Back to Journals » Infection and Drug Resistance » Volume 15
Late-Onset Sepsis in a Premature Infant Mediated by Breast Milk: Mother-to-Infant Transmission of Group B Streptococcus Detected by Whole-Genome Sequencing
Authors Li A, Fang M, Hao D, Wu Q, Qian Y, Xu H, Zhu B
Received 15 July 2022
Accepted for publication 7 September 2022
Published 9 September 2022 Volume 2022:15 Pages 5345—5352
DOI https://doi.org/10.2147/IDR.S381466
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Aiyun Li,1,* Ming Fang,2,* Dongjie Hao,1 Qiaoai Wu,1 Yaqi Qian,1 Hao Xu,3,4 Bo Zhu1
1Department of Clinical Medicine, The Women’s Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China; 2Institute of Infection Disease Control, Shandong Center for Disease Control and Prevention, Jinan, People’s Republic of China; 3State Key Laboratory for Diagnosis and Treatment of Infectious Disease, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China; 4Department of Structure and Morphology, Jinan Microecological Biomedicine Shandong Laboratory, Jinan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hao Xu; Bo Zhu, Email [email protected]; [email protected]
Background: Late-onset group B Streptococcus (LOGBS) sepsis is a cause of infection and death in infants. Infected breast milk has been considered a source of neonatal GBS infection and invasive infection. However, mother-to-infant transmission of GBS detected by the high-resolution diagnostic method is rarely reported.
Methods: This study describes a low-weight premature infant who developed late-onset GBS septicemia 21 days after birth. GBS strains isolated from the mother’s cervical secretion, the mother’s milk, and the baby’s blood were cultured to identify the source of GBS infection. We further confirmed the GBS isolates through matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS). Finally, we performed whole-genome sequencing (WGS) and phylogenetic analyses on the GBS strains recovered.
Results: GBS isolates were cultured from the bloodstream of the premature infant and the mother’s milk, respectively. Subsequently, WGS and phylogenetic analyses on three GBS isolates demonstrated that the GBS strain from the infant’s bloodstream was 100% homologous to that from the mother’s breast milk, which had some different gene fragments from the GBS strain from the mother’s cervical secretion. It provided evidence that this infant’s late-onset GBS septicemia originated from his mother’s breast milk instead of the vertical mother-to-infant transmission.
Conclusion: Through WGS and phylogenetic analysis of the GBS strains, we proved in this study that the late-onset GBS sepsis in a premature infant was derived from his mother’s breast milk. It indicated that WGS diagnosis is an effective tool for infection tracing. Furthermore, this report provides direction for preventing late-onset GBS infection.
Keywords: group B Streptococcus, late-onset GBS sepsis, breast milk, whole-genome sequencing, phylogenetic analysis
Introduction
Late-onset neonatal bloodstream infections that occur after the first seven days of age in a preterm infant are illnesses in which a credible pathogen is recovered.1 This disease is associated with some risks, such as low birth weight in infants, long-term child development in NICU (A neonatal intensive care unit), brain injury, and bronchopulmonary dysplasia.2,3 Late-onset neonatal bloodstream infections have different pathogenic bacteria, including gram-negative bacilli, gram-positive cocci, and fungi.4,5
Group B Streptococcus (GBS) or Streptococcus agalactiae infection remains one of the most significant causes of late-onset neonatal sepsis and meningitis among young infants.1,6–8 Thus far, there is insufficient population-based data on GBS late-onset disease (LOD). Previous studies showed that GBS, generally associated with early-onset infections with specific risk factors, has become a pathogenic bacteria of late-onset neonatal bloodstream infections with rising incidence.9 However, transmission routes and risk factors for LOGBS sepsis are not yet fully understood.7,10 It is speculated that 50% of colonized mothers may transfer GBS to their fetuses.11 One proposed transmission route for GBS late-onset disease is infection through contaminated breast milk.11–14 Mother’s breast milk is a source of GBS late-onset disease.14 Interestingly, mechanisms associated with GBS transmission in breast milk remain poorly understood.15
This work provides high-resolution evidence of mother-to-infant GBS transmission by integrating genomic and epidemiological data. Our findings strongly evidenced the transmission of GBS sepsis via maternal breast milk.
Materials and Methods
Bacterial Isolation and Identification
Cervical secretion and breast milk samples from the mother, and the blood sample from the baby were collected separately. Each sample was innoculate on a Columbia Blood agar plate (Autobio, China) to incubated for 18–24 hours at 37℃. Bacterial identification was conducted by matrix-assisted laser desorption/ionization-time of flight mass spectrometry (MALDI-TOF-MS) (Bruker, Leipzig, Germany) and further confirmed by PCR and 16S rRNA sequencing.16
Whole-Genome Sequencing (WGS) and Analysis
Whole-genome sequencing was performed on the GBS strains. Genomic DNA was extracted using a commercial kit (MPBIO, FastDNA SPIN Kit for Soil, USA) and then subjected to WGS with an Illumina Novaseq 6000 platform (Novogene Co., China). Paired reads were assembled into several scaffolds using Spades (version 3.9.1).17 In silico multilocus sequence typing (MLST) (https://pubmlst.org/bigsdb?db=pubmlst_sagalactiae_seqdef) analysis was performed as described previously.18 Virulence genes were identified using the Virulence Factor database.19
Phylogenetic Analysis of GBS
We created a core genome-based phylogenetic tree using three S. agalactiae genomes sequenced in this study. We randomly selected 122 public S. agalactiae genomes from China to further characterize the evolutionary relationship among GBS isolates (Supplementary Table 1). All collection genomes were annotated using RAST.20 The core genes in S. agalactiae genomes were identified using Rast and Roary.21 Core genes were defined previously.22 A maximum-likelihood phylogenetic tree based on the core single nucleotide polymorphism alignments was generated using MEGA X.23 Phylogenetic tree visualizations were produced using the Interactive Tree of Life (version 6.5.8) (https://itol.embl.de/).
Results
Case Presentation
A 29-year-old pregnant woman was presented to the emergency department on May 10, 2021, complaining of amenorrhoea for 31 + 4 weeks and vaginal discharge for more than 3 hours. The critical nodes in the diagnosis and treatment process are shown in Figure 1. She was diagnosed with premature rupture of fetal membranes. Dexamethasone was applied to promote fetal lung maturation by intramuscular injection. To prevent infection, the pregnant woman was administered cefuroxime sodium (1.5g, qd) and azithromycin (0.5g, qd) intravenously for two days, followed by oral cefuroxime axetil tablets (0.25g, bid) and azithromycin tablets (0.5g, qd) for four days. Once admission, we collected her vaginal and rectal swabs for the nucleic acid test of group B streptococcus (GBS), which both reported GBS-DNA positive. In addition, the examination of cervical secretion revealed ureaplasma urealyticum (UU) DNA positive and the growth of Candida albicans and S. agalactiae.
 |
Figure 1 The critical nodes in the diagnosis and treatment process. Abbreviations: GBS, Group B streptococcal; WGS, whole-genome sequencing; UU, ureaplasma urealyticum. |
On the sixth day after admission, the pregnant woman appeared to have regular contractions with an opened uterine orifice. Considering that she was a GBS carrier and strived for vaginal delivery, the pregnant woman was administrated with penicillin G intravenously at the dose of 4800 mg/kg for the first time and 2400 mg/kg in the follow-up until childbirth.
At 32 + 2 weeks gestation, a male preterm infant weighed 1710 g and was delivered via the vagina. Newborn Apgar scores at 5 and 10 minutes were 9 and 10, respectively. The infant was diagnosed with neonatal respiratory distress syndrome (NRDS) in the following first hour, presenting with a less rosy complexion, tachypnea, and groan. Umbilical cord blood culture at birth was negative. Intermittent nasogastric feeding (INGF) with breast milk was started on the second day of the infant’s life, with full feeding achieved within ten days.
At 20 days after birth, the infant’s weight had increased to 2310 g. However, he developed groan, with fluctuant percutaneous SPO2, apnea of 2 times, the body temperature of 37.9 ℃, and the highest heart rate of 210 times/min. His whole body was light spotted, with abdominal distention. Endotracheal intubation, artificial ventilator support, fasting, gastrointestinal decompression, and intravenous rehydration were immediately initiated for this infant. Besides, meropenem and vancomycin were intravenously used for anti-infective therapy. We collected the infant’s blood and cerebrospinal fluid for culture. The microbiological cerebrospinal fluid showed no abnormality. Three days later, two vials of blood cultures reported positive results for GBS, which was susceptible to penicillin G. The infant was diagnosed with neonatal late-onset septicemia. Next, vancomycin was stopped, and penicillin G (100 mg/kg, Q8H) was used instead. After four days of anti-infection therapy, the child’s temperature returned to normal. The ventilator was stopped. The infant presented light shortness of breath under atmospheric inhalation, without abnormal blood oxygen saturation and apnea fluctuations.
The infant’s mother was a GBS carrier before delivery. She had been treated with penicillin G. After childbirth, her temperature was normal, and no apparent signs of infection were observed at first. Nevertheless, on the ninth day postpartum, she developed recurrent fevers with the highest temperature of 39.5℃, accompanied by galactostasis, breast tension, and breast pain. In the beginning, she adopted the physical cooling method herself. After ten days of fever, she visited the hospital and was given penicillin G intravenously for two days until her temperature returned to normal. It’s worth noting that her baby has continually been fed breast milk during her fever. We speculated that the late-onset GBS septicemia of the infant might be caused by contaminated breast milk. Thus, we collected the mother’s breast milk for bacterial culture, and the result showed that GBS was positive.
WGS and Comparative Analysis for GBS
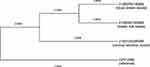
Three GBS strains isolated from the mother’s cervical secretion, the mother’s breast milk, and the child’s blood were culture-purified. Next, we performed WGS and phylogenetic analysis on the three GBS strains. The GBS isolates from the infant’s bloodstream and maternal breast milk had a high consistency with 100% homology in homology analysis (Figure 2). In contrast, the GBS isolates from the mother’s perinatal cervical secretion differed in homology analysis, although they all belonged to the clade IV (Figure 3). Thus, we concluded that the infant’s infection with GBS was more likely to have originated from his mother’s breast milk instead of the vertical transmission from the birth canal to the infant.
Phylogenetic Relationship Between GBS Population
Of the total of 125 isolates analysis in this work, 96 (76.8%) were recovered from human sources. In detail, 45 (45/96, 46.9%) isolates were derived from human blood and cerebrospinal fluid, and thus considered invasive. The remaining 51 isolates, mainly from the cervix, vaginal, and urine (51/96, 53.1%), were considered colonizing isolates (Supplementary Table 1). The phylogenetic analysis resulting from the different SNP calling methods are highly similar and exhibited that the tree topology of the GBS collection is highly robust with respect to the SNP calling method. The results showed that the GBS collection analyzed in this work was clearly classified into five main clades (Figure 3). Notably, three isolates recovered from this study were phylogenetically closely related, indicating potential clonal dissemination in the hospital. Surprisingly, sepsis and breast milk isolates from this work are highly related to food-related strain (GCA_002104835), isolated from Schizothorax prenanti from Sichuan Province, China (Supplementary Table 1).
Discussion
Late-onset GBS transmission routes are not fully understood. Transmission routes underlying late-onset diseases are more complex and have been linked to various sources, including breast milk, household contacts, nosocomial, or community sources.24 Nosocomial transmission has been confirmed for infants born to GBS-negative mothers.25 Community transmission of GBS can also occur in formula-fed babies.26 However, the relationship between GBS in breast milk and LOS with GBS is still vastly debated. Thus far, limited case studies detect GBS transmission to the neonate through breast milk.27 It is worthy to note that previous investigations suggested that breast milk GBS may be from a newborn colonized with GBS at birth and then infecting the breast during feeds, which leads to increased bacteria in breast milk and then causes LOGBS.15,28
S. agalactiae is an important mastitis pathogen because of its highly contagious nature and ability to degrade milk quality.29 GBS infection is the most common occurrence in cattle.30,31 Hence, its species name S. agalactiae is derived. Most infected cattle show no overt signs of disease, such as abnormal milk but have high somatic cell counts and decreased milk production.29
Recently, some reports have provided no significant shifts in the incidence of late-onset GBS disease, in contrast to the considerable decline in early-onset GBS disease seen from 2003 to 2010.32 However, among infants with onset disease on day >7 of life, intrapartum antibiotics do not seem to prevent the development of late-onset GBS disease, which causes a substantial financial burden and causes severe disability syndrome, long-term sequelae, and even death.33 Prevention of late-onset GBS disease warrants renewed attention, particularly in preterm and low birth weight infants.34
Maternal milk as a source of neonatal GBS disease has been reported rarely.35,36 Recent advances in molecular techniques have enhanced the capabilities of epidemiological analysis of the origin of GBS isolates.11,37 In this case, our results showed that the GBS isolates from the infant bloodstream and maternal breast milk had a high consistency in homology analysis. However, the GBS isolates from the mother’s perinatal cervical secretion exhibited differences in homology analysis. These results showed that GBS isolates that caused infant bloodstream infection were derived from maternal breast milk instead of the mother’s perinatal cervical secretion. The heavy vaginal colonization of GBS from the mother was a recognized factor in the pathogenesis of the early-onset disease. Therefore, the infant lost the presents of early-onset disease. This indicated that the GBS colonization of the mother genital tract is not the cause of late-onset bloodstream disease but the cause of early-onset illness. As a result, the well-known circular process was broken in this case.
The limitation of our study is that the source of GBS strain from breast milk is unidentified. We speculated that maternal milk ducts are infected during breastfeeding, and the infant is re-infected during feeding. This may occur with or without mastitis, which depends on many factors. In this report, the mother showed obvious mastitis, and a high bacterial load in the breast milk may be a factor in the pathogenesis of late-onset neonatal disease. Therefore, the assertion that it is safe to continue breastfeeding during episodes of mastitis might only be valid in cases of milk stasis instead of when there is an infection. Breast milk culture might be necessary to discriminate between the two stages. Preterm infants have a less developed immune system and more frequently have transient hypogammaglobulinemia than term babies.
Conclusion
Through WGS and phylogenetic analysis of the GBS strains, we proved in this study that the late-onset GBS sepsis in a premature infant was derived from his mother’s breast milk. It indicated that WGS diagnosis is an effective tool for infection tracing. Furthermore, this report provides the direction for early diagnosis of late-onset GBS infection.
Data Sharing Statement
The whole-genome sequences of three GBS isolates were deposited in GenBank with the following Biosample accession numbers: SAMN28129759, SAMN28129760, and SAMN28129761.
Ethics Approval and Informed Consent
This report was conducted following the Declaration of Helsinki and approved by the ethics committee of The Women’s Affiliated Hospital, Zhejiang University School of Medicine. The patient provided written informed consent and agreed to the publication of the case.
Funding
This work was partly supported by the National Natural Science Foundation of China (82072314), the Research Project of Jinan Microecological Biomedicine Shandong Laboratory (JNL-2022011B), and CAMS Innovation Fund for Medical Sciences (2019-I2M-5-045).
Disclosure
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Bekker V, Bijlsma MW, van de Beek D, Kuijpers TW, van der Ende A. Incidence of invasive group B streptococcal disease and pathogen genotype distribution in newborn babies in the Netherlands over 25 years: a nationwide surveillance study. Lancet Infect Dis. 2014;14(11):1083–1089. doi:10.1016/S1473-3099(14)70919-3
2. Jauneikaite E, Kapatai G, Davies F, et al. Serial clustering of late-onset group B Streptococcal infections in the neonatal unit: a genomic re-evaluation of causality. Clin Infect Dis. 2018;67(6):854–860. doi:10.1093/cid/ciy174
3. Heath PT, Jardine LA. Neonatal infections: group B streptococcus. BMJ Clin Evid. 2014;2014:0323.
4. Kaufman D, Fairchild KD. Clinical microbiology of bacterial and fungal sepsis in very-low-birth-weight infants. Clin Microbiol Rev. 2004;17(3):638–680. doi:10.1128/CMR.17.3.638-680.2004
5. Laurent F, Butin M. Staphylococcus capitis and NRCS-A clone: the story of an unrecognized pathogen in neonatal intensive care units. Clin Microbiol Infect. 2019;25(9):1081–1085. doi:10.1016/j.cmi.2019.03.009
6. Madrid L, Seale AC, Kohli-Lynch M, et al. Infant group B streptococcal disease incidence and serotypes worldwide: systematic review and meta-analyses. Clin Infect Dis. 2017;65(suppl_2):S160–S172. doi:10.1093/cid/cix656
7. Karampatsas K, Davies H, Mynarek M, Andrews N, Heath PT, Le Doare K. Clinical risk factors associated with late-onset invasive group B Streptococcal disease: systematic review and meta-analyses. Clin Infect Dis. 2022. doi:10.1093/cid/ciac206
8. O’Sullivan CP, Lamagni T, Patel D, et al. Group B streptococcal disease in UK and Irish infants younger than 90 days, 2014-15: a prospective surveillance study. Lancet Infect Dis. 2019;19(1):83–90. doi:10.1016/S1473-3099(18)30555-3
9. Wojkowska-Mach J, Chmielarczyk A, Strus M, Lauterbach R, Heczko P. Neonate bloodstream infections in organization for economic cooperation and development countries: an update on epidemiology and prevention. J Clin Med. 2019;8:10. doi:10.3390/jcm8101750
10. Kolter J, Henneke P. Codevelopment of microbiota and innate immunity and the risk for group b streptococcal disease. Front Immunol. 2017;8:1497. doi:10.3389/fimmu.2017.01497
11. Ager EPC, Steele ED, Nielsen LE, Nestander MA, Mende K, Spencer SE. Hypervirulent Streptococcus agalactiae septicemia in twin ex-premature infants transmitted by breast milk: report of source detection and isolate characterization using commonly available molecular diagnostic methods. Ann Clin Microbiol Antimicrob. 2020;19(1):55. doi:10.1186/s12941-020-00396-6
12. Zimmermann P, Gwee A, Curtis N. The controversial role of breast milk in GBS late-onset disease. J Infect. 2017;74(Suppl 1):S34–S40. doi:10.1016/S0163-4453(17)30189-5
13. Nicolini G, Borellini M, Loizzo V, Creti R, Memo L, Berardi A. Group B streptococcus late-onset disease, contaminated breast milk and mothers persistently GBS negative: report of 3 cases. BMC Pediatr. 2018;18(1):214. doi:10.1186/s12887-018-1192-x
14. Widger J, O’Connell NH, Stack T. Breast milk causing neonatal sepsis and death. Clin Microbiol Infect. 2010;16(12):1796–1798. doi:10.1111/j.1469-0691.2010.03071.x
15. Le Doare K, Kampmann B. Breast milk and Group B streptococcal infection: vector of transmission or vehicle for protection? Vaccine. 2014;32(26):3128–3132. doi:10.1016/j.vaccine.2014.04.020
16. Zheng B, Feng C, Xu H, et al. Detection and characterization of ESBL-producing Escherichia coli expressing mcr-1 from dairy cows in China. J Antimicrob Chemother. 2019;74(2):321–325. doi:10.1093/jac/dky446
17. Bankevich A, Nurk S, Antipov D, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19(5):455–477. doi:10.1089/cmb.2012.0021
18. Khan UB, Jauneikaite E, Andrews R, Chalker VJ, Spiller OB. Identifying large-scale recombination and capsular switching events in Streptococcus agalactiae strains causing disease in adults in the UK between 2014 and 2015. Microb Genom. 2022;8(3):000783.
19. Liu B, Zheng D, Zhou S, Chen L, Yang J. VFDB 2022: a general classification scheme for bacterial virulence factors. Nucleic Acids Res. 2022;50(D1):D912–D917. doi:10.1093/nar/gkab1107
20. Overbeek R, Olson R, Pusch GD, et al. The SEED and the Rapid Annotation of microbial genomes using Subsystems Technology (RAST). Nucleic Acids Res. 2014;42(Databaseissue):D206–D214. doi:10.1093/nar/gkt1226
21. Page AJ, Cummins CA, Hunt M, et al. Roary: rapid large-scale prokaryote pan genome analysis. Bioinformatics. 2015;31(22):3691–3693. doi:10.1093/bioinformatics/btv421
22. Holt KE, Wertheim H, Zadoks RN, et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A. 2015;112(27):E3574–E3581.
23. Kumar S, Stecher G, Li M, Knyaz C, Tamura K. MEGA X: molecular evolutionary genetics analysis across computing platforms. Mol Biol Evol. 2018;35(6):1547–1549. doi:10.1093/molbev/msy096
24. Furuta A, Brokaw A, Manuel G, et al. Bacterial and host determinants of group B Streptococcal infection of the neonate and infant. Front Microbiol. 2022;13:820365. doi:10.3389/fmicb.2022.820365
25. Kim HJ, Kim SY, Seo WH, et al. Outbreak of late-onset group B streptococcal infections in healthy newborn infants after discharge from a maternity hospital: a case report. J Korean Med Sci. 2006;21(2):347–350. doi:10.3346/jkms.2006.21.2.347
26. Morinis J, Shah J, Murthy P, Fulford M. Horizontal transmission of group B streptococcus in a neonatal intensive care unit. Paediatr Child Health. 2011;16(6):e48–e50. doi:10.1093/pch/16.6.e48
27. Tsai MH, Hsu JF, Lai MY, et al. Molecular characteristics and antimicrobial resistance of group B Streptococcus strains causing invasive disease in neonates and adults. Front Microbiol. 2019;10:264. doi:10.3389/fmicb.2019.00264
28. Kotiw M, Zhang GW, Daggard G, Reiss-Levy E, Tapsall JW, Numa A. Late-onset and recurrent neonatal Group B streptococcal disease associated with breast-milk transmission. Pediatr Dev Pathol. 2003;6(3):251–256. doi:10.1007/s10024-001-0276-y
29. Crestani C, Forde TL, Lycett SJ, et al. The fall and rise of group B Streptococcus in dairy cattle: reintroduction due to human-to-cattle host jumps? Microb Genom. 2021;7(9). doi:10.1099/mgen.0.000648
30. Cobo-Angel CG, Jaramillo-Jaramillo AS, Palacio-Aguilera M, et al. Potential group B Streptococcus interspecies transmission between cattle and people in Colombian dairy farms. Sci Rep. 2019;9(1):14025. doi:10.1038/s41598-019-50225-w
31. Hernandez L, Bottini E, Cadona J, et al. Multidrug resistance and molecular characterization of Streptococcus agalactiae isolates from dairy cattle with mastitis. Front Cell Infect Microbiol. 2021;11:647324. doi:10.3389/fcimb.2021.647324
32. Berardi A, Rossi C, Lugli L, et al. Group B streptococcus late-onset disease: 2003–2010. Pediatrics. 2013;131(2):e361–e368. doi:10.1542/peds.2012-1231
33. Seale AC, Bianchi-Jassir F, Russell NJ, et al. Estimates of the burden of group B Streptococcal disease worldwide for pregnant women, stillbirths, and children. Clin Infect Dis. 2017;65(suppl_2):S200–S219. doi:10.1093/cid/cix664
34. Puopolo KM, Mukhopadhyay S, Hansen NI, et al. Group B Streptococcal infection in extremely preterm neonates and neurodevelopmental outcomes at 2 years. Clin Infect Dis. 2022. doi:10.1093/cid/ciac222
35. Dangor Z, Khan M, Kwatra G, et al. The association between breast milk group B Streptococcal capsular antibody levels and late-onset disease in young infants. Clin Infect Dis. 2020;70(6):1110–1114. doi:10.1093/cid/ciz360
36. Tazi A, Plainvert C, Anselem O, et al. Risk factors for infant colonization by hypervirulent CC17 group B Streptococcus: toward the understanding of late-onset disease. Clin Infect Dis. 2019;69(10):1740–1748. doi:10.1093/cid/ciz033
37. Slotved HC, Fuursted K, Kavalari ID, Hoffmann S. Molecular identification of invasive non-typeable group B streptococcus isolates from Denmark (2015 to 2017). Front Cell Infect Microbiol. 2021;11:571901. doi:10.3389/fcimb.2021.571901
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.