Back to Journals » OncoTargets and Therapy » Volume 11
LDH-A promotes epithelial–mesenchymal transition by upregulating ZEB2 in intestinal-type gastric cancer
Authors Zhang Y , Lin S, Chen Y, Yang F, Liu S
Received 24 January 2018
Accepted for publication 11 March 2018
Published 27 April 2018 Volume 2018:11 Pages 2363—2373
DOI https://doi.org/10.2147/OTT.S163570
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Geoffrey Pietersz
Video abstract presented by Shenlin Liu.
Views: 783
Yongjie Zhang,1,2,* Sen Lin,3,* Yan Chen,2,* Fei Yang,2 Shenlin Liu1
1Department of Gastroenterology, Affiliated Hospital of Nanjing University of Traditional Chinese Medicine, Nanjing, Jiangsu, People’s Republic of China; 2Department of Medical Oncology, Huai’an Hospital to Xuzhou Medical University, Huai’an, Jiangsu, People’s Republic of China; 3Clinical Laboratory, Huai’an Hospital to Xuzhou Medical University, Huai’an, Jiangsu, People’s Republic of China
*These authors contributed equally to this work
Introduction: Epithelial-mesenchymal transition (EMT) is regarded as a crucial process of invasion and metastasis, which contribute greatly to cancer-related relapse and death. Based on research results that hypoxia can trigger gastric cancer EMT and decreasing lactate production can selectively kill hypoxic cancer cells, we infer that lactate dehydrogenase A (LDH-A) transforming pyruvate into lactate is at least in part responsible for poor prognosis of gastric cancer.
Materials and methods: We used siRNA to knock down LDH-A in intestinal-type gastric cancer (ITGC) cell lines SGC7901 and BGC823. Western blot and RT-PCR were applied to detect mRNA and protein expression of EMT-related genes, respectively. Transwell invasion assay and migration assay were applied to study invasive and migratory abilities, respectively. Survival analysis was used to evaluate prognostic values.
Results and conclusion: The results of in vitro experiment demonstrated that LDH-A facilitates ITGC cells’ invasion and migration by upregulating ZEB2. The positive correlation between LDH-A and ZEB2 was verified in 371 ITGC specimens. Survival analysis indicated that co-expression of LDH-A/ZEB2 had synergetic power to predict overall survival. Thus, we conclude that the close relationship between LDH-A and ZEB2 may offer a potential therapeutic strategy for ITGC.
Keywords: intestinal-type gastric cancer, LDH-A, ZEB2, epithelial–mesenchymal transition, Warburg effect, glycolysis
Introduction
Despite improved response rates, thanks to great progress of chemoradiotherapy and targeted therapy in recent years,1–4 gastric cancer (GC) remains one of the most lethal malignancies worldwide.5,6 Invasion and metastasis impede the treatment efficiency and bear a major share of the responsibility for GC-related relapse and death.7 A better understanding of the mechanism contributing to GC invasion and metastasis is conducive to improving treatment efficacy and prognosis.
Epithelial–mesenchymal transition (EMT) is a process in which epithelial cells are transformed into cells with mesenchymal phenotypes with enhanced invasive and migratory abilities, characterized by downregulation of epithelial marker of E-cadherin and upregulation of mesenchymal markers including N-cadherin, fibronectin and vimentin. Transcriptional regulators of EMT consist of the snail family, the basic helix-loop-helix factors, the zinc finger E-box binding proteins ZEB1 and ZEB2 and so on. Profound insight into the role of EMT in GC could further deepen our understanding of GC invasion and metastasis. Diverse molecular pathways associated with cancer stem cells (CSCs), epigenetic modification and transcriptional regulation have been deeply elucidated during aberrant activation and regulation of GC EMT.8
Tumors are composed of malignant cells and the microenvironment. The components of complicated tumor microenvironment, such as tumor stromal cells and cellular factors, can regulate malignant behaviors of cancer cells via EMT. In contrast to normal cells, cancer cells preferentially take advantage of glycolysis rather than mitochondrial oxidative phosphorylation even in the presence of oxygen, which means pyruvate is transformed into lactate and therefore avoids excess reactive oxygen species.9 This phenomenon is known as Warburg effect. Increased lactate production leads to acidification of tumor microenvironment, which promotes tumor invasion directly and indirectly by killing normal cells and by degradation of the extracellular matrix. Pyruvate dehydrogenase kinase 1, which serves as inhibitor of pyruvate dehydrogenase, can promote liver metastasis in breast cancer by increasing conversion of glucose-derived pyruvate into lactate.10 In view of the importance of making full use of Warburg effect to decrease lactate production in tumor microenvironment, it is indispensable to elucidate driver genes in upstream and downstream signal pathway of lactate for more precise and efficacious treatment. Lactate dehydrogenase A (LDH-A) is a crucial rate-limiting enzyme responsible for catalyzing pyruvate into lactate irreversibly, which is the final step of the aerobic glycolysis. Sonveaux et al not only found that lactate is the bedrock of symbiotic relationship between glycolytic and oxidative tumor cells during the course of energy metabolites, but also demonstrated that targeting lactate-fueled respiration can specifically kill hypoxic tumor cells in mice.11 Considering hypoxia is a significant GC EMT inducer,12–16 it is therefore reasonable to deduce that silencing LDH-A in tumors could downregulate EMT.
In this study, the correlation of LDH-A and ZEB2 was evaluated in intestinal-type gastric cancer (ITGC) cell lines and clinical specimens. We then examined if the combined expression of LDH-A and ZEB2 had synergetic power to predict overall survival (OS). Our results indicate that the significantly close correlation of LDH-A and ZEB2 underlies the inhibitory effect of LDH-A silencing on invasion and metastasis and the important role of LDH-A in the poor prognosis of human ITGC.
Materials and methods
Cell lines
The human ITGC cell lines MKN28, SGC7901 and BGC823 and the human immortalized gastric mucosa epithelial cell line GES were purchased from the American Type Culture Collection (Manassas, VA, USA) and maintained in RPMI 1640 medium.
LDH-A-targeting siRNAs and transfection
We designed three siRNAs targeting LDH-A messenger RNA (mRNA) and a scrambled siRNA used as a negative control with software found on the Ambion website and synthesized by Shanghai GenePharma Co., Ltd (Shanghai, People’s Republic of China). The siRNA sequences are listed in Table S1. Cell transfection was performed using Lipofectamine 2000 transfection reagent (Thermo Fisher Scientific, Waltham, MA, USA) according to the manufacturer’s instructions. Briefly, siRNALDH-A or siRNANC diluted with Opti-MEM was mixed with transfection reagent and the mixture was added into 2 mL Opti-MEM medium per well of a 6-well plate at about 30%–50% cell confluence. Six hours later, cells were re-fed with fresh Opti-MEM medium and continued for culture. The transfection efficiency was examined after 24 h of transfection. Cells were collected for the transwell invasion and migration assay after 24 h for RNA extraction and after 48 h for protein extraction.
Real-time reverse transcription polymerase chain reaction
We first extracted total RNA from the cells with Trizol reagent (Thermo Fisher Scientific), and then carried out the cDNA synthesis based on the protocol of the PrimeScript RT reagent Kit (TaKaRa, Kyoto, Japan) with 1 μg RNA as the starting amount. The real-time quantitative PCR reaction was performed using the SYBR Premix Ex Taq™ (TaKaRa) and an ABI PRISM 7300 PCR detection system (Applied Biosystems, Carlsbad, CA, USA). The primers for these transcripts are listed in Table S2.
Western blot
We used the Bradford assay to quantify the protein content of the cellular extracts. The extracted protein was first subjected to SDS–PAGE, then transferred onto polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA, USA) and finally immunoblotted with antibodies for LDH-A (Cell Signaling Technology, Danvers, MA, USA), ZEB2, E-cadherin and vimentin (Abcam, AB Biotech Company Ltd., Cambridge, MA, USA).
Transwell invasion assay
We used a Transwell membrane (8 μm pore size; Corning-Costar, Cambridge, MA, USA) coated with Matrigel (BD Biosciences, Bedford, MA, USA) for invasion assay. We first transfected SGC7901 and BGC823 cells with siRNALDH-A; 24 h later we trypsinized the cells, then washed and resuspended cells in serum-free medium and added cells to the upper chamber of pre-coated transwells at a density of 5.0×104 cells per well. After incubation for 24 h, we swabbed the cells present on the upper chamber with a cotton-tipped swab. The invasive cells attached to the lower surface of the membrane were fixed with methanol and stained with 0.1% crystal violet (Sigma-Aldrich Co., St Louis, MO, USA). Finally, we counted the number of invasive cells (five fields per filter) under an inverted microscope and calculated the mean numbers of invasive cells. The experimental procedures were repeated three times.
Transwell migration assay
We used a Transwell membrane (8 μm pore size; Corning-Costar) for migration assay. The experimental procedures were identical to the Transwell invasion assay except that the time of incubation was 12 h.
Patients, clinical specimens and preparation of TMAs
A total of 371 patients (mean age 56 years; 266 males, 105 females) histologically diagnosed with primary ITGC receiving no pre-operative adjuvant chemotherapy were enrolled in this study following curative surgery at Changhai Hospital from 2001 to 2005. Matched non-neoplastic gastric tissues were sampled from the resection margins in the furthest distance from the tumor borderline of all patients. All patients had follow-up information and the follow-up deadline was March 2010. All of the tissue specimens were obtained with patient’s written informed consent and used with approval of the Changhai Hospital Institutional Review Board. The tissue microarray (TMA) was constructed as described previously.17
Immunohistochemistry
Protein expression of LDH-A, ZEB2 and vimentin in sections of human ITGC specimens was detected with antibodies for LDH-A (Cell Signaling Technology), ZEB2 and vimentin (Abcam, AB Biotech Company Ltd), respectively. Control staining of LDH-A, ZEB2 and vimentin was conducted by substituting PBS for the primary antibody.
Evaluation of immunostaining
The immunostaining outcome was evaluated by two independent pathologists. For LDH-A, ZEB2 and vimentin staining, percentage of stained cells and staining intensity were taken into consideration. Percentage of staining cells was evaluated semiquantitatively as follows: 0, <5%; 1, 5%–25%; 2, 25%–50%; 3, 50%–75%; 4, >75% of the cells in the respective lesions. Staining intensity was evaluated semiquantitatively as follows: 0, no staining; 1, weak; 2, moderate; 3, strong. The final score achieved by multiplying the intensity value and the percentage value (ranging 0–12)18 was divided into negative (final scores, <4) and positive (final scores, >4).
Statistical analysis
Pearson’s χ2 test was applied to study the relation between different variables. Kaplan–Meier method and the log-rank test were used to assess univariate survival analysis of OS and the difference of survival rates, respectively, while the Cox proportional hazards model for multivariate survival analysis was used to evaluate the prognostic values of different clinicopathological parameters. A P-value <0.05 was considered to be statistically significant. All data were analyzed using the SPSS statistical software program 16.0 for Microsoft Windows (SPSS Inc., Chicago, IL, USA).
Results
siRNA mediated knockdown of LDH-A in SGC7901 and BGC823 cells
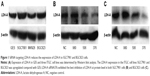
We used Western blot analysis to detect LDH-A expression in ITGC cell lines SGC7901, MKN28 and BGC823 and the human immortalized gastric mucosa epithelial cell line GES. SGC7901 and BGC823 showed higher levels of LDH-A expression than GES cells (Figure 1A). In order to explore the role of LDH-A in ITGC, we used siRNA to selectively knockdown LDH-A expression in SGC7901 and BGC823 cells. Western blot analysis outcome indicated that siRNA376 exhibited the best inhibition of LDH-A expression in both SGC7901 (Figure 1B) and BGC823 (Figure 1C) cells and was therefore selected for subsequent experiments.
LDH-A promotes EMT in ITGC by upregulating ZEB2 expression in vitro
We investigated the role of LDH-A in invasion and migration of ITGC cells. LDH-A knockdown (KD) decreased invasion and migration ability of SGC7901 by 46.63% and 52.48%, respectively, compared with KD control (KDC) (Figure 2A), while decreased invasion and migration ability of BGC823 cells by 66.11% and 64.80%, respectively (Figure 2B). To explore whether LDH-A promotes the metastasis of ITGC cells by EMT, we detected the mRNA expression of EMT-related genes including Snail, ZEB1, ZEB2, E-cadherin, N-cadherin, fibronectin and vimentin. RT-PCR results indicated that after LDH-A knockdown in SGC7901 cells, mRNA expression of ZEB2 and vimentin was significantly decreased (P<0.001), coupled with remarkable upregulation of epithelial marker E-cadherin (P<0.001) (Figure 3A). In BGC823 cells, mRNA expression of ZEB2, vimentin (P<0.001) and fibronectin (P<0.01) was remarkably decreased, coupled with the downregulation of epithelial marker E-cadherin (P<0.001) (Figure 3B). The expression of Snail, ZEB1 and N-cadherin at mRNA lever remained unchanged in both SGC7901 and BGC823 cells. The downregulation of ZEB2 and vimentin and upregulation of E-cadherin at protein level in both SGC7901 (Figure 3A) and BGC823 cells (Figure 3B) were also confirmed by Western blot analysis.
Positive correlation between LDH-A and ZEB2 expression in ITGC specimens
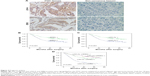
Immunohistochemistry (IHC) results of TMA demonstrated that both LDH-A and ZEB2 were significantly upregulated in ITGC specimens compared to adjacent non-cancerous tissues (ANTs) (Figure 4A). Positive expression of LDH-A was detected in 193 of 371 (52.0%) ITGC cases and in 48 of 371 (12.9%) ANTs. Positive expression of ZEB2 was detected in 207 of 371 (55.8%) ITGC cases and in 45 of 371 (12.1%) ANTs. Next, we analyzed IHC results to confirm if positive relationship between ZEB2 and LDH-A exists. As shown in Table 1, LDH-A expression was significantly related with ZEB2 (P=0.003) and vimentin expression (P=0.024), as well as tumor size (P=0.033), invasion depth (P<0.001) and lymph node metastasis (P<0.001), but not with primary tumor location, distant metastasis, TNM stage and differentiation (P>0.05).
  | Table 1 Correlation between LDH-A expression and clinicopathological characteristics |
LDH-A protein in combination with ZEB2 protein expression correlated with poorer clinical results in ITGC
IHC analysis revealed that the LDH-A positive expression accounted for 43.3% of ZEB2-negative tumor and 58.9% of ZEB2-positive tumor. There existed a strikingly positive correlation between LDH-A and ZEB2 expression (P=0.003, Table 1). Univariate analysis demonstrated that ZEB2 expression may predict poor prognosis in ITGC (P<0.001, log-rank test; Figure 4B), and so is LDH-A expression (P<0.001, log-rank test; Figure 4C). In order to explore the potential relationship between the expression of LDH-A/ZEB2 and the prognosis of ITGC, we divided patients into four groups: one subgroup with both ZEB2 and LDH-A positive expression, either ZEB2 or LDH-A negative expression and both ZEB2 and LDH-A negative expression. Univariate analysis demonstrated that OS of patients with both ZEB2 and LDH-A positive expression was remarkably worse than that of other combinations in ITGC (P<0.001, Figure 4D). According to results of multivariate survival analysis, ZEB2 expression was an independent prognostic factor (P<0.001), and so was tumor size (P=0.001), distant metastasis (P=0.002), regional lymph node involvement (P=0.015) and invasion depth (P=0.017) (Table 2).
  | Table 2 Multivariate analysis of the prognostic significance of individual clinicopathologic factors in ITGC |
Discussion
Reprogramming of energy metabolism is regarded as the seventh hallmark of cancer. The most distinctive metabolic phenotype of tumor cells is Warburg effect. Despite being overshadowed by gene mutations for a long time, Warburg effect has become a noticeable focus for its great contribution to cancer progression. Increased Glut1 expression and glucose uptake were verified in both pancreatic ductal adenocarcinoma cell line Panc-1 and breast cancer cell line MCF-7 undergoing TGFβ-induced EMT.19,20 The enhancement of glycolysis and downregulation in gluconeogenesis was described as a predominant EMT-related change in breast cancer.21,22 Thus, the induction of EMT is accompanied by augmentations of Warburg effect. LDH-A, as a downstream molecule of hypoxia-inducible factor 1α (HIF-1α) and the vital rate-limiting enzyme in Warburg effect, is responsible for transforming pyruvate into lactate, which is identified as the motor of malignant tumor,23 and promotes aggressiveness in multiple types of tumors.24–27 However, there is no enough evidence to support the direct correlation between lactate and EMT up to now.
EMT is a multistage reprogramming process in which the epithelial cells switch to mesenchymal phenotype with strong mobility. ZEB2 expression has been regarded as an important marker for GC migration and invasion.28 Many genes regulating ZEB2 in GC have been revealed. FoxM1 overexpression induces EMT by upregulating ZEB2 and vimentin and therefore promotes GC migration and invasion.29 The same rationale is applied to the homeobox B8, a sequence-specific transcription factor.30 MicroRNA-145-5p inhibits GC invasiveness through suppression of EMT by targeting N-cadherin and ZEB2.31 Furthermore, miR-200c could increase the sensitivity of GC cells to DDP by downregulating ZEB2.32,33 All these data suggest that ZEB2 may play an important role in metastasis and treatment resistance of GC. However, very little information is available on ZEB2-regulating Warburg effect-related enzyme in GC, although Warburg effect is very essential in tumor progression.
Recent studies have demonstrated that HIF-1α can drive EMT through upregulation of ZEB2 in many types of cancer including GC.34–36 We confirmed that LDH-A silencing can markedly downregulate ZEB2 expression at both the mRNA and protein levels, suggesting LDH-A is an upstream regulator of ZEB2 expression. Accordingly, it is tempting to deduce that decreased capabilities of invasion and migration in ITGC upon LDH-A silencing is at least in part attributable to downregulation of ZEB2-mediated EMT. The transcription factor HIF-1 can increase vascularization and enhance Warburg effect by upregulating downstream target genes vascular endothelial growth factor and LDH-A, respectively. Enhancement of Warburg effect motivated by LDH-A overexpression brings about a high production rate of lactate, which elevates HIF-1 by preventing degradation under both anaerobic and aerobic conditions.37 Therefore, we speculate that it is the interruption of the positive feedback loop between HIF and LDH-A upon LDH-A silencing that leads to ZEB2 downregulation. Considering that targeting lactate-fueled respiration can specifically eliminate hypoxic tumor cells,11 which overexpress markers of CSCs including Oct4 and Sox2, LDH-A silencing can suppress malignancy progression through downregulation of CSCs markers by energy starvation of hypoxic tumor cells. Furthermore, HIF can induce cancer stemness.38–40 Recent research indicated that EMT and CSC serve as reciprocal co-activators in GC.41,42 Altogether, LDH-A, HIF, CSCs and EMT constitute a positive cycle to collaboratively promote the invasion and metastasis of ITGC, which results in these four genes downregulating ZEB2 in a cooperative manner upon LDH-A silencing.
IHC result of ITGC clinical specimens revealed significantly close association of ZEB2 with LDH-A, which is consistent with in vitro results. In other words, our data from both clinical specimens and in vitro experiments indicated that ITGC aggressiveness is at least in part due to LDH-A/ZEB2 signal pathway. Univariate analysis demonstrated that both LDH-A and ZEB2 positive expression can predict significantly worse OS, while multivariate analysis indicated that only positive ZEB2 was an independent prognostic factor. Considering the positive correlation between the expression of LDH-A and ZEB2, it is necessary to explore if the co-expression of LDH-A/ZEB2 had synergetic power to predict OS. As we expected, the ITGC patients with both LDH-A and ZEB2 positive expression exhibited poorer OS. Taken together, the predictive power of combination of ZEB2 and LDH-A expression outperformed that of ZEB2 or LDH-A alone. Therefore, we draw a conclusion that LDH-A and ZEB2 may promote ITGC progression in a cooperative manner.
Conclusion
Our study demonstrated that the mechanism of the significantly inhibitory effects of LDH-A silencing on ITGC metastasis is attributable to the downregulation of ZEB2 and vimentin, as well as upregulation of E-cadherin in vitro experiments. The intimate correlation of ZEB2 and LDH-A was further confirmed by analysis of ITGC tissue specimens and OS. As far as we know, this study is the first to verify the strikingly close correlation of energy metabolism-related enzyme with ZEB2 in ITGC. EMT is related with vital processes of GC initiation and progression such as tumor cell stemness, proliferation, invasion and migration. We believe that inhibition of EMT via LDH-A silencing could provide efficient therapy for ITGC patients.
Disclosure
The authors report no conflicts of interest in this work.
References
Memon MA, Subramanya MS, Khan S, Hossain MB, Osland E, Memon B. Meta-analysis of D1 versus D2 gastrectomy for gastric adenocarcinoma. Ann Surg. 2011;253(5):900–911. | ||
Xu AM, Huang L, Zhu L, Wei ZJ. Significance of peripheral neutrophil-lymphocyte ratio among gastric cancer patients and construction of a treatment-predictive model: a study based on 1131 cases. Am J Cancer Res. 2014;4(2):189–195. | ||
Huang L, Xu A, Li T, Han W, Wu S, Wang Y. Detection of perioperative cancer antigen 72-4 in gastric juice pre- and post-distal gastrectomy and its significances. Med Oncol. 2013;30(3):651. | ||
Xu AM, Huang L, Han WX, Wei ZJ. Monitoring of peri-distal gastrectomy carbohydrate antigen 19-9 level in gastric juice and its significance. Int J Clin Exp Med. 2014;7(1):230–238. | ||
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90. | ||
Xu AM, Huang L, Liu W, Gao S, Han WX, Wei ZJ. Neoadjuvant chemotherapy followed by surgery versus surgery alone for gastric carcinoma: systematic review and meta-analysis of randomized controlled trials. PLoS One. 2014;9(1):e86941. | ||
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. | ||
Zhao L, Li W, Zang W, et al. JMJD2B promotes epithelial-mesenchymal transition by cooperating with β-catenin and enhances gastric cancer metastasis. Clin Cancer Res. 2013;19(23):6419–6429. | ||
Lu J, Tan M, Cai Q. The Warburg effect in tumor progression: mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015;356(2 Pt A):156–164. | ||
Dupuy F, Tabariès S, Andrzejewski S, et al. PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 2015;22(4):577–589. | ||
Sonveaux P, Végran F, Schroeder T, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118(12):3930–3942. | ||
Guo J, Wang B, Fu Z, Wei J, Lu W. Hypoxic microenvironment induces EMT and upgrades stem-like properties of gastric cancer cells. Technol Cancer Res Treat. 2016;15(1):60–68. | ||
Zhou J, Li K, Gu Y, et al. Transcriptional upregulation of RhoE by hypoxia-inducible factor (HIF)-1 promotes epithelial to mesenchymal transition of gastric cancer cells during hypoxia. Biochem Biophys Res Commun. 2011;415(2):348–354. | ||
Matsuoka J, Yashiro M, Doi Y, et al. Hypoxia stimulates the EMT of gastric cancer cells through autocrine TGFβ signaling. PLoS One. 2013;8(5):e62310. | ||
Liu N, Wang Y, Zhou Y, et al. Krüppel-like factor 8 involved in hypoxia promotes the invasion and metastasis of gastric cancer via epithelial to mesenchymal transition. Oncol Rep. 2014;32(6):2397–2404. | ||
Shen X, Xue Y, Si Y, et al. The unfolded protein response potentiates epithelial-to-mesenchymal transition (EMT) of gastric cancer cells under severe hypoxic conditions. Med Oncol. 2015;32(1):447. | ||
Yu G, Wang J, Chen Y, et al. Overexpression of phosphorylated mammalian target of rapamycin predicts lymph node metastasis and prognosis of Chinese patients with gastric cancer. Clin Cancer Res. 2009;15(5):1821–1829. | ||
Li YZ, Zhao P, Han WD. Clinicopathological significance of LRP16 protein in 336 gastric carcinoma patients. World J Gastroenterol. 2009;15(38):4833–4837. | ||
Li W, Wei Z, Liu Y, Li H, Ren R, Tang Y. Increased 18F-FDG uptake and expression of Glut1 in the EMT transformed breast cancer cells induced by TGF-β. Neoplasma. 2010;57(3):234–240. | ||
Liu M, Quek LE, Sultani G, Turner N. Epithelial-mesenchymal transition induction is associated with augmented glucose uptake and lactate production in pancreatic ductal adenocarcinoma. Cancer Metab. 2016;4:19. | ||
Dong C, Yuan T, Wu Y, et al. Loss of FBP1 by Snail-mediated repression provides metabolic advantages in basal-like breast cancer. Cancer Cell. 2013;23(3):316–331. | ||
Kondaveeti Y, Guttilla Reed IK, White BA. Epithelial–mesenchymal transition induces similar metabolic alterations in two independent breast cancer cell lines. Cancer Lett. 2015;364(1):44–58. | ||
Walenta S, Mueller-Klieser WF. Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol. 2004;14(3):267–274. | ||
Wang J, Wang H, Liu A, Fang C, Hao J, Wang Z. Lactate dehydrogenase A negatively regulated by miRNAs promotes aerobic glycolysis and is increased in colorectal cancer. Oncotarget. 2015;6(23):19456–19468. | ||
Xian ZY, Liu JM, Chen QK, et al. Inhibition of LDHA suppresses tumor progression in prostate cancer. Tumour Biol. 2015;36(10):8093–8100. | ||
Xie H, Valera VA, Merino MJ, et al. LDH-A inhibition, a therapeutic strategy for treatment of hereditary leiomyomatosis and renal cell cancer. Mol Cancer Ther. 2009;8(3):626–635. | ||
Sun X, Sun Z, Zhu Z, et al. Clinicopathological significance and prognostic value of lactate dehydrogenase A expression in gastric cancer patients. PLoS One. 2014;9(3):e91068. | ||
Dai YH, Tang YP, Zhu HY, et al. ZEB2 promotes the metastasis of gastric cancer and modulates epithelial mesenchymal transition of gastric cancer cells. Dig Dis Sci. 2012;57(5):1253–1260. | ||
Miao L, Xiong X, Lin Y, et al. Down-regulation of FoxM1 leads to the inhibition of the epithelial-mesenchymal transition in gastric cancer cells. Cancer Genet. 2014;207(3):75–82. | ||
Ding WJ, Zhou M, Chen MM, Qu CY. HOXB8 promotes tumor metastasis and the epithelial-mesenchymal transition via ZEB2 targets in gastric cancer. J Cancer Res Clin Oncol. 2017;143(3):385–397. | ||
Jiang SB, He XJ, Xia YJ, et al. MicroRNA-145-5p inhibits gastric cancer invasiveness through targeting N-cadherin and ZEB2 to suppress epithelial–mesenchymal transition. Onco Targets Ther. 2016;9:2305–2315. | ||
Geng DM, Kan XM, Zhang WW. Effect of ZEB2 silencing on cisplatin resistance in gastric cancer. Eur Rev Med Pharmacol Sci. 2017;21(8):1746–1752. | ||
Jiang T, Dong P, Li L, et al. MicroRNA-200c regulates cisplatin resistance by targeting ZEB2 in human gastric cancer cells. Oncol Rep. 2017;38(1):151–158. | ||
Yoo YG, Christensen J, Gu J, Huang LE. HIF-1α mediates tumor hypoxia to confer a perpetual mesenchymal phenotype for malignant progression. Sci Signal. 2011;4(178):pt4. | ||
Terry S, Buart S, Tan TZ, et al. Acquisition of tumor cell phenotypic diversity along the EMT spectrum under hypoxic pressure: consequences on susceptibility to cell-mediated cytotoxicity. 2017;6(2):e1271858. | ||
Depner C, Zum Buttel H, Böğürcü N, et al. EphrinB2 repression through ZEB2 mediates tumour invasion and anti-angiogenic resistance. Nat Commun. 2016;7:12329. | ||
Lu H, Forbes RA, Verma A. Hypoxia-inducible factor 1 activation by aerobic glycolysis implicates the Warburg effect in carcinogenesis. J Biol Chem. 2002;277(26):23111–23115. | ||
Myszczyszyn A, Czarnecka AM, Matak D, et al. The role of hypoxia and cancer stem cells in renal cell carcinoma pathogenesis. Stem Cell Rev. 2015;11(6):919–943. | ||
Inukai M, Hara A, Yasui Y, Kumabe T, Matsumoto T, Saegusa M. Hypoxia-mediated cancer stem cells in pseudopalisades with activation of hypoxia-inducible factor-1α/Akt axis in glioblastoma. Hum Pathol. 2015;46(10):1496–1505. | ||
Cui CP, Wong CC, Kai AK, et al. SENP1 promotes hypoxia-induced cancer stemness by HIF-1α deSUMOylation and SENP1/HIF-1α positive feedback loop. Gut. 2017;66(12):2149–2159. | ||
Yoon C, Cho SJ, Chang KK, Park DJ, Ryeom SW, Yoon SS. Role of Rac1 pathway in epithelial-to-mesenchymal transition and cancer stem-like cell phenotypes in gastric adenocarcinoma. Mol Cancer Res. 2017;15(8):1106–1116. | ||
Wang B, Chen Q, Cao Y, et al. LGR5 is a gastric cancer stem cell marker associated with stemness and the EMT signature genes NANOG, NANOGP8, PRRX1, TWIST1, and BMI1. PLoS One. 2016;11(12):e0168904. |
Supplementary materials
  | Table S1 Design of siRNA sequences |
  | Table S2 PCR primers used in this study |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.