Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 11 » Issue 1
Left ventricular structure and remodeling in patients with COPD
Authors Pelà G, Li Calzi M, Pinelli S, Andreoli R, Sverzellati N, Bertorelli G, Goldoni M, Chetta AA
Received 19 December 2015
Accepted for publication 19 February 2016
Published 13 May 2016 Volume 2016:11(1) Pages 1015—1022
DOI https://doi.org/10.2147/COPD.S102831
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Richard Russell
Giovanna Pelà,1 Mauro Li Calzi,1 Silvana Pinelli,1 Roberta Andreoli,1 Nicola Sverzellati,2 Giuseppina Bertorelli,1 Matteo Goldoni,1 Alfredo Chetta1
1Department of Clinical and Experimental Medicine, 2Department of Surgery, University Medical School, University Hospital Parma, Parma, Italy
Background: Data on cardiac alterations such as left ventricular (LV) hypertrophy, diastolic dysfunction, and lower stroke volume in patients with COPD are discordant. In this study, we investigated whether early structural and functional cardiac changes occur in patients with COPD devoid of manifest cardiovascular disease, and we assessed their associations with clinical and functional features.
Methods: Forty-nine patients with COPD belonging to all Global Initiative for Chronic Obstructive Lung Disease (GOLD) classes were enrolled and compared with 36 controls. All subjects underwent clinical history assessment, lung function testing, blood pressure measurement, electrocardiography, and conventional and Doppler tissue echocardiography. Patients were also subjected to computed tomography to quantify emphysema score.
Results: Patients with COPD had lower LV cavity associated with a marked increase in relative wall thickness (RWT), suggesting concentric remodeling without significant changes in LV mass. RWT was significantly associated with ratio of the forced expiratory volume in 1 second to the forced vital capacity and emphysema score and was the only cardiac parameter that – after multivariate analysis – significantly correlated with COPD conditions in all individuals. Receiver operating characteristic curve analysis showed that RWT (with a cutoff point of 0.42) predicted the severity of COPD with 83% specificity and 56% sensitivity (area under the curve =0.69, 95% confidence interval =0.59–0.81). Patients with COPD showed right ventricular to be functional but no structural changes.
Conclusion: Patients with COPD without evident cardiovascular disease exhibit significant changes in LV geometry, resulting in concentric remodeling. In all individuals, RWT was significantly and independently related to COPD. However, its prognostic role should be determined in future studies.
Keywords: COPD, Doppler tissue echocardiography, left ventricular remodeling, emphysema score, right ventricular function, left ventricular function
Introduction
COPD is a major cause of mortality and morbidity.1 Extrapulmonary alterations, notably cardiac complications such as cor pulmonale, can be frequently observed in patients with COPD. In addition, coronary artery disease and heart failure may play important roles in mortality and morbidity in patients with COPD.2 Cardiovascular complications might not only be related to CODP itself but could also be due to a chronic systemic inflammatory condition, in which heart and lungs are differentially involved.3 Interestingly, changes in left ventricle function and structure such as left ventricular hypertrophy (LVH), diastolic dysfunction, and reduction in cardiac chambers and stroke volume have been described but might not be exclusively due to COPD.4–6 The lower preload caused by hyperinflation may be responsible for reduced left ventricular (LV) and right ventricular (RV) dimensions4,5 yet, a causal link between COPD and increased LV mass (LVM)6 has not been clearly established. Notably, these authors demonstrated a higher (30% versus 20% in controls) prevalence of LVH in normoxemic, normotensive patients with COPD, unrelated to forced expiratory volume in 1 second (FEV1). In that study, relative wall thickness (RWT) value (an index of LV geometry) was similar between patients with COPD and controls.6 Chronic inflammation or pronounced activation of the renin–angiotensin–aldosterone system rather than airway obstruction could play major yet unclear roles.
It is worth noting that an echocardiographic-detected LVH is a strong predictor of cardiovascular events, especially when combined with concentric remodeling revealed by an increased RWT.7,8 Therefore, the assessment of LV geometry in patients with COPD could have relevant clinical impacts in terms of improving risk stratification for future cardiovascular events and augmenting prognosis. Thus far, this issue remains poorly investigated.
With the aim of characterizing cardiac remodeling in COPD, we selected patients from all Global Initiative for Chronic Obstructive Lung Disease (GOLD) classes, without history of cardiovascular disease, in order to exclude any confounding factor. The aims of the present work were twofold: 1) to evaluate the influence of different degrees of airway obstruction and lung hyperinflation on cardiac structures by analyzing both the right and the left ventricles. By evaluating LVM and RWT, we wanted to quantify LV remodeling and the prevalence of LVH in COPD and 2) to study the relationship between LV remodeling and morbidity of a COPD cohort.
Materials and methods
Study population
This study conforms to the Declaration of Helsinki and was approved by the Institutional Medical Ethics Committee of the University of Parma. All participants provided signed informed consent before recruitment (Protocol number 41361).
From 2008 to April 2015, we screened inpatients and outpatients of both sexes, affected by COPD ranging from mild-to-severe airflow obstruction, who were referred to the Lung Unit of the University Hospital of Parma. We selected only patients with COPD without previous diagnosis of cardiac disorders such as coronary artery disease, heart failure, significant valvular dysfunction, cardiomyopathies, chronic atrial fibrillation, or pacemaker and left bundle branch blocks. Patients with systemic arterial hypertension higher than stage I and insulin-dependent diabetes were also excluded. Patients with COPD were diagnosed and classified according to the GOLD criteria. As control group, we recruited sex- and age-matched healthy subjects. Participants underwent spirometry and conventional and Doppler tissue echocardiography (DTE). Patients with COPD also underwent computed tomography (CT) scan.
Clinical examination
After recording their personal and parental medical history, study subjects underwent a physical examination and 12-lead resting electrocardiography; their blood pressure (BP) was determined in triplicate at 2-minute intervals (OMRON 705 IT, Omron, Kyoto, Japan). A normal office BP was defined as ≤140/90 mmHg.9 Body mass index (BMI) was calculated in kg/m2.
Pulmonary function testing
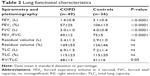
Pulmonary function was measured by a flow-sensing spirometer connected to a computer for data analysis (Vmax 22 and 6200, Sensor Medics, Yorba Linda, CA, USA). Functional residual capacity was measured by body plethysmography (Vmax 22 and 6200, Sensor Medics). Total lung capacity (TLC) was calculated as the sum of functional residual capacity and linked inspiratory capacity. Residual volume was obtained by subtracting vital capacity (VC) from TLC. At least three measurements were taken for each spirometry and lung volume variable to ensure reproducibility. Forced vital capacity (FVC), FEV1, RV, and TLC were recorded and expressed as percent of predicted value. FEV1/FVC and residual volume/TLC were also recorded and expressed as a ratio.
Conventional and Doppler tissue echocardiography
M-mode, two-dimensional, and Doppler echocardiography were performed by one ultrasonography-experienced cardiologist (GP), using commercially available, multihertz sector, 2–4 MHz probe-equipped machines (Acuson Aspen, Siemens, Mountain View, CA, USA; Vivid S5, GE Healthcare, Wauwatosa, WI, USA). The examination was feasible for screening cardiac parameters in all the patients with COPD, despite concerns that echocardiography windows might be poor.
The interventricular septal (SWT) and posterior wall (PWT) thicknesses, systolic (ESD) and diastolic (EDD) LV diameters, absolute LVM, and indexed-to-body-surface-area (LVM/BSA) were calculated as previously described.10,11 LVH was defined as an LVM/BSA of >95 g/m2 in women and >115 g/m2 in men.12 RWT was calculated as (SWT + PWT)/EDD, using the 0.42 cutoff to define eccentric (≤0.42) or concentric (≥0.42) remodeling.12 Simpson’s biplane rule-based end-diastolic (EDV) and systolic (ESV) volumes and ejection fraction (EF) were calculated, while fractional shortening (FS) was calculated as ([EDV – ESV]/EDV) ×100.
The RV cavity was measured from the long-axis parasternal view to extract the transversal dimension at end-diastole and end-systole. Using the apical four-chamber view, the longitudinal axis (as the distance between the apex of the right ventricle and the center of the tricuspid ring) and the maximal transversal diameter, in the basal one-third of the RV inflow, were measured at the end-diastole.12 Pulmonary pressure was calculated directly by sampling the tricuspid insufficiency and indirectly by the acceleration time on pulmonary flow.
Mitral and tricuspidal inflow patterns were analyzed from apical four-chamber view and E- and A-wave, and their ratios were considered as peak flow velocity and time velocity integral, to evaluate the conventional diastolic function of both ventricles. From the same projection, DTE analysis was performed at the lateral site and posteroseptum of mitral annulus and lateral site of tricuspidal annulus to assess myocardial systolic (S) and diastolic (E′, A′) waves of LV and RV. All measurements were usually made in triplicate during different heart cycles, as previously reported.11,13
CT scan
Forty-three patients with COPD have been recently studied with 6- and 64-detector row CT scanners (Siemens Medical Solutions, Forchheim, Germany). All whole-lung CT scans were acquired during one deep inspiratory breath-hold without the use of any contrast medium. Both scanners were daily calibrated on air to allow reliable measurements and comparison between examinations. Standard 6-detector row CT scanner parameters were as follows: 120 kV, effective 120 mAs, individual detector collimation 1 mm, gantry rotation time of 0.5 second, and pitch 1.25. The 64-detector row CT scanner parameters were as follows: 120 kV, effective 100 mAs, individual detector collimation 0.6 mm, gantry rotation time of 0.5 second, and pitch 1.
CT images data were reconstructed at 1-mm-thick sections with a reconstruction increment of 1 mm and a sharp kernel (B70f). They were transferred to a personal computer running MevisPULMO software (version 1.4, Fraunhofer MEVIS, Bremen, Germany) and analyzed for quantitative emphysema assessment by one operator (NS). A 3×3 kernel-based axial Gauss smoothing was applied to minimize the noise in sharp kernel images. For the whole lung, emphysema extent was defined as percentage of lung voxels ≤−950 Hounsfield units.14
Statistical analysis
Data are expressed as mean ± standard deviation (SD) or median (interquartile range) depending on the normality of data, assessed by using the Kolmogorov–Smirnov test. Consequently, controls and patients with COPD were compared for each variable using parametric or nonparametric univariate statistics (Student’s t-test or Mann–Whitney test). Pearson correlations were performed to analyze the associations between variables of lung function (such as GOLD class, spirometry data, and emphysema score) and cardiac chamber size.
Multiple regression analysis was used to analyze the relationship between COPD disease and the echocardiographic-based variables (LVM, RWT, and LV EDD), with age, heart rate (HR), BMI, and systolic BP or pulmonary pressure, respectively, for LV or RV parameters as the covariates. Smoking habits, FEV1, and FEV1/FVC were not included in the analysis as collinear with the presence of COPD disease (R-values >0.5 and variance inflation factor in multivariate models >2).
To test if RWT was predictive of the severity of COPD disease in patients with COPD, receiver operating characteristic (ROC) curve was done reporting the area under curve (AUC) value as general diagnostic tool, and the flex point, ie, where the sum of sensitivity and specificity reached a maximum, was used to divide the patients with COPD into two groups.
Hospitalizations for patients with COPD were considered as 1) overall number of hospitalizations for COPD and/or CV events normalized for observation time, rounding the number as integer in 10 years and 2) time elapsing from the start of the study and the first hospitalization. In the first case, because of the distribution of hospitalization, an overdispersed Poisson regression model was performed. In the second case, a Cox regression model was applied. In both cases, the categorical variable RWT ≤ cutoff point and > cutoff point was used.
A two-tailed P-value <0.05 was always considered as significant. SPSS 22.0 (IBM Corporation, Armonk, NY, USA) was used for all the statistical analysis.
Results
Patients’ characteristics
We scrutinized 56 COPD inpatients and outpatients. Seven patients with negative cardiovascular history were excluded during enrollment because of a mild valvular and/or dilative heart disease as detected by echocardiography. A total of 49 patients (45–85 years old) were finally enrolled. In 43 patients, CT scans were available. We screened 36 healthy volunteers without previous history of respiratory, cardiovascular, and metabolic disorders as the control group.
The clinical data of patients with COPD and of their controls are presented in Table 1. Patients were slightly older than controls, but both groups matched for sex and anthropometric parameters such as BMI and BSA. Systolic and diastolic BPs of the two groups were superimposable, but HR was higher in patients than in controls. Smoking habits and medical history of hypertension were greater in COPD (P<0.001 and P<0.05, respectively). No significant difference was observed in the history of diabetes mellitus (Table 1).
Pulmonary function values are listed in Table 2. All the GOLD-4 classes were homogenously (21%, 35%, 29%, and 15%) represented. The emphysema score was 18%±13% ranging from 1% to 53%. A total of 33 (68%) patients with COPD received inhaled β2 agonist agents, 26 (53%) received inhaled steroids, and 17 (35%) received inhaled muscarinic antagonists. The majority of hypertensive individuals were treated with a monotherapy of antihypertensive agents, while only six patients with COPD received a combination therapy (four COPD and one control with β-blockers, 18 COPD and three controls with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers, and five COPD and one control with calcium antagonists).
During follow-up (42.2 [SD: 20.7] months), seven patients with COPD died and 16 were hospitalized once or more for respiratory or cardiac complications (only two because of cardiovascular events).
Echocardiography
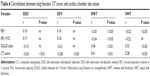
Patients with COPD, as compared with controls, exhibited a significant decrease of LV chamber size as assessed by EDD and EDV, which was associated with a significant increase in SWT and a mild, nonsignificant increase in PWT. Therefore, RWT was markedly higher in patients, suggesting that concentric remodeling occurs in COPD (Table 3). The absolute and indexed LVM were similar in the two groups as was the percentage of patients with positive ECHO criteria for LVH.
LV systolic functions, assessed by FS and EF, were similar between the two groups, but stroke volume was lower in COPD versus controls (Table 4). The mitral inflow pattern showed significant reduction of the E/A ratios in patients with COPD, but no significant differences in systolic and diastolic functions were detected by DTE between the two groups (Table 4).
Table 5 reports the results of RV assessment. A mild but nonsignificant increase in ventricular dimensions was observed in patients with COPD. The tricuspidal E-wave and the myocardial E′, E′/A′ ratios and S′-wave, all assessed as time–velocity integral, were reduced, suggesting impairments of both systolic and diastolic functions. Pulmonary pressure estimated by tricuspidal insufficiency was increased, and the acceleration time of pulmonary ejection was reduced in patients with COPD (Table 5).
After univariate analysis, RWT was strongly correlated with FEV1, FEV1/FVC, GOLD class, and emphysema scores. EDD and EDV showed significant correlations only with the emphysema score. Finally, SWT correlated with FEV1, GOLD class, and CT score but not with FEV1/FVC (Table 6).
Multiple regression analysis was performed to test the association between LV echocardiographic-derived parameters (RWT, SWT, EDD, and EDV) as outcomes and age, sex, BMI, systolic BP, HR, and the clinical condition (controls or COPD) as predictors (Table 7). Only RWT was associated with COPD. All echocardiographic variables – but not SWT – were related to HR. Sex, as expected,15 influenced LV structure. No association was found between the RV parameters and COPD.
ROC curve analysis showed that RWT, with a cutoff point of 0.40, predicted the severity of the disease with an 83% specificity and a 56% sensitivity (AUC =0.69, 95% CI =0.59–0.81). Patients with COPD with RWT >0.40 versus patients with RWT ≤0.40 (RWT_CAT variable) showed a significant reduction of FEV1/FVC (P=0.017) and a significant increase in emphysema score (P=0.033), whereas FEV1 and residual volume/TLC were not significantly different.
Univariate statistics showed that RWT was associated with a higher number of hospitalizations (P=0.034). However, in the multivariate Poisson regression model including age, systolic BP, HR, body surface area, and FEV1/FVC, we did not detect significant differences, with FEV1/FVC (exp[B] =0.87 [0.79–0.95], P=0.003) and age (exp[B] =1.11 [1.01–1.21], P=0.023) being the only significantly different variables.
Data from the previous analysis were confirmed when the time between enrollment and first hospitalization was considered. Specifically, in a Cox regression model with the same covariates, age (HR =1.08 [1.00–1.17], P=0.047) and FEV1/FVC (HR =0.88 [0.80–0.96], P=0.005) were the only significantly different variables.
Finally, overall patient survival was not considered in the statistical analysis because only seven patients died during our study.
Discussion
The main finding of our study is that COPD is associated with LV structural changes characterized by a decrease in EDD and EDV as well as an alteration of ventricular geometry, notably concentric remodeling. Other authors who used echocardiographic techniques and nuclear magnetic resonance,5,16 reported the relationship between COPD and decreased LV size, but this is the first report that provides evidence of direct links between pulmonary function, CT score, and LV geometry.
In our study, patients with COPD had an increased RWT compared with controls; on average, the former showed RWT values >0.42, which was considered as the cutoff point beyond which concentric remodeling was diagnosed.17 In addition, a more severe degree of respiratory disease was associated with a greater increase of concentric remodeling, as indicated by the significant relationship between LV geometry and GOLD class, spirometry parameters, and CT emphysema score. Furthermore, the outcome of a multivariate statistical analysis including LV echocardiographic-derived parameters (RWT, SWT, EDD, and EDV) as dependent variables and age, sex, BMI, BP, HR and the clinical COPD condition as predictors showed that only RWT was independently related to COPD, whereas no relationship was observed with the other echocardiographic parameters.
Our study does not confirm the LVH in COPD reported by Anderson et al.6 The absolute and indexed LVM was unchanged in our patients as compared with controls. It is noteworthy that our results are in agreement with those previously reported by echocardiography18 with the highly sophisticated technique – nuclear magnetic resonance.4 Differences in the selection criteria may explain these discrepancies. For example, unlike the study by Anderson et al,6 our patients belonged to all GOLD classes. Moreover, our population included only patients with grade 1 hypertension, treated mostly by monotherapy. We excluded hypertensive patients affected by grades 2 and 3 hypertension. A greater systolic BP in COPD versus controls, as demonstrated by the ambulatory and 24-hour monitoring and the number of antihypertensive-per-treated patients with COPD (median =2), has also been reported by Anderson et al.6 In addition, the age differences in our cohort were low (mean difference ~3 years), albeit weakly statistically significant. By contrast, in the study by Anderson et al,6 the COPD group was, on average, 10 years older than the controls. This ~10-year age difference between the two groups may be critical because population studies report that age is a strong determinant of LVM, independent of height, weight, and sex, both in childhood and in adults.19,20 In turn, the previously observed differences in LVM between COPD and controls could be mainly related to the different ages of the two groups.
Although we did not find any difference in LVH between the two groups, our patients with COPD exhibited a significant increase in RWT as compared with controls. This is crucial to stratify cardiovascular risk in the general population, as a concentric LV remodeling (normal LV mass with increased RWT) is a well-known negative prognostic factor.7,17
As previously demonstrated by Barr et al,16 the extent of emphysema on CT scanning was linearly related to cardiac dimensions expressed by EDD and EDV: a greater magnitude of emphysema corresponds to a greater reduction in LV cavity size. Several explanations have been proposed to explain the relationship between COPD and microcardia. First, there is a lower preload in left cardiac chambers mediated by an intrathoracic hypovolemia and a decrease of the pulmonary vascular bed caused by dynamic hyperinflation.4 This mechanism may explain not only the reductions in LV diameters and volumes but also the lower stroke volumes16 and the supposed LV diastolic dysfunction found at the transmitral Doppler analysis.21 Indeed, the inflow pattern is not only an index of ventricular relaxation and compliance, but it is also influenced by others variables such as the preload and the HR. A lower preload can mimic an impaired LV relaxation in normal functioning heart.22 More sophisticated techniques, such as DTE, which analyzes the contraction and relaxation velocities directly on the myocardium, are less influenced by ventricular loads and provide more sensitive indexes of diastolic function.22–24 In our study, the E/A ratio from the mitral inflow pattern was significantly reduced in COPD, but the myocardial S- and E′-waves were preserved, excluding an impairment in the LV systolic and diastolic function.
A leftward shift of the interventricular septum consequent to the increased right ventricular afterload could cause a decrease in LV cavity size.21,25 However, this premise is not entirely convincing because, in our study, the configuration of this wall did not differ between the two groups, and we did not find any significant RV enlargement despite the significant increase in pulmonary artery pressure. Our findings are in agreement with previous studies4,5 that found even smaller RV secondary to the hyperinflation and subsequent lower preload to both heart chambers. In addition, patients with COPD had impaired RV systolic and diastolic functions, as demonstrated by the significant reductions of S- and E′-waves, which were significantly related only to pulmonary artery pressure but not to CT score or FEV1/FVC. In summary, we report structural and functional cardiac changes in COPD, but only RWT was independently related to the COPD condition (after multivariate analysis).
To verify if RWT predicted the severity of the disease, we divided the COPD cohort into two groups according to the ROC curves analysis, and we observed that patients with RWT >0.40 were sicker, with more pronounced reduction in FEV1/FVC and more increase in CT score. The prognostic role of RWT was also assessed in relation to respiratory and cardiac hospitalizations: only after the univariate statistics, but not after multivariate Poisson analysis, cardiac geometry was significantly related to this event, with age and FEV1/FVC being the only strong predictors. These analyses did not show LV geometry as morbidity predictor, confirming the role of age and clinical variables; however, we speculate that, in a larger series of patients, this structural cardiac index may prove a useful marker of hospitalization risk. During the follow-up, seven deaths occurred, but, owing to the limited number of fatal events, overall patient survival was not considered in the statistical analysis. However, the comparisons between survivors and nonsurvivors revealed that RWT was significantly higher (0.52 versus 0.42, P=0.014) in nonsurvivors, suggesting its potential prognostic value as mortality predictor, which has to be confirmed in the future with a greater number of fatal events.
Limitations and strengths
The first limitation of our study is the limited number of patients. We must emphasize that all enrolled subjects were thoroughly screened and selected for the absence of any significant comorbidities with effects on the structure and heart function. The average age of the two groups (>60 years) is the one in which it is difficult to encounter subjects free of cardiovascular disease, hypertension, or diabetes.
The strength of the present study is that a single expert investigator recruited and appraised patients and performed all echocardiographies, thus reducing analytical variability.
Conclusion
We demonstrated that significant alterations – typified by a concentric remodeling – exist in the LV geometry of patients with COPD. LV geometry, compared with LVM, is a little-known marker of cardiovascular risk. Such structural LV modifications are not associated with an increased LVM or with LV systolic and diastolic dysfunctions. RWT appears to be the only cardiac parameter independently related to COPD and could help identify those patients at greatest risk of cardiac complications. Further studies are warranted to ascertain whether LV geometry is related to morbidity and mortality in COPD.
Acknowledgment
The authors would like to acknowledge Dr Francesco Visioli, who critically revised the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
Vestbo J, Hurd SS, Agustí AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347–365. | ||
Sin DD, Wu L, Man SF. The relationship between reduced lung function and cardiovascular mortality: a population-based study and a systematic review of the literature. Chest. 2005;127(6):1952–1959. | ||
MacNee W, Maclay J, McAllister D. Cardiovascular injury and repair in chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008; 5(8):824–833. | ||
Jörgensen K, Müller MF, Nel J, Upton RN, Houltz E, Ricksten S-E. Reduced intrathoracic blood volume and lef and right ventricular dimensions in patients with severe emphysema: an MRI study. Chest. 2007;131(4):1050–1057. | ||
Watz H, Waschki B, Meyer T, et al. Decreasing cardiac chamber sizes and associated heart dysfunction in COPD: role of hyperinflation. Chest. 2010;138(1):32–38. | ||
Anderson WJ, Lipworth BJ, Rekhraj S, Struthers AD, George J. Left ventricular hypertrophy in COPD without hypoxemia. The elephant in the room? Chest. 2013;143(1):91–97. | ||
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham Heart Study. N Engl J Med. 1990;322(22):1561–1566. | ||
Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114(5):345–352. | ||
Mancia G, Fagard R, Narkiewicz K, et al. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the management of arterial hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). J Hypertens. 2013;31(7):1281–1357. | ||
Devereux RB, Reichek N. Echocardiographic determination of left ventricular mass in man: anatomic validation of the method. Circulation. 1977;55(4):613–618. | ||
Pelà G, Bruschi G, Montagna L, Manara M, Manca C. Left and right ventricular adaptation assessed by Doppler tissue echocardiography in athletes. J Am Soc Echocardiogr. 2004;17(3):205–211. | ||
Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39. | ||
Pelà G, Tirabassi G, Pattoneri P, Pavone L, Garini G, Bruschi G. Cardiac involvement in the Churg-Strauss syndrome. Am J Cardiol. 2006;97(10):1519–1524. | ||
Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152(2):653–657. | ||
Pelà G, Crocamo A, Li Calzi M, et al. Sex-related differences in left ventricular structure in early adolescent non-professional athletes. Eur J Prev Cardiol. 2016;23(7):777–784. | ||
Barr RG, Bluemke DA, Ahmed FS, et al. Percent emphysema, airflow obstruction, and impaired left ventricular filling. N Engl J Med. 2010;362(3):217–227. | ||
Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. | ||
Sabit R, Bolton CE, Fraser AG, et al. Sub-clinical left and right ventricular dysfunction in patients with COPD. Respir Med. 2010;104(8):1171–1178. | ||
de Simone G, Devereux R, Daniels SR, Meyer RA. Gender differences in left ventricular growth. Hypertension. 1995;26(6 Pt 1):979–983. | ||
de Simone G, Devereux R, Kimball TR, et al. Interaction between body size and cardiac workload. Influence on left ventricular mass during body growth and adulthood. Hypertension. 1998;31(5):1077–1082. | ||
Funk GC, Lang I, Schenk P, Valipour A, Hartl S, Burghuber OC. Left ventricular diastolic dysfunction in patients with COPD in the presence and absence of elevated pulmonary arterial pressure. Chest. 2008; 133(6):1354–1359. | ||
Sohn DW, Chai IH, Lee DJ, et al. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30(2):474–480. | ||
Nagueh SF, Middleton KJ, Kopelen HA, Zoghbi WA, Quiñones MA. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30(6):1527–1533. | ||
Pelà G, Bruschi G, Cavatorta A, Manca C, Cabassi A, Borghetti A. Doppler tissue echocardiography: myocardial wall motion velocities in essential hypertension. Eur J Echocardiogr. 2001;2(2):108–117. | ||
Schena M, Clini E, Errera D, Quadri A. Echo-Doppler evaluation of left ventricular impairment in chronic cor pulmonale. Chest. 1996;109(6):1446–1451. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.