local hospital due to repeated headaches, fever, and convulsions. His CD4 count gradually increased to 131 cells/μL, the HIV viral load decreased to undetectable level, ICP was between 150 and 220 mmH2O, CSF WBC count was 15–50 cells/μL, CSF glucose was between 2.2 and 2.3 mmol/L, and CSF: serum glucose ratio was between 0.43 and 0.53. The CSF protein increased up to 12.625 g/L at October 2019, no Cryptococcus was found in India ink staining, the CSF Cryptococcus antigen was positive, and Cryptococcus culture was negative. He was treated at local hospitals with dexamethasone of 5–10 mg QD for 10 days, and the dosage was then reduced gradually. The same anti-Cryptococcus and ART treatment were continued. The patient’s headache improved, and he was discharged from the hospital with methylprednisolone (4 mg/day) for 2 weeks.
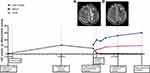
In May 2020, although an undetectable plasma viral load and CD4 count of 122 cells/μL, the patient’s reactions were more sluggish at our hospital (the First Affiliated Hospital of Zhejiang University, China). On physical examination, he had a T of 36.9°C, BP of 111/79 mmHg, P of 108 bpm, and R of 18 breaths/min. He was well oriented, responded to questions slowly, and had a negative meningeal irritation sign. CSF analysis revealed an opening pressure of 150 mmH2O, WBC count of 120 cells/μL, glucose of 2.1 mmol/L, total protein level of 7.89 g/L, and CSF: serum glucose ratio was 0.41. Furthermore, no Cryptococcus was found in India ink staining, the CSF Cryptococcus antigen was positive, and Cryptococcus culture was negative. Culture and polymerase chain reaction testing for Epstein-Barr virus, cytomegalovirus, and herpes simplex virus-1 and −2 were negative. A T-SPOT test for Mycobacterium tuberculosis in blood was negative. Further, acid-fast staining of a sputum smear and the CSF culture for bacteria and fungi were negative. However, multiple intracranial lesions with meningeal enhancement were observed in brain magnetic resonance imaging (MRI) (Figure 1A).
On admission, the patient’s Montreal Cognitive Assessment (MoCA; Beijing version; cut-off score, 26)6 and International HIV Dementia Scale (IHDS; cut-off score, 10)7 scores were 13 and 2.5, respectively. Although he was on standard anti-cryptococcal treatment and HAART, he had aseptic meningitis with cognitive impairment; he was diagnosed with paradoxical IRIS. After providing written informed consent, he received oral lenalidomide (one cycle: 25 mg/day for 21 days and no lenalidomide for 7 days according to the manufacturer’s instructions) simultaneously with fluconazole and HAART. The patient was followed up at 0, 1, 2, 3, 6, and 12 cycles after lenalidomide treatment initiation. Blood tests (blood routine, liver function, kidney function, blood lipid, serum glucose, electrolyte, coagulation function, CD4 count, HIV viral load) and CSF examinations (CSF routine, biochemistry, culture, India ink staining, Cryptococcus antigen detection, Cryptococcus culture) were performed during each follow-up. Neuroimaging and cognitive evaluations were performed at baseline,1, 2, 6, and 12 cycles. Plasma and CSF samples were stored at −80°C for testing.
One cycle after this treatment, his headache was relieved, his cognitive function had recovered rapidly (MoCA and IHDS scores increased to 20 and 6.5, respectively), and he was able to communicate normally. CSF testing revealed a WBC count of 50 cells/μL, glucose of 1.9 mmol/L, CSF: serum glucose was 0.42 and total protein level of 1.64 g/L, which suggested an inflammation. Furthermore, CSF cultures were negative. After 6 cycles of lenalidomide therapy, his cognitive status returned to normal (MoCA and IHDS scores increased to 26 and 11, respectively). The CSF WBC count (14 cells/μL) and total protein levels (0.55 g/L) were reduced, and CSF cultures were negative. T2-weighted MRI revealed a significant reduction in the signal in the left frontal lobe lesion (Figure 1B). Lenalidomide treatment was terminated after 12 cycles, and the patient was followed up for 18 months. There were no abnormalities in the routine blood tests, liver and kidney function tests, and myocardial zymogram. As of the time of this report, he has remained asymptomatic and has resumed his normal duties.
Case 2
In June 2018, a 39-year-old male, one day after the diagnosis of HIV infection, was admitted to our hospital for dizziness and vomiting, which had been ongoing for two weeks prior to his admission. On physical examination, his T was 37.5°C, BP 119/92 mmHg, P 112 bpm, and R 20 breaths/min. Additionally, he had a stiff neck, positive meningeal irritation sign. He had a CD4 cell count and plasma HIV load of 7 cells/μL and 131,000 copies/mL, respectively. CSF analysis revealed a pressure of >400 mmH2O, WBC count of 3 cells/μL, glucose of 2.3 mmol/L, chlorine of 121 mmol/L, and protein of 0.426 g/L, CSF: serum glucose ratio was 0.41. CSF India ink staining revealed Cryptococcus, and CSF culture showed Cryptococcus neoformans. Based on these findings, he was diagnosed with CM. The dizziness and vomiting disappeared after a VP shunt was placed. Antifungal treatment was administered similarly to Case 1 (for 2 weeks; amphotericin B [0.7 mg/kg/day] and 5-flucytosine [100 mg/kg/day], followed by consolidation with fluconazole [400 mg/day]) for 8 weeks. CSF cultures were negative after 10 days. HAART (Tenofovir disoproxil fumarate + Lamivudine + Dolutegravir) was initiated on day 28 of the admission, and he was discharged from the hospital. The patient had visited the hospital regularly for follow-ups. By March 2019, the patient did not feel uncomfortable, ICP was 115–200 mmH2O, CSF protein was 1.06–2.53 g/L, CSF WBC was 5–10 cells/μL, CSF glucose was 2.0–2.8 mmol/L, CSF: serum glucose ratio was 0.46–0.58, CD4 count increased to 82 cells/μL, and the HIV viral load was 0 copies/mL.
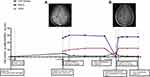
However, in April 2019, the patient developed headache and dizziness. One month later, he developed sluggishness. The patient felt that he could endure the headache and did not come to the hospital until June 2019. He was re-admitted with an undetectable viral load and CD4 cell count of 132 cells/μL. On physical examination, he had a T of 38.2°C, P of 92 bpm, BP of 112/74 mmHg, and R of 18 breaths/min. He was conscious and responded slowly. The ICP was 150 mmH2O, CSF protein was 4.67 g/L, CSF WBC was 68 cells/μL, CSF glucose was 1.8 mmol/L, and CSF: serum glucose ratio was 0.38. Etiological investigations revealed an unremarkable CSF analysis. He exhibited a slight decrease in cognitive ability (MoCA score, 26; IHDS score, 8). Brain MRI revealed multiple regions of thickening and meningeal enhancement (Figure 2A). Based on these findings, he was diagnosed with IRIS. The patient responded well to lenalidomide (regimen as in Case 1; one cycle: 25 mg/day for 21 days and no lenalidomide for 7 days according to the manufacturer’s instructions), and after one cycle of treatment, he was able to communicate normally. CSF examination revealed the CSF WBC count of 2 cells/μL, CSF glucose of 2.2 mmol/L, CSF: serum glucose ratio was 0.49, CSF protein of 4.08 g/L, and negative CSF cultures. He had a MoCA score of 30 and an IHDS score of 10 after two cycle of treatment. The follow-up plan of the patient was the same as that of Case 1. There was no discomfort during the follow-up after 6 cycles of treatment. The ICP was 150 mmH2O, CSF protein was 1.24 g/L, CSF WBC was 2.0 cells/μL, CSF glucose was 2.5 mmol/L, CSF: serum glucose ratio was 0.53, CD4 count was 111 cells/μL, and HIV viral load was undetectable at 6 cycles of treatment. The patient stopped the treatment in April 2020 after 10 cycles at his discretion. One month later, his CSF analysis results were suggestive of inflammation (CSF WBC count of 5 cells/μL, CSF glucose of 2.1 mmol/L, CSF: serum glucose ratio was 0.41, CSF protein of 1.16 g/L, and negative CSF cultures). However, his cognitive status remained normal (MoCA and IHDS scores increased to 29 and 12, respectively.)
In July 2020, three months after the termination of lenalidomide treatment, the patient developed involuntary right limb tremors and expressive aphasia. On physical examination, he was conscious, mute, and negative for meningeal irritation sign. The patient’s MoCA and IHDS scores were both zero. His CSF analysis results were suggestive of inflammation (CSF pressure of 140 mmH2O, CSF WBC of 20 cells/μL, CSF glucose of 2.6 mmol/L, CSF protein of 5.19 g/L, and CSF: serum glucose ratio was 0.45); however, India ink staining yielded negative results, and the etiological investigation was negative. Consequently, paradoxical IRIS was diagnosed. Lenalidomide (25 mg/day) was re-introduced with levetiracetam (500 mg BID). The patient responded rapidly to treatment; 1 cycle following lenalidomide re-introduction, he was communicating well and doing his routine activities unaided (CSF pressure of 175 mm H2O, CSF WBC of 30/μL, CSF glucose of 2.5 mmol/L, CSF protein of 2.0 g/L, CSF: serum glucose ratio was 0.53, MoCA score of 27, and IHDS score of 12). Brain MRI revealed a significantly reduced degree of meningeal enhancement (Figure 2B). After 6 cycles of repeat lenalidomide treatment, his CSF analysis results were normal (CSF pressure of 120 mmH2O, CSF WBC of 10 cells/μL, CSF glucose of 2.4 mmol/L, CSF protein of 0.9 g/L, CSF: serum glucose was 0.51, and negative India ink staining); therefore, lenalidomide treatment was discontinued. At the time of this report, the patient has been followed up for 27 months and has returned to work.
Correlation Between Psychocognitive Assessment Scores and Cerebrospinal Fluid Parameters
We analyzed the correlation between MoCA and IHDS scores with CSF parameters (WBC, glucose, chlorine, protein, albumin, and immunoglobulin G [IgG]) in both patients through a linear regression analysis. MoCA and IHDS had a negatively correlated trend with CSF protein (Case 1: MoCA: R2=0.66, P = 0.093; IHDS: R2=0.79, P = 0.044; Case 2: MoCA: R2=0.43, P = 0.039; IHDS: R2=0.71, P = 0.002) and CSF IgG (Case 1: MoCA: R2=0.66, P = 0.096; IHDS: R2=0.77, P = 0.050; Case 2: MoCA: R2=0.53, P = 0.017; IHDS: R2=0.81, P = 0.0004) (Figure 3).
Thirty-two CSF cytokines were analyzed using the Human Cytokine/Chemokine/Growth Factor Panel A (Merck Millipore, St. Louis, USA): α-2-macroglobulin, apolipoprotein A-I (Apo-AI), Apolipoprotein E (Apo-E), Complement C3, Complement Factor H, fibroblast growth factor 2 (FGF-2), granulocyte-colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), interferon-γ (IFN-γ), growth-regulated oncogene protein (GRO), soluble CD40 ligand (sCD40L), platelet-derived growth factor-AA (PDGF-AA), interleukin (IL)-12p40, IL-12p70, IL-13, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-6, IL-8, IL-10, IL-17α, monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein-1α (MIP-1α), MIP-1β, tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), amyloid-β (1–42) (αβ42), total Tau (tTau), and phosphorylated tau 181 (pTau181).
Before lenalidomide treatment, the CSF cytokines of the two patients had a similar Th1 profile: TNF-α, G-CSF, and IL-6 increased significantly above the normal value, while the Th2 profile (IL-2, IL-3, IL-4, IL-13) was low. In both cases, the cytokine levels that were related to neuroinflammation cognition (α-2-macroglobulin, Apo-AI, Complement C3, Complement Factor H, G-CSF, IL-10, GRO, IL-6, IL-8, and TNF-α) significantly decreased to less than 20% of pre-treatment levels. Among them, levels of α-2-macroglobulin, G-CSF, IL-17α, and IL-6 rebounded more than five times after drug withdrawal in Case 2. Spearman correlation test was used to retrospectively analyze the correlation of MoCA and IHDS scores with these cytokine levels. There was a significant negative correlation between both MoCA and IHDS with the following inflammation factors: MoCA: GRO (R2=−0.748, P = 0.005), IL-10 (R2=−0.625, P = 0.03), IL-2 (R2=−0.648, P = 0.023), IL-8 (R2=−0.776, P = 0.003), MIP-1β (R2=−0.660, P = 0.02), TNF-α (R2=−0.687, P = 0.014); IHDS: GRO (R2=−0.827, P = 0.001), IL-10 (R2=−0.789, P = 0.002), IL-2 (R2=−0.688, P = 0.013), IL-8 (R2=−0.870, P < 0.001), MIP-1β (R2=−0.732, P = 0.007), TNF-α (R2=−0.766, P = 0.004). Furthermore, there was a significant positive correlation of MoCA and IHDS with dementia-related factors (MoCA: αβ42 [R2=0.684, P = 0.014] and tTau [R2=0.628, P = 0.029]; IHDS: αβ42 [R2=0.609, P = 0.036] and tTau [R2=0.624, P = 0.03]).
Discussion
To our knowledge, this is the first report on lenalidomide for treating cognitive impairment caused by immune-mediated inflammation in patients with HIV-CM. The possible mechanism was preliminarily explored by analyzing the cytokines in CSF. Based on our results, we speculate that the cognitive impairment of the patients was related to immune-mediated intracranial inflammation, such as IRIS. Lenalidomide can regulate intracranial inflammation via multiple pathways including TNF-α blocking, which can reduce the recruitment of leukocytes and alleviate tissue damage,8,9 leading to improved cognitive and motor function.
The patients did not have obvious symptoms of intracranial hypertension after IRIS, which was mainly due to the optimal control of intracranial pressure by the VP shunt. The main clinical manifestation of our two patients was cognitive impairment, which is a common neurological symptom in patients with AIDS. These patients suffer from cognitive impairment from four mechanisms: direct effects of HIV,10,11 opportunistic infection, adverse effects of ART, and IRIS.12 First, HIV can cause neuronal damage and cognitive decline directly,13 such as HIV-associated neurocognitive disorders (HAND), especially in people with advanced stages of AIDS and low CD4 count. ART is an effective treatment for AIDS-related cognitive impairment;10 however, our patients did not improve but developed severe cognitive impairment after the ART initiation, which was not one of the HAND. Second, opportunistic infections in the central nervous system, such as cryptococcal infection, tuberculosis, and viral infection, can cause cognitive impairment. In such cases, an effective treatment of pathogens improves cognition. However, our two patients had aseptic meningitis when they had cognitive impairment, and no suspected pathogen infection was found. Third, our patients received oral protease and integrase inhibitors. The toxic side effects on the nerves are significantly lower than those of efavirenz, and the antiviral regimen was not changed during the whole treatment process; this could not explain the cognitive impairment. Fourth, the abnormal immune response after ART initiation may cause further damage,12,14 such as IRIS. Both of our patients had cognitive impairment after the successful clearance of Cryptococcus and ART initiation. CSF analysis findings were recommended for intracranial inflammation, but no infectious etiology was found. MoCA and IHDS scores were also found to have a significant negative correlation trend with CSF protein and CSF IgG levels, which are associated with intracranial inflammation. Therefore, we speculate that immune-mediated damage, such as IRIS, may be the cause of cognitive impairment in our patients.
IRIS is characterized by the clinical deterioration of infectious diseases (paradoxical IRIS) or the emergence of new symptoms (unmasking IRIS) after the reversal of immune deficiency.15 Approximately 25% of patients with HIV-CM develop IRIS within 4 months, which presents as recurrent aseptic meningitis associated with headache, convulsions, or blurred consciousness.15,16 Although the clinical manifestations of cryptococcal related-IRIS usually begin within the first 4 weeks of ART initiation,17 it occurs after more than 12 weeks in some cases,18,19 and some studies have reported that it may still occur after 12 months.8,9,20
Short courses of corticosteroids are the main treatment of IRIS and have been used to treat severe symptoms, but the evidence supporting optimal therapeutics is limited.21 Studies have shown that IRIS is an imbalance between the pro-inflammatory Th1/Th17 and anti-inflammatory Th2/Treg axes, which are responsible for the secretion of large amounts of pro-inflammatory cytokines, resulting in over-activation of Th1 profile cytokines and leading to an inflammation burst.22,23 TNF-α, which is mainly produced by active macrophages, can recruit immune cells and form granulomas.24 TNF-α inhibitors can specifically block the Th1 signaling pathway; therefore, anti-TNF-α therapy may be an effective treatment option for IRIS. Thalidomide has been used to treat IRIS and achieved good therapeutic effects;9 however, there is no further relevant research due to its side effects.
Lenalidomide is a Thalidomide antagonist. Its inhibitory effect on TNF-α is 50,000 times that of thalidomide,25 and it is associated with a lower incidence of teratogenicity, peripheral neurotoxicity, and other side effects.26 Our report shows that lenalidomide can quickly alleviate clinical manifestations, such as fever, headache, and cognitive impairment, without the occurrence of any serious adverse events and with a good therapeutic effect on the patients.
Considering that the immune environment of the end-organ site of infection may better reflect the actual immune status of the body27 and that studies have found that lenalidomide can pass through the blood-brain barrier and has been used in the treatment of central tumors,28,29 we further analyzed 32 CSF cytokines in our two patients. We found that the high Th1 profile and significantly low Th2 profile before lenalidomide treatment may be related to IRIS. MoCA and IHDS had a significantly negatively correlated trend with inflammation-related factors, which suggests that the cognitive function of the patients is closely related to intracranial inflammation. The levels of these cytokines related to neuroinflammation were significantly lower than those before the treatment. Among them, cytokines related to Th1 and Th17 types showed a significant rebound after the drug withdrawal in Case 2. Therefore, we speculate that lenalidomide regulates intracranial inflammation in patients with HIV-CM via multiple pathways, not only by TNF-α blocking.
In Case 2, meningitis recurred after the drug was discontinued. We found that the levels of G-CSF, IL-17α, and IL-6 in CSF were still high around the withdrawal time. The CSF protein levels were 2.5 times higher than the normal level, suggesting that the intracranial inflammation was not well controlled. After the six cycles of treatment in the second round of therapy, although the CSF protein level was higher than the normal level, all cytokine levels were low, and there were no symptoms of meningitis after the drug withdrawal. The CSF cytokine analysis was a sensitive means to monitor the level of intracranial inflammation and might be useful in monitoring the timing of drug withdrawal. However, the specific time point of drug withdrawal was not clear.
In addition, we used the classical MoCA and IHDS for cognitive assessment. Different from the classical neuropsychological assessment, the two assessment methods are simple, do not require trained experts, and are widely used in neurocognitive impairment assessments.30,31 In our study, the sensitivity and specificity of the MoCA and IHDS were high before the intervention; however, the sensitivity of IHDS was significantly lower than that of MoCA after 6 cycles of treatment. The MoCA is simple and feasible and might be used as a screening tool by non-psychiatrists.
Despite the encouraging clinical effects, issues such as drug interaction with antifungal and antiretroviral therapy, treatment course, and consequences of drug withdrawal need to be addressed. A prospective multicenter study is needed to elucidate the potential value of this approach in the future.
In summary, our study reveals the potential effect of lenalidomide in treating IRIS-related cognitive impairment in patients with HIV-CM. This is the first report on the effect of lenalidomide on cytokines in the patients with CM. Our research provides new insights and a basis for future studies of CM treatment using lenalidomide and its mechanism.
Data Availability Statement
The original contributions generated for the study are included in the article. Further inquiries can be directed to the corresponding author.
Acknowledgments
We are very grateful to the staff in the HIV/AIDS ward of the First Affiliated Hospital, College of Medicine, Zhejiang University.
Funding
This work was supported by a grant from the National Special Research Program for Important Infectious Diseases [grant number 2017ZX10202102]. The funding organization had no involvement in the study or in the decision to submit the article for publication.
Disclosure
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
1. Balasko A, Keynan Y. Shedding light on IRIS: from pathophysiology to treatment of cryptococcal meningitis and immune reconstitution inflammatory syndrome in HIV-infected individuals. HIV Med. 2019;20(1):1–10. doi:10.1111/hiv.12676
2. Elsegeiny W, Marr KA, Williamson PR. Immunology of cryptococcal infections: developing a rational approach to patient therapy. Front Immunol. 2018;9:651. doi:10.3389/fimmu.2018.00651
3. Steff M, Joly V, Di Lucca J, et al. Clinical activity of Lenalidomide in visceral human immunodeficiency virus–related Kaposi sarcoma. JAMA Dermatol. 2013;149(11):1319–1322. doi:10.1001/jamadermatol.2013.5751
4. Bibas M, Grisetti S, Alba L, Picchi G, Del Nonno F, Antinori A. Patient with HIV-associated plasmablastic lymphoma responding to bortezomib alone and in combination with dexamethasone, gemcitabine, oxaliplatin, cytarabine, and pegfilgrastim chemotherapy and lenalidomide alone. J Clin Oncol. 2010;28(34):e704–e708. doi:10.1200/JCO.2010.30.0038
5. Denman J, Manavi K, Cook M. Lenalidomide as a treatment for relapsed AL amyloidosis in an HIV-positive patient. Int J STD AIDS. 2017;28(10):1045–1047. doi:10.1177/0956462417694561
6. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699. doi:10.1111/j.1532-5415.2005.53221.x
7. Sacktor NC, Wong M, Nakasujja N, et al. The International HIV Dementia Scale: a new rapid screening test for HIV dementia. AIDS. 2005;19(13):1367–1374.
8. Sitapati AM, Kao CL, Cachay ER, Masoumi H, Wallis RS, Mathews WC. Treatment of HIV-related inflammatory cerebral cryptococcoma with Adalimumab. Clin Infect Dis. 2010;50(2):e7–e10. doi:10.1086/649553
9. Brunel AS, Reynes J, Tuaillon E, et al. Thalidomide for steroid-dependent immune reconstitution inflammatory syndromes during AIDS. AIDS. 2012;26(16):2110–2112. doi:10.1097/QAD.0b013e328358daea
10. Heaton RK, Clifford DB, Franklin DR, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi:10.1212/WNL.0b013e318200d727
11. Habib AG, Yakasai AM, Owolabi LF, et al. Neurocognitive impairment in HIV-1-infected adults in Sub-Saharan Africa: a systematic review and meta-analysis. Int J Infect Dis. 2013;17(10):e820–e831. doi:10.1016/j.ijid.2013.06.011
12. Saylor D. Neurologic complications of human immunodeficiency virus infection. Continuum. 2018;24(5):1397–1421. doi:10.1212/CON.0000000000000647
13. Sutherland EJ, Brew BJ. Human immunodeficiency virus and the nervous system. Neurol Clin. 2018;36(4):751–765. doi:10.1016/j.ncl.2018.07.002
14. Levine AJ, Hinkin CH, Ando K, et al. An exploratory study of long-term neurocognitive outcomes following recovery from opportunistic brain infections in HIV+ adults. J Clin Exp Neuropsychol. 2008;30(7):836–843. doi:10.1080/13803390701819036
15. Haddow LJ, Colebunders R, Meintjes G, et al. Cryptococcal immune reconstitution inflammatory syndrome in HIV-1-infected individuals: proposed clinical case definitions. Lancet Infect Dis. 2010;10(11):791–802. doi:10.1016/S1473-3099(10)70170-5
16. Müller M, Wandel S, Colebunders R, et al. Immune reconstitution inflammatory syndrome in patients starting antiretroviral therapy for HIV infection: a systematic review and meta-analysis. Lancet Infect Dis. 2010;10(4):251–261. doi:10.1016/S1473-3099(10)70026-8
17. Lawn SD, Bekker LG, Myer L, Orrell C, Wood R. Cryptococcocal immune reconstitution disease: a major cause of early mortality in a South African antiretroviral programme. AIDS. 2005;19(17):2050–2052. doi:10.1097/01.aids.0000191232.16111.f9
18. Vlasova-St Louis I, Chang CC, Shahid S, French MA, Bohjanen PR. Transcriptomic predictors of paradoxical cryptococcosis-associated immune reconstitution inflammatory syndrome. Open Forum Infect Dis. 2018;5(7):ofy157. doi:10.1093/ofid/ofy157
19. Jenny-Avital ER, Abadi M. Immune reconstitution cryptococcosis after initiation of successful highly active antiretroviral therapy. Clin Infect Dis. 2002;35(12):e128–e133. doi:10.1086/344467
20. Lortholary O, Fontanet A, Mémain N, et al. Incidence and risk factors of immune reconstitution inflammatory syndrome complicating HIV-associated cryptococcosis in France. AIDS. 2005;19(10):1043–1049. doi:10.1097/01.aids.0000174450.70874.30
21. Beardsley J, Hoang NLT, Kibengo FM, et al. Do intracerebral cytokine responses explain the harmful effects of dexamethasone in human immunodeficiency virus-associated cryptococcal meningitis? Clin Infect Dis. 2019;68(9):1494–1501. doi:10.1093/cid/ciy725
22. Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13. doi:10.12703/P6-13
23. Boulware DR, Meya DB, Bergemann TL, et al. Clinical features and serum biomarkers in HIV immune reconstitution inflammatory syndrome after cryptococcal meningitis: a prospective cohort study. PLoS Med. 2010;7(12):e1000384. doi:10.1371/journal.pmed.1000384
24. Dellière S, Guery R, Candon S, et al. Understanding pathogenesis and care challenges of immune reconstitution inflammatory syndrome in fungal infections. J Fungi. 2018;4(4):139. doi:10.3390/jof4040139
25. Muller GW, Chen R, Huang SY, et al. Amino-substituted thalidomide analogs: potent inhibitors of TNF-alpha production. Bioorg Med Chem Lett. 1999;9(11):1625–1630. doi:10.1016/S0960-894X(99)00250-4
26. Kumar S, Rajkumar SV. Thalidomide and lenalidomide in the treatment of multiple myeloma. Eur J Cancer. 2006;42(11):1612–1622. doi:10.1016/j.ejca.2006.04.004
27. Okafor EC, Hullsiek KH, Williams DA, et al. Correlation between blood and CSF compartment cytokines and chemokines in subjects with cryptococcal meningitis. Mediators Inflamm. 2020;2020:8818044. doi:10.1155/2020/8818044
28. Lee YP, Hong JY, Yoon SE, et al. Real-world, single-center data for lenalidomide plus rituximab in relapsed or refractory diffuse large B-cell lymphoma and transformed follicular lymphoma. Cancer Manag Res. 2021;13:4241–4250. doi:10.2147/CMAR.S309092
29. Goldfinger M, Xu M, Bertino JR, Cooper DL. Checking in on lenalidomide in diffuse large B cell lymphoma. Clin Lymphoma Myeloma Leuk. 2019;19(6):e307–e311. doi:10.1016/j.clml.2019.02.012
30. Almeida F, Macedo A, Trigo D, et al. Neurocognitive evaluation using the International HIV Dementia Scale (IHDS) and Montreal Cognitive Assessment Test (MoCA) in an HIV 2 population. HIV Med. 2021;22(3):212–217. doi:10.1111/hiv.12963
31. Aita SL, Kaewpoowat Q, Yasri S, et al. Psychometric utility of the international HIV dementia scale and Montreal Cognitive Assessment in HIV-associated asymptomatic neurocognitive impairment. J Neurovirol. 2021;27(4):568–578. doi:10.1007/s13365-021-00991-z
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.