Back to Journals » Clinical Interventions in Aging » Volume 18
Lasso-Based Machine Learning Algorithm for Predicting Postoperative Lung Complications in Elderly: A Single-Center Retrospective Study from China
Authors Liu J, Ma Y, Xie W, Li X, Wang Y, Xu Z, Bai Y, Yin P, Wu Q
Received 1 February 2023
Accepted for publication 7 April 2023
Published 14 April 2023 Volume 2023:18 Pages 597—606
DOI https://doi.org/10.2147/CIA.S406735
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Zhi-Ying Wu
Jie Liu,1,* Yilei Ma,2,* Wanli Xie,1,* Xia Li,1 Yanting Wang,1 Zhenzhen Xu,1 Yunxiao Bai,1 Ping Yin,2 Qingping Wu1
1Department of Anesthesiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China; 2School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Qingping Wu, Email [email protected]
Background: The predictive effect of systemic inflammatory factors on postoperative pulmonary complications in elderly patients remains unclear. In addition, machine learning models are rarely used in prediction models for elderly patients.
Patients and Methods: We retrospectively evaluated elderly patients who underwent general anesthesia during a 6-year period. Eligible patients were randomly assigned in a 7:3 ratio to the development group and validation group. The Least logistic absolute shrinkage and selection operator (LASSO) regression model and multiple logistic regression analysis were used to select the optimal feature. The discrimination, calibration and net reclassification improvement (NRI) of the final model were compared with “the Assess Respiratory Risk in Surgical Patients in Catalonia” (ARISCAT) model.
Results: Of the 9775 patients analyzed, 8.31% developed PPCs. The final model included age, preoperative SpO2, ANS (the Albumin/NLR Score), operation time, and red blood cells (RBC) transfusion. The concordance index (C-index) values of the model for the development cohort and the validation cohort were 0.740 and 0.748, respectively. The P values of the Hosmer–Lemeshow test in two cohorts were insignificant. Our model outperformed ARISCAT model, with C-index (0.740 VS 0.717, P = 0.003) and NRI (0.117, P < 0.001).
Conclusion: Based on LASSO machine learning algorithm, we constructed a prediction model superior to ARISCAT model in predicting the risk of PPCs. Clinicians could utilize these predictors to optimize prospective and preventive interventions in this patient population.
Keywords: older adult, postoperative complications, ANS, the albumin/NLR score, risk factors
Introduction
PPCs are the leading cause of morbidity and mortality, increasing the risk of intensive care admissions, prolonging hospital stays, and raising the economic burden.1–6 Worldwide, more than 310 million surgical procedures are performed each year,7 with the proportion of older adults increasing.8 In comparison with young adults, older adults are at a greater risk of developing postoperative PPCs.9,10 In nonthoracic surgery, the risk of postoperative PPCs in patients aged 65 years or older was almost 6 times among other adults.11 As the global aging process intensifies,12 the global burden of postoperative pulmonary complications will increase further. Hence, developing a prediction tool for PPCs in older patients is particularly necessary.
Although some risk prediction models about PPCs have been developed,9,13–15 such as ARISCAT model, the lack of specific targeting of elderly patients and single type of surgery limit their application and generalization in elderly patients. In addition, recent studies have shown that some markers of the systemic inflammatory response: albumin, the neutrophil-to-lymphocyte ratio (NLR), ANS, lymphocyte-to-monocyte ratio (LMR), the platelet-to-lymphocyte ratio (PLR) and systemic immune-inflammatory index (SII) are significantly associated with postoperative complications.16–19 These factors can be easily detected by blood tests, which are simple and common in clinical practice. The lack of data assessing the ability of these indicators to predict the risk of PPCs in older patients may compromise the accuracy of predicting the likelihood of PPCs in patients. To achieve the goal of predicting PPCs risk in elderly patients, a comprehensive and applicable tool that includes biomarkers is urgently needed.
More recently, machine learning has played an important role in the construction of clinical predictive models. LASSO regression is a powerful machine learning tool that minimizes the potential collinearity of predictive variables and filters out the most influential ones.20
The purpose of this study was to develop and validate a machine learning predictive model incorporating multiple dimensions of variables. The model could assess the likelihood of PPCs in elderly patients, promoting rational prevention and effective treatment.
Materials and Methods
Study Design
Based on the electronic health record of our institution, the retrospective cohort study analyzed older patients who received general anesthesia in the hospital from January 1, 2014, to December 31, 2019. Two authors systematically checked and confirmed all clinical data for each study participant. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Wuhan Union Hospital (Approval No.2021–0986). In order to protect the patient’s privacy, all personal information was anonymous. Due to the absence of the patient’s identifying information, the requirement for informed consent was waived. The analysis and reporting of this study followed the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) reporting guideline.21
Study Population
Patients aged 65 years or older, and receiving invasive ventilation for surgery who underwent general anesthesia were included in the study. Patients were excluded if they met one or more of the following criteria: (1) preoperative mechanical ventilation; (2) operations related to previous postoperative complications; (3) a second operation after surgery; (4) organ transplantation; (5) discharged within 24 hours after surgery; (6) cardiac and thoracic surgery. In addition, we excluded patients with missing data in data analysis.
Data Collection
Based on a review of previous research and available variables, we collected potential characteristics. Preoperative factors included age, gender, body mass index (BMI), history of smoking and alcohol, respiratory infection < 30 days, preoperative SpO2, cancer, chronic obstructive pulmonary disease (COPD), hypertension, coronary artery disease and diabetes.
Intraoperative factors were listed below: type of surgery, surgical incision, operation time, emergency procedure and red blood cell transfusion (RBC transfusion).
Preoperative laboratory test results included hemoglobin, platelet, urea nitrogen, creatinine, alanine aminotransferase (ALT), aspartate aminotransferase (AST), total bilirubin (TBil), albumin, NLR, ANS, PLR, LMR, SII. Anemia was defined as the hemoglobin concentration < 130 g/L in men or < 120 g/L in women. ANS was calculated by assigning a point of 0 or 1 to albumin and NLR levels over or below the threshold, with scores ranging from 0 to 2. The primary outcome was the composite of PPCs (atelectasis, pneumonia, respiratory failure and aspiration pneumonia) in the first 7 days after surgery.
Machine Learning Predictive Model
The whole cohort was randomly entered into a development cohort and validation cohort at a ratio of 7:3. A prediction model was developed using the development group, and its performance was tested in the validation group.
We developed the model in the training set using a machine-learning algorithm. The machine learning algorithm, the LASSO regression model, avoids overfitting and reduces model complexity through L1 regularization to enhance robustness.22 Lasso regression is a compression estimation method with the core idea of reducing the set of variables. By constructing a penalty function, LASSO regression compresses the variable coefficients. It makes the regression coefficients of some insignificant variables zero, thus selecting the critical variables for the following analysis step. The optimal variables were determined by the optimal lambda value for 10-fold cross-validation.
We employed LASSO regression for variable selection using the GLMNET package in R. Subsequently, the features selected in LASSO regression were included in the multiple logistic regression analysis. Finally, predictors with P values <0.05 were used to construct the model to predict PPCs.
Data-Analysis
For baseline characters, continuous variables were expressed as median (interquartile range) using the t-test or Mann–Whitney test, and categorical variables were presented as number (percentage) using χ2 test or Fisher's exact test. Continuous variables were transformed into categorical variables according to clinically relevant thresholds or the Youden index of the receiver operating characteristic (ROC) curve analysis.
The discrimination of the model was assessed by C-index and calibration was evaluated by the Hosmer–Lemeshow goodness-of-fit test. For different AUC comparisons, we used the DeLong test.23 All statistical analyses were performed in R version 4.1.1. It was considered statistically significant when a 2-sided P < 0.05.
Results
Clinical Characteristics
A total of 13,413 patients aged 65 years and older were screened in the initial assessment, and 3638 patients were excluded (Figure 1). Finally, 9775 patients were randomly assigned to the development cohort (6842 patients) and validation cohort (2933 patients). The overall length of postoperative hospital stay was 8.96 (6.04 to 13.39) and the incidence of PPCs was 8.31%. Table 1 shows the baseline characteristics of the development and validation sets. No significant differences were found in key domains.
 |
Table 1 Clinical Characteristics of the Study Population in the Development Cohort and Validation Cohort |
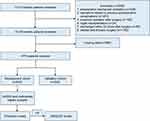 |
Figure 1 Patient Flow Diagram. |
Predictors Selection
In the development group, we performed a LASSO regression analysis to evaluate the 29 variables (Figure 2A and B). After selection by LASSO regression, the six factors were incorporated into a multivariable logistic regression analysis. Finally, five variables were independently statistically significant predictors: age, preoperative SpO2, ANS, operation time and RBC transfusion. The model based on these variables was displayed in a forest diagram (Figure 3).
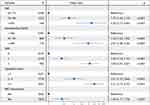 |
Figure 3 The forest diagram based on the multivariable regression model. |
Validation and Assessment of the Prediction Model
The C-index of the prediction model was 0.740 (95% CI, 0.720–0.760) in the development group and 0.748 (95% CI, 0.717–0.779) in the validation group, which indicated moderate discrimination. The model showed good calibration, with insignificant Hosmer–Lemeshow chi-square values of (χ2 = 10.392, P = 0.239) and (χ2 = 8.232, P = 0.411). Our model performed better than the ARISCAT model in predicting PPCs (AUC: 0.717; 95% CI, 0.696–0.738), P =0.003 (Figure 4). The NRI of comparison between our model and ARISCAT model was 0.117 (95% CI, 0.073–0.161; P < 0.001). ARISCAT model had poor calibration, with a Hosmer–Lemeshow chi-square yielding a P-value of < 0.001.
 |
Figure 4 The receiver operating characteristics curve of the model (with and without ANS) and ARISCAT model. |
Comparison of Inflammatory Biomarkers
The ROC curves of the individual biomarker were plotted (Figure 5) and their predictive ability was assessed by AUC values. Among six potential biomarkers, ANS had the best predictive ability and was the independent predictor. Besides, the AUC of model (incorporating the ANS) was significantly better than the model without the ANS (0.740 vs 0.715, P < 0.001) (Figure 4).
 |
Figure 5 The receiver operating characteristics curves of six systemic inflammatory markers. |
Discussion
In the analysis of elderly patients under general anesthesia, a model based on LASSO regression with five variables was developed and validated to predict PPCs. Five independent risk factors that were easy to collect included age, preoperative SpO2, ANS, operation time, and RBC transfusion. The model had moderate discrimination and good calibration for identifying individuals at risk for PPCs. In addition, ANS as a biomarker was added to our model to provide more information about patients and increase the predictive value of the model.
With advances in surgical techniques and perioperative management, the demand for surgery in elderly patients is increasing. Elderly patients with multiple interrelated risk factors, such as poor performance, underlying comorbidities, and impaired lung function are considered to be at high risk for PPCs.24 Therefore, there is an urgent need to develop and validate a simple predictive model in older patients to help physicians assess the risk of PPCs.
Postoperative pulmonary complications are widely defined and have different mechanisms. Complications based on pulmonary collapse and airway contamination include atelectasis, pneumonia, respiratory failure and aspiration pneumonia, while pleural effusion and pneumothorax have different mechanisms.25 In this case, we developed a machine learning model and compared it with ARISCAT model, which is currently the most widely used model. Lasso regression is a modern approach that takes the form of a penalty on the absolute scale of the regression coefficients. The application of LASSO’s powerful shrinkage technique in this study was able to reduce variability and minimize the problem of multicollinearity, improving the accuracy of the model.
Previous studies have shown that several biomarkers associated with systemic inflammation are valuable in predicting postoperative complications.18,26 However, there is limited evidence on the predictive value of systemic inflammation for PPCs in elderly patients undergoing general anesthesia. In this study, ANS was identified as an independent predictor of PPCs. In addition, ANS significantly increased the ability of the model to predict PPCs. NLR is a simple systemic inflammatory biomarker that uses only differential results from blood cell counts; Albumin is a negative acute phase protein independently associated with inflammation and increased nutritional risk.27 ANS is a combination of albumin and NLR, which represents a multisystem inflammatory response. Pulmonary inflammation is a common pathological process of atelectasis, pneumonia and respiratory failure. With the progress of the disease, the inflammation is aggravated.28,29 In this study, ANS was compared with other inflammatory blood cell parameters and found to have the highest predictive ability for PPCs. Probably due to the use of the machine learning method and the introduction of the new marker, our model showed better performance than ARISCAT model.
Although some risk factors, such as age, preoperative SpO2 and operation time, resembled those reported in the previous models,9,10,24 RBC transfusion was ignored. A study exploring the association between RBC transfusion and PPCs found that RBC transfusion was associated with the activation of pulmonary inflammation/coagulation and systemic coagulation disorders, increasing the incidence of PPCs.30 In addition, blood transfusion can inhibit host immunity, which has certain immune risks.31 Elderly patients have decreased organ function and poor immune function, and intraoperative blood transfusion may further increase the risk of PPCs. Our model results further confirmed that intraoperative RBC transfusion was an independent risk factor for PPCs.
Despite the model reported in this paper having certain clinical significance, our study still has some limitations. First, despite the large sample size, it was designed retrospectively. Second, the model was not externally validated and its international replicability should be confirmed. Third, some detailed parameters related to mechanical ventilation were not included in our analysis.
Conclusion
In this study, we developed and validated a model based on LASSO regression that included an inflammatory biomarker as well as other clinical features to predict the risk of PPCs in elderly patients. It could help clinicians assess the likelihood of PPCs in older patients and make individualized clinical decisions about management options.
Acknowledgments
All authors would like to sincerely thank all participants who contributed to this study, and the reviewers for their insightful suggestions.
Funding
This study was supported by the National Key Research and Development Program of China (Grant No. 2018YFC2001903) and the National Natural Science Foundation of China (Grant No. 81873952) and the National Natural Science Foundation of China (Grant No. 81901948).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005;242(3):326–41; discussion 41–3. doi:10.1097/01.sla.0000179621.33268.83
2. Fernandez-Bustamante A, Frendl G, Sprung J, et al. Postoperative pulmonary complications, early mortality, and hospital stay following noncardiothoracic surgery: a multicenter study by the perioperative research network investigators. JAMA Surg. 2017;152(2):157–166. doi:10.1001/jamasurg.2016.4065
3. Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA
4. Pearse RM, Moreno RP, Bauer P, et al. Mortality after surgery in Europe: a 7 day cohort study. Lancet. 2012;380(9847):1059–1065. doi:10.1016/S0140-6736(12)61148-9
5. Serpa Neto A, Hemmes SN, Barbas CS, et al. Incidence of mortality and morbidity related to postoperative lung injury in patients who have undergone abdominal or thoracic surgery: a systematic review and meta-analysis. Lancet Respir Med. 2014;2(12):1007–1015. doi:10.1016/S2213-2600(14)70228-0
6. Shander A, Fleisher LA, Barie PS, Bigatello LM, Sladen RN, Watson CB. Clinical and economic burden of postoperative pulmonary complications: patient safety summit on definition, risk-reducing interventions, and preventive strategies. Crit Care Med. 2011;39(9):2163–2172. doi:10.1097/CCM.0b013e31821f0522
7. Meara JG, Leather AJ, Hagander L, et al. Global Surgery 2030: evidence and solutions for achieving health, welfare, and economic development. Lancet. 2015;386(9993):569–624. doi:10.1016/S0140-6736(15)60160-X
8. Becher RD, Wyk BV, Leo-Summers L, Desai MM, Gill TM. The incidence and cumulative risk of major surgery in older persons in the United States. Ann Surg. 2021;271:87–92.
9. Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population-based surgical cohort. Anesthesiology. 2010;113(6):1338–1350. doi:10.1097/ALN.0b013e3181fc6e0a
10. Smetana GW, Lawrence VA, Cornell JE. Preoperative pulmonary risk stratification for noncardiothoracic surgery: systematic review for the American College of Physicians. Ann Intern Med. 2006;144(8):581–595. doi:10.7326/0003-4819-144-8-200604180-00009
11. McAlister FA, Bertsch K, Man J, Bradley J, Jacka M. Incidence of and risk factors for pulmonary complications after nonthoracic surgery. Am J Respir Crit Care Med. 2005;171(5):514–517. doi:10.1164/rccm.200408-1069OC
12. Partridge L, Deelen J, Slagboom PE. Facing up to the global challenges of ageing. Nature. 2018;561(7721):45–56. doi:10.1038/s41586-018-0457-8
13. Neto AS, da Costa LGV, Hemmes SNT, et al. The Las Vegas risk score for prediction of postoperative pulmonary complications: an observational study. Eur J Anaesthesiol. 2018;35(9):691–701. doi:10.1097/EJA.0000000000000845
14. Bilimoria KY, Liu Y, Paruch JL, et al. Development and evaluation of the universal ACS NSQIP surgical risk calculator: a decision aid and informed consent tool for patients and surgeons. J Am Coll Surg. 2013;217(5):833–42.e1–e3. doi:10.1016/j.jamcollsurg.2013.07.385
15. Amar D, Munoz D, Shi W, Zhang H, Thaler HT. A clinical prediction rule for pulmonary complications after thoracic surgery for primary lung cancer. Anesth Analg. 2010;110(5):1343–1348. doi:10.1213/ANE.0b013e3181bf5c99
16. Shen CJ, Miao T, Wang ZF, et al. Predictive value of post-operative neutrophil/lymphocyte count ratio for surgical site infection in patients following posterior lumbar spinal surgery. Int Immunopharmacol. 2019;74:105705. doi:10.1016/j.intimp.2019.105705
17. Liu Z, Wu H, Liufu N, et al. Development and validation of a nomogram incorporating selected systemic inflammation-based prognostic marker for complication prediction after vascularized fibula flap reconstruction. Oral Oncol. 2019;99:104467. doi:10.1016/j.oraloncology.2019.104467
18. Bora Makal G, Yıldırım O. Are the C-reactive protein/albumin ratio (CAR), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (NLR) novel inflammatory biomarkers in the early diagnosis of postoperative complications after laparoscopic sleeve gastrectomy? Obes Res Clin Pract. 2020;14(5):467–472. doi:10.1016/j.orcp.2020.07.003
19. Lin ZX, Ruan DY, Li Y, et al. Lymphocyte-to-monocyte ratio predicts survival of patients with hepatocellular carcinoma after curative resection. World J Gastroenterol. 2015;21(38):10898–10906. doi:10.3748/wjg.v21.i38.10898
20. Tibshirani R, Tibshirani R. Regression shrinkage via the lasso; 1996.
21. Collins GS, Reitsma JB, Altman DG, Moons KG. Transparent Reporting of a multivariable prediction model for Individual Prognosis Or Diagnosis (TRIPOD): the TRIPOD Statement. Br J Surg. 2015;102(3):148–158. doi:10.1002/bjs.9736
22. Xu CJ, van der Schaaf A, Schilstra C, Langendijk JA, Van’t Veld AA. Impact of statistical learning methods on the predictive power of multivariate normal tissue complication probability models. Int J Radiat Oncol Biol Phys. 2012;82(4):e677–e684. doi:10.1016/j.ijrobp.2011.09.036
23. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. doi:10.2307/2531595
24. Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth. 2017;118(3):317–334. doi:10.1093/bja/aex002
25. Abbott TEF, Fowler AJ, Pelosi P, et al. A systematic review and consensus definitions for standardised end-points in perioperative medicine: pulmonary complications. Br J Anaesth. 2018;120(5):1066–1079. doi:10.1016/j.bja.2018.02.007
26. Mungan İ, Dicle ÇB, Bektaş Ş, et al. Does the preoperative platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio predict morbidity after gastrectomy for gastric cancer? Mil Med Res. 2020;7(1):9. doi:10.1186/s40779-020-00234-y
27. Eckart A, Struja T, Kutz A, et al. Relationship of nutritional status, inflammation, and serum albumin levels during acute illness: a prospective study. Am J Med. 2020;133(6):713–22.e7. doi:10.1016/j.amjmed.2019.10.031
28. Zeng C, Lagier D, Lee JW, Vidal Melo MF. Perioperative pulmonary atelectasis: part I. biology and mechanisms. Anesthesiology. 2022;136(1):181–205. doi:10.1097/ALN.0000000000003943
29. O’Gara B, Talmor D. Perioperative lung protective ventilation. BMJ. 2018;362:k3030. doi:10.1136/bmj.k3030
30. Tuinman PR, Vlaar AP, Cornet AD, et al. Blood transfusion during cardiac surgery is associated with inflammation and coagulation in the lung: a case control study. Crit Care. 2011;15(1):R59. doi:10.1186/cc10032
31. Hart S, Cserti-Gazdewich CM, McCluskey SA. Red cell transfusion and the immune system. Anaesthesia. 2015;70(Suppl 1):38–45, e13–e16. doi:10.1111/anae.12892
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

