Back to Journals » Cancer Management and Research » Volume 12
LCN2 Mediated by IL-17 Affects the Proliferation, Migration, Invasion and Cell Cycle of Gastric Cancer Cells by Targeting SLPI
Authors Xu J, Lv S, Meng W, Zuo F
Received 26 August 2020
Accepted for publication 11 November 2020
Published 14 December 2020 Volume 2020:12 Pages 12841—12849
DOI https://doi.org/10.2147/CMAR.S278902
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kenan Onel
Jing Xu,1,2 ShengXiang Lv,1,2 Wei Meng,3 Fang Zuo4
1Department of Gastroenterology, The First People’s Hospital of Lianyungang, Lianyungang, Jiangsu Province, 222000, People’s Republic of China; 2Department of Gastroenterology, First Affiliated Hospital of Kangda College of Nanjing Medical University, Lianyungang, Jiangsu Province 222000, People’s Republic of China; 3Department of Functional Examination, Jinan Central Hospital, Jinan, Shandong Province 250014, People’s Republic of China; 4Department of Gastroenterology, Jinan Central Hospital, Jinan, Shandong Province 250014, People’s Republic of China
Correspondence: Fang Zuo
Department of Gastroenterology, Jinan Central Hospital, Jinan, Shandong Province 250014, People’s Republic of China
Tel +86 13370582853
Email [email protected]
Introduction: Gastric cancer occurred in China and even the whole East Asia with high incidence. The objective of this study was to investigate the role of IL-17 in gastric cancer cells mediated by LCN2 binding to SLPI.
Methods: The expression of LCN2 and SPLI in gastric cancer cells and transfection effects were confirmed by RT-qPCR analysis. The proliferation, clone formation ability, invasion, migration, apoptosis, and cell cycle of gastric cancer cells were in turn detected by CCK-8 assay, clone formation assay, transwell assay, wound healing assay, and flow cytometry analysis. The combination between LCN2 and SLPI was determined by co-immunoprecipitation assay. The expression of Caspase-3, Bcl-2, cyclinB1, cyclinD1, MMP9, and SLPI in gastric cancer cells was detected by Western blot analysis.
Results: LCN2 and SPLI exhibited the highest levels in AGS cells, and thus AGS cells were selected for the next experiments. Down-regulation of LCN2 suppressed the proliferation and clone formation ability of AGS cells treated with IL-17. IL-17 promoted the invasion and migration of AGS cells, which was partially reversed by the down-regulation of LCN2. Down-regulation of LCN2 mediated by IL-17 promoted apoptosis and suppressed the cell cycle of AGS cells.
Discussion: Down-regulation of LCN2 mediated by IL-17 suppressed the proliferation and suppressed the migration and invasion and cell cycle of gastric cancer cells by targeting SLPI.
Keywords: lipocalin-2, LCN2, interleukin-17, IL-17, gastric cancer cells, SLPI
Introduction
Gastric cancer is one of the most common malignant gastrointestinal tumors. According to relevant statistics, the incidence of gastric cancer ranks fourth while its mortality rate ranks second among all malignant tumors. Meanwhile, China’s statistics illustrated that both the incidence and the mortality of gastric cancer rank first among gastrointestinal malignant tumors.1 Every year, about 700,000 patients in the world die from gastric cancer, and there are about 1 million newly developed gastric cancer cases.2 China, as a country with a high incidence of gastric cancer, accounts for 25% of the new gastric cancer cases in the world. The prognosis of gastric cancer patients is very poor, with the 5-year survival rate <25%.3 Therefore, it is urgent to explore the molecular mechanism of the occurrence and development of gastric cancer for the further development of new therapeutic strategies for gastric cancer.
Interleukin-17 (IL-17) is a cytokine mainly produced by Th17 cells with multiple biological effects. It can induce the production of a variety of cytokines, chemokines, inflammatory effector substances and antimicrobial proteins, and thus participate in a variety of autoimmune diseases and infectious inflammation.4 A number of clinical studies have shown that IL-17 or IL-17 mRNA levels can be detected in peripheral blood or tumor tissues of patients with gastric cancer, medulloblastoma, ovarian cancer, colorectal carcinoma, lung cancer, and breast cancer.5–9 IL-17 can promote the carcinogenesis in breast cancer, lung cancer, colon cancer, and gastric cancer.1 By KEGG (https://www.genome.jp/kegg/pathway.html), LCN2 is found to be a downstream protein of the IL-17/NF-κB pathway, and it can be activated by IL-17.
Lipocalin-2 (LCN2) is a neutrophil gelatinase-associated lipocalin mainly secreted by hepatocytes.15 It has been found that LCN2 plays a key role in the differentiation, proliferation, angiogenesis, invasion, and metastasis of tumor cells.16,17 Furthermore, it is abnormally expressed in cervical cancer, oral squamous cell carcinoma, colorectal cancer, and breast cancer.16–19 High expression of LCN2 can promote the invasion and metastasis of tumor cells by enhancing MMP9 activity17,20 and inducing epithelial-mesenchymal transformation (EMT).21,22 By STRING (https://string-db.org), LCN2 can combine with secretory leukocyte peptidase inhibitor (SLPI), which is highly expressed in gastric cancer, and can promote the proliferation, invasion, and metastasis of gastric cancer cells.23
Overall, we speculated that IL-17 might affect the proliferation, migration and invasion, and cell cycle of AGS cells, and it could mediate LCN2 which binds to SLPI.
Materials and Methods
Cell Culture and Cell Transfection
Gastric parietal cell line (HGT-1 cells) and gastric cell lines (N87, Fu97, AGS and MKN-45 cells) were provided from Shanghai Zishi Biotechnology Co., Ltd. (Shanghai, China). The cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 10 μg/mL streptomycin, and 100 U/mL penicillin, and they were incubated at 37°C with 5% CO2 in a humidified atmosphere. Routine liquid exchange and subculture were conducted. Gastric cancer cells at the logarithmic growth stage were re-suspended after digestion, counted, and planked. The plasmids of shRNA-NC, shRNA-LCN2-1, and shRNA-LCN2-2 were transfected with the Lipofectamine®2000 Reagent at 70%~80% cell density on the next day.
RT-qPCR Analysis
The total RNA from gastric cancer cells was extracted with Trizol reagent. According to the manufacturer’s instructions, a reverse transcription kit was used to reverse transcribe the RNA into cDNA. AceQ qPCR SYBR Green Master Mix kit was used for the quantitative analysis of mRNA in qPCR. β-Actin was used as the internal reference.
CCK-8 Assay
Gastric cancer cells were treated with IL-17, and gastric cancer cells transfected with shRNA-NC or shRNA-LCN2 were also treated with IL-17. All cells were planted in 96-well plates at the density of 5000 cells/mL and incubated in a 5% CO2 and 37°C incubator for 24 h. 10 μL CCK-8 reagent was added to each well and the optical density at 450 nm was determined 2 h later.
Clone Formation Assay
After indicated treatment, all cells were planted in a culture dish containing 10 mL culture medium, cultured at 37°C, incubated for 14 days, and cloned successfully. The cells were fixed with 4% paraformaldehyde for 15 min, and the crystal violet staining solution was added for 20 min, followed by the microscopic observation.
Wound Healing Assay
After indicated treatment, cell suspension at the density of 5×105 cells/mL was routinely cultured to monolayer cells. Sterile 100 μL yellow shot was used to draw a straight line on monolayer-cultured cells to form a cell-free growth area (scratch). The width of scratch area was recorded, and then the shed cells were washed with serum-free medium. After 24 h of conventional culture, the images were observed and obtained under an inverted microscope.
Transwell Assay
Matrigel was pre-coated in the transwell chamber. After indicated treatment, cells were re-suspended to a concentration of 1×105 cells/mL in culture medium of 1% fetal bovine serum. And, 100 μL cell suspension was added to the upper chamber, and 900 μL culture medium containing 10% serum was added to the lower chamber. After 24 h, the transwell chamber was taken out, fixed with 4% paraformaldehyde for 30 min and stained with crystal violet for 20 min. Finally, the images were observed and obtained under an optical microscope.
Flow Cytometry Analysis
Gastric cancer cells at the logarithmic phase were incubated in the six-well plates with each well containing 5×105 cells. After treatment of IL-17, cisplatin (DDP), and transfection, cells were cultured in an incubator for another 48 h. The cells were digested with EDTA-free pancreatic enzyme and collected into a centrifuge tube. The cells were centrifuged at 1000 r/min for 5 min and the supernatant was discarded. Then, cells were washed with cold PBS, centrifuged at 1000 r/min for 5 min to discard the supernatant and blended with binding buffer to be transferred to a flow tube. Five microlitres of Annexin V and propidium iodide (PI) were added to the cells, which were mixed gently and kept away from light at 4°C for 20 min. After staining, 0.5 mL binding buffer was added to each flow tube, and the apoptotic ratio and cell cycle of each tube were determined by flow cytometry.
Co-Immunoprecipitation Assay
The gastric cells at the logarithmic growth phase were added with cell lysis buffer and placed on the ice for lysis. After centrifugation at 4°C, the supernatant was taken and corresponding antibodies were added, respectively. After incubation at 4°C for 1 h, protein A/G agarose beads were added. Then, the protein A/G glycoprotein beads in the precipitation were gently washed and rinsed with cell lysis buffer for three times. Finally, the protein loading buffer was added and boiled in boiling water for 5 min. Western blot was used to detect the target proteins and confirm the binding proteins.
Western Blot Analysis
The logarithmic growth phase cells were collected, treated with IL-17 and DDP, and transfected. The total cell protein was extracted with RIPA on ice. After full lysis, the cells were isolated at 10,000 r/min at 4°C for 10 min. The supernatant was taken and the protein concentration was determined according to the instructions of the BCA kit. After being separated by 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), 30 μg total protein was transferred to cellulose nitrate film and sealed with 5% skim milk at room temperature for 1 h. After incubation with Caspase-3, Bcl-2, cyclinB1, cyclinD1, MMP9, SLPI and GAPDH at 4°C overnight. HRP-labeled secondary antibody was added to the cellulose nitrate film on the second day, which was incubated at room temperature for 1 h. The protein bands were observed by an enhanced chemiluminescence detection system.
Statistical Analysis
SPSS 23.0 statistical software was applied for statistical analysis and GraphPad Prism 5 was used to make figures. Experimental data are represented as mean ± standard deviation. One-way analysis of variance coupled with Tukey post hoc was used to evaluate intergroup differences. P<0.05 was considered statistically significant.
Results
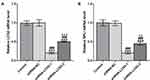
The Expression of LCN2 and SPLI in Gastric Cancer Cell Lines
The expression of LCN2 in gastric cancer cell lines (SCG-7901, Fu97, AGS and HST2) was increased compared with that in HGT-1 cells (Figure 1A). Likewise, the expression of SPLI in gastric cancer cell lines (SCG-7901, Fu97, AGS and HST2) was increased compared with that in HGT-1 cells (Figure 1B).LCN2 and SPLI showed the highest levels in AGS cells among gastric cancer cell lines, and thus AGS cell line was chosen for the subsequent experiments.
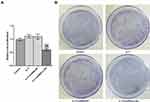
AGS Cells are Transfected
AGS cells were respectively transfected with shRNA-NC, shRNA-LCN2-1, and shRNA-LCN2-2. The expression of LCN2 in AGS cells transfected with shRNA-LCN2-1/2 was decreased compared with that in the control group and the shRNA-NC group. There was no obvious difference in LCN2 expression in AGS cells between the control group and the shRNA-NC group (Figure 2A). The changes of SPLI in these four groups were consistent with that of LCN2 (Figure 2B). AGS cells transfected with shRNA-LCN2-1 exhibited the lowest level of LCN2 and SPLI, and shRNA-LCN2-1 was selected for the follow-up experiments.
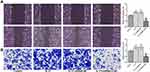
Down-Regulation of LCN2 Mediating IL-17 Suppresses the Proliferation and Clone Formation of AGS Cells
The proliferation of AGS cells was not obviously changed by IL-17. Down-regulation of LCN2 mediating IL-17 suppressed the proliferation of AGS cells. The proliferation of AGS cells treated with IL-17 was not obviously changed compared with those treated with IL-17 and transfected with shRNA-NC (Figure 3A). As shown in Figure 3B, IL-17 did not affect the clone formation of AGS cells and shRNA-NC also had no obvious effect on the clone formation of IL-17-treated AGS cells. Down-regulation of LCN2 mediating IL-17 suppressed the clone formation of AGS cells (Figure 3B).
Down-Regulation of LCN2 Mediated by IL-17 Suppresses the Migration and Invasion of AGS Cells
IL-17 promoted the migration (Figure 4A) and invasion (Figure 4B) of AGS cells. There is no obvious difference in migration and invasion of AGS cells between the IL-17 group and the IL17+shRNA-NC group. Down-regulation of LCN2 weakened the promotion effect of IL-17 on the migration and invasion of AGS cells.
Down-Regulation of LCN2 Mediated by IL-17 Promotes Apoptosis and Suppresses Cell Cycle of AGS Cells
DDP enters into cells and blocks transcription and DNA replication, leading to cell cycle arrest and cell apoptosis.24 In 1965, DDP was confirmed to have a broad-spectrum anticancer effect,25 showing remarkable efficacy in the treatment of various cancers, including lung cancer, ovarian cancer, and gastric cancer. However, long-term use of DDP in gastric cancer patients will increase the risk of developing drug resistance to it. Therefore, how to treat gastric cancer with DDP resistance is worth studying. IL-17 had no obvious effect on apoptosis of AGS cells while DDP promoted the apoptosis of AGS cells. IL-17 suppressed the apoptosis of AGS cells treated with DDP, and down-regulation of LCN2 mediated by IL-17 promoted the apoptosis of AGS cells treated with DDP (Figure 5A). IL-17 did not cause the activation and acceleration of cell cycle of AGS cells, and DDP could induce G2/M arrest and block the G2 phase of AGS cells. The inhibition of DDP on cell cycle was antagonized by IL-17, and the antagonistic effect of IL-17 was reversed by the down-regulation of LCN2 (Figure 5B).
LCN2 Can Combine with SLPI
When LCN2 antibody was added to lysates of AGS cells, protein expression of LCN2 was observed in the lysates. The protein expression of SLPI still existed in lysates of AGS cells added with LCN2 antibody (Figure 6).
 |
Figure 6 LCN2 can combine with SLPI. The interaction between LCN2 and SLPI was determined by co-immunoprecipitation assay. ***P<0.001 vs input group. ###P<0.001 vs IgG group. |
Down-Regulation of LCN2 Affects Cell Phenotype-Associated Proteins in AGS Cells
As shown in Figure 7, IL-17 promoted the expression of MMP9 and Bcl-2 in AGS cells. DDP increased the Caspase-3 expression and decreased the expression of Bcl-2, cyclinB1, MMP9, and SLPI in AGS cells. The expression of Bcl-2 and MMP9 was increased in AGS cells treated with DDP and IL-17. Down-regulation of LCN2 promoted the Caspase-3 expression and suppressed the expression of Bcl-2, cyclinD1, MMP9 and SLPI in AGS cells treated with DDP and IL-17.
Discussion
Gastric cancer is characterized by tumor metastasis and invasiveness. The existing treatments for gastric cancer are relatively limited, and tremendous efforts have been devoted to developing new therapeutic strategies for gastric cancer. In the present study, expression of LCN2 and SPLI was increased in gastric cancer cells. In addition, down-regulation of LCN2 mediated by IL-17 suppressed the proliferation, migration and invasion, and cell cycle of gastric cancer cells by targeting SLPI.
Inflammatory response has been described as 1 of the top 10 characteristics of tumors.26 Chronic inflammatory response can damage gastric mucosa and induce the formation of gastric cancer.27 Various inflammatory factors secreted by inflammatory cells promote the occurrence and metastasis of tumors in different ways, so inhibiting inflammatory response is one of the key links in the prevention and treatment of gastric cancer. IL-17 is a pro-inflammatory cytokine that stimulates the NF-κB signaling pathway for the transcription of downstream effectors and promotes cell proliferation and angiogenesis. It is also involved in inflammatory response, which promotes the development of gastric cancer.28 LCN2 is a downstream protein of the IL-17/NF-κB pathway. In this study, IL-17 has no obvious effect on the proliferation, clone formation ability, apoptosis, and cell cycle of gastric cancer cells, which could be affected by the down-regulation of LCN2.
SLPI belongs to the Kazal serine protease inhibitor family and has the ability to regulate cell differentiation and proliferation.29 Relevant studies have shown that SLPI mRNA and protein were significantly elevated in gastric cancer tissues.31,32 SLPI expression in ovarian cancer cells was significantly higher than that in normal cells, and thus it was associated with tumor progression.30 Therefore, SLPI could be a potentially useful tissue-specific promoter (TSP) for ovarian cancer.33 SLPI inhibition suppressed the proliferation, migration, and invasion of colorectal cancer cells.34 In addition, IL-17 promoted the DDP resistance of cells in colorectal cancer and ovarian cancer.35,36 In this study, DDP promoted the apoptosis of gastric cancer cells, and SLPI expression was decreased accordingly after DDP treatment. IL-17 up-regulated the SLPI expression and antagonized the inhibitory effect of DDP on gastric cancer cells. Down-regulation of LCN2 could inhibit the proliferation and cell cycle, and could promote the apoptosis of gastric cancer cells by decreasing the SLPI expression.
In conclusion, IL-17 suppressed the proliferation, clone formation, migration, invasion, and cell cycle, and promoted the apoptosis of AGS cells by down-regulating LCN2 expression and SLPI expression.
Funding
There is no funding to report.
Disclosure
The authors declare they have no conflicts of interest.
References
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7–30.
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
3. Feng W, Ding Y, Zong W, Ju S. Non-coding RNAs in regulating gastric cancer metastasis. Clinica Chimica Acta. 2019;496:125–133.
4. Onishi R, Gaffen S. IL-17 and its target genes: mechanisms of IL-17 function in disease. Immunology. 2010;129(3):311–321.
5. Zhang B, Rong G, Wei H, et al. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374(3):533–537.
6. Zhou P, Sha H, Zhu J. The role of T-helper 17 (Th17) cells in patients with medulloblastoma. J Int Med Res. 2010;38(2):611–619.
7. Kato T, Furumoto H, Ogura T, et al. Expression of IL-17 mRNA in ovarian cancer. Biochem Biophys Res Commun. 2001;282(3):735–738.
8. Liu J, Duan Y, Cheng X, et al. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407(2):348–354.
9. Chen X, Wan J, Liu J, et al. Increased IL-17-producing cells correlate with poor survival and lymphangiogenesis in NSCLC patients. Lung Cancer. 2010;69(3):348–354.
10. Amara S, Majors C, Roy B, et al. Critical role of SIK3 in mediating high salt and IL-17 synergy leading to breast cancer cell proliferation. PLoS One. 2017;12(6):e0180097.
11. Cao Y, Zhao D, Li P, et al. MicroRNA-181a-5p Impedes IL-17-induced nonsmall cell lung cancer proliferation and migration through targeting VCAM-1. Cell Physiol Biochem. 2017;42(1):346–356.
12. Chen Z, Cao J, Yang P, et al. Effect of interleukin 17 on invasion of human colon cancer cells. Chin j Gastrointestinal Surg. 2016;19(6):695–701.
13. Iida T, Iwahashi M, Katsuda M, et al. Tumor-infiltrating CD4+ Th17 cells produce IL-17 in tumor microenvironment and promote tumor progression in human gastric cancer. Oncol Rep. 2011;25(5):1271–1277.
14. Wu X, Yang T, Liu X, et al. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumour Biol. 2016;37(4):5493–5501.
15. Goetz D, Holmes M, Borregaard N, Bluhm M, Raymond K, Strong R. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore-mediated iron acquisition. Mol Cell. 2002;10(5):1033–1043.
16. Hiromoto T, Noguchi K, Yamamura M, et al. Up-regulation of neutrophil gelatinase-associated lipocalin in oral squamous cell carcinoma: relation to cell differentiation. Oncol Rep. 2011;26(6):1415–1421.
17. Hu C, Yang K, Li M, Huang W, Zhang F, Wang H. Lipocalin 2: a potential therapeutic target for breast cancer metastasis. OncoTargets Ther. 2018;11:8099–8106.
18. Scotto L, Narayan G, Nandula S, et al. Identification of copy number gain and overexpressed genes on chromosome arm 20q by an integrative genomic approach in cervical cancer: potential role in progression. Genes Chromosomes Cancer. 2008;47(9):755–765.
19. Kim S, Lee S, Min I, et al. Lipocalin 2 negatively regulates cell proliferation and epithelial to mesenchymal transition through changing metabolic gene expression in colorectal cancer. Cancer Sci. 2017;108(11):2176–2186.
20. Koh S, Lee K. HGF mediated upregulation of lipocalin 2 regulates MMP9 through nuclear factor-κB activation. Oncol Rep. 2015;34(4):2179–2187.
21. Ding G, Fang J, Tong S, et al. Over-expression of lipocalin 2 promotes cell migration and invasion through activating ERK signaling to increase SLUG expression in prostate cancer. The Prostate. 2015;75(9):957–968.
22. Chung I, Wu T, Liao C, et al. Overexpression of lipocalin 2 in human cervical cancer enhances tumor invasion. Oncotarget. 2016;7(10):11113–11126.
23. Du X, Liu X, Wang Z, Wang Y. SLPI promotes the gastric cancer growth and metastasis by regulating the expression of P53, Bcl-2 and Caspase-8. Eur Rev Med Pharmacol Sci. 2017;21(7):1495–1501.
24. Louvet C, André T, Tigaud JM, et al. Phase II study of oxaliplatin, fluorouracil, and folinic acid in locally advanced or metastatic gastric cancer patients. J Clin Oncol. 2002;20(23):4543–4548.
25. Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358(1):36–46.
26. Hanahan D, Weinberg Robert A. Hallmarks of Cancer: the Next Generation. Cell. 2011;144(5):646–674.
27. Nguyen PM, Putoczki TL. Could the inhibition of IL-17 or IL-18 be a potential therapeutic opportunity for gastric cancer? Cytokine. 2019;118:8–18.
28. Li C, Su Y, Gong H, et al. Immune regulation of IL-17 through NF-κB signaling pathway involved in the development of gastric cancer. Basic Clin Med. 2020;40(07):923–928.
29. Bouchard D, Morisset D, Bourbonnais Y, Tremblay GM. Proteins with whey-acidic-protein motifs and cancer. Lancet Oncol. 2006;7(2):167–174.
30. Shigemasa K, Tanimoto H, Underwood L, et al. Expression of the protease inhibitor antileukoprotease and the serine protease stratum corneum chymotryptic enzyme (SCCE) is coordinated in ovarian tumors. Int j Gynecol Cancer. 2001;11(6):454–461.
31. Cheng W-L, Wang C-S, Huang Y-H, et al. Overexpression of a secretory leukocyte protease inhibitor in human gastric cancer. Int J Cancer. 2008;123:1787–1796.
32. Choi B-D, Jeong S-J, Wang G, et al. Secretory leukocyte protease inhibitor is associated with MMP-2 and MMP-9 to promote migration and invasion in SNU638 gastric cancer cells. Int J Mol Med. 2011;28:527–534.
33. Barker S, Coolidge C, Kanerva A, et al. The secretory leukoprotease inhibitor (SLPI) promoter for ovarian cancer gene therapy. J Gene Med. 2003;5(4):300–310.
34. Wei Z, Liu G, Jia R, et al. Targeting secretory leukocyte protease inhibitor (SLPI) inhibits colorectal cancer cell growth, migration and invasion via downregulation of AKT. PeerJ. 2020;8:e9400.
35. Sui G, Qiu Y, Yu H, Kong Q, Zhen B. Interleukin-17 promotes the development of cisplatin resistance in colorectal cancer. Oncol Lett. 2019;17(1):944–950.
36. Zhang H, Niu X, Duan H, et al. Study of the effects and underlying mechanisms of IL −17A on the cisplatin -based resistance of ovarian cancer. J Tianjin Med Univ. 2017;23(03):
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.