Back to Journals » Drug Design, Development and Therapy » Volume 15
Lactobacillus plantarum ZS62 Alleviates Alcohol-Induced Gastric Injury in Mice via an Anti-Oxidative Mechanism
Authors Wu Y, Hu J, Long X, Pan Y, Mu J, Park KY, Zhao X
Received 27 November 2020
Accepted for publication 17 March 2021
Published 22 April 2021 Volume 2021:15 Pages 1667—1676
DOI https://doi.org/10.2147/DDDT.S292243
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Georgios Panos
Ya Wu,1,2,* Jing Hu,3,* Xingyao Long,1,4 Yanni Pan,1,4 Jianfei Mu,1 Kun-Young Park,1,4 Xin Zhao1
1Chongqing Collaborative Innovation Center for Functional Food, Chongqing Engineering Research Center of Functional Food, Chongqing Engineering Laboratory for Research and Development of Functional Food, Chongqing University of Education, Chongqing, 400067, People’s Republic of China; 2College of Biological and Chemical Engineering, Chongqing University of Education, Chongqing, 400067, People’s Republic of China; 3Department of Pharmacy, Southwest Hospital, Army Medical University (Third Military Medical University), Chongqing, 400038, People’s Republic of China; 4Department of Food Science and Biotechnology, Cha University, Seongnam, 13488, South Korea
*These authors contributed equally to this work
Correspondence: Kun-Young Park; Xin Zhao Tel +86-23-6265-3650
Email [email protected]; [email protected]
Aim: Gastric mucosal injury is a typical characteristic of gastric diseases. The prevalence of gastric mucosal injury caused by alcohol has been on the rise, which has been considered a serious problem. The purpose of this study is to explore the protective effect on gastric injury of Lactobacillus plantarum ZS62 (LP-ZS62) isolated from naturally fermented yak yoghurt.
Methods: We established a gastric injury model through alcohol and evaluated the protective effect of LP-ZS62 on gastric injury in mice. The injury to the gastric mucosa, histopathological sections, related biochemical indicators, and related genes were examined to evaluate the protective effect of LP-ZS62.
Results: LP-ZS62 effectively alleviated alcohol-induced gastric injury according to visual observations of gastric tissue and pathological tissue sections. The experimental results revealed that LP-ZS62 decreased malondialdehyde (MDA) level, and elevated superoxide dismutase (SOD) and glutathione (GSH) levels in gastric tissues. Additionally, LP-ZS62 increased glutathione peroxidase (GSH-Px), prostaglandin E2 (PGE2), and somatostatin (SS) levels. LP-ZS62 also decreased inflammatory cytokines interleukin (IL)-1β, tumor necrosis factor-α (TNF-α) and IL-6 levels, and increased the anti-inflammatory cytokine IL-10 level. The quantitative polymerase chain reaction results showed that LP-ZS62 upregulated mRNA expression of nuclear factor E2-related factor 2 (Nrf2), copper/zinc superoxide dismutase (SOD1), manganese superoxide dismutase (SOD2), catalase (CAT), gamma-glutamylcysteine synthetase (GSH1), and glutathione peroxidase (GSH-Px).
Conclusion: This study confirmed that LP-ZS62 alleviated alcohol-induced gastric injury by regulating antioxidant capacity. Therefore, LP-ZS62 could be developed as a probiotic product to treat alcoholic gastric injury.
Keywords: Lactobacillus plantarum ZS62, alcohol-induced gastric injury, inflammation, antioxidant
Introduction
Alcohol is a common beverage in daily life. Long-term drinking or one-time intake of large amounts of alcohol causes systemic inflammation and damage to organs followed by chronic diseases, including chronic liver disease, neurogenic disease, stomach cancer, and inflammatory bowel syndrome.1–3 As a common ulcerative factor, high-concentration ethanol not only directly damages gastric mucosal tissues but also induces the production and release of free radicals and inflammatory mediators. These substances damage the gastric mucosal barrier and cause inflammation, oozing, edema, erosion, bleeding, and other injuries, which damage gastric tissue.4 It has been demonstrated that alcohol drinkers are more likely to suffer from gastrointestinal diseases, particularly the occurrence of stomach cancer is closely related to alcohol.5 Therefore, it is crucial to prevent and treat alcoholic gastric injury and develop products that protect the gastrointestinal tract.
Probiotics are microorganisms that are beneficial to the human body and are an important part of the micro-ecosystem, mainly including yeasts, lactobacillus, and bifidobacterium.6 The gastric cavity is an important microecological region in the digestive system. Many studies have confirmed that lactobacillus and yeasts are the main residents in gastric tissue. Lactobacillus maintains the microecological balance in the stomach, which is colonized in the non-secretory area.7 Lactobacillus regulates the mucosal immune system, thereby helping to maintain a complete gastrointestinal barrier;8,9 and inhibits the adhesion and colonization of pathogenic bacteria on the gastrointestinal mucosa.10 Moreover, lactobacillus directly or indirectly affects the functions of monocytes, macrophages, T cells, and other immune cells, thereby regulating immunity and suppressing inflammation to prevent and treat gastrointestinal-related diseases.11 It has been reported that Lactobacillus plantarum could protect against alcohol-induced gastric injury via inhibiting inflammation and oxidative stress.12–14 Therefore, the application of lactobacillus in alcoholic gastric injury should be investigated based on its unique physiological characteristics.
We performed experiments with Lactobacillus plantarum ZS62 (LP-ZS62), which was isolated from natural fermented yoghurt, and explored its effect on alcohol-induced gastric injury in mice. We examined the injury to the gastric mucosa and used histopathological sections and related biochemical indicators to evaluate the protective effect of LP-ZS62 on gastric injury. Then, the possible mechanism of LP-ZS62 was explained by measuring mRNA expression by quantitative polymerase chain reaction (qPCR). This project supports the data and theory of probiotics for the treatment of gastrointestinal problems and enriches the application of probiotics in the field of medicine.
Materials and Methods
Source of Strain
The strain used in the experiment was Lactobacillus plantarum ZS62 (LP-ZS62), which was screened from naturally fermented yak yoghurt in Zhaosu County, Xinjiang, China, and identified using the NCBI’s Basic Local Alignment Search Tool (BLAST). LP-ZS62 was preserved in the China General Microbiological Culture Collection Center (CGMCC, Beijing, China; CGMCC No. 18228). The comparative strain was Lactobacillus delbrueckii subsp. bulgaricus (CGMCC No. 1.16075) from the CGMCC.
Tolerance to Artificial Gastric Juice
To prepare artificial gastric juice, 1 mol/L HCl solution was added to a solution of 0.2% NaCl and 0.35% pepsinase, which was adjusted to pH 3.0. Five µL of the activated strain supernatant was discarded after centrifugation and the remainder was kept. A bacterial suspension was obtained by adding 5 mL sterile saline to the remainder of the strain. Nine mL of artificial gastric juice and 1 mL of the bacterial suspension were mixed, and then cultured and incubated at 37°C. After diluting the cultivating 0-h and 3-h samples to the adequate concentration, they were plate-coated on de Man, Rogosa and Sharpe (MRS) solid medium. The viable cells were measured after the diluent was cultured at 37°C for 48 h. The following formula was used to calculate survival rate: survival rate (%) = 3-h viable count (CFU/mL)/0-h viable count (CFU/mL) × 100.
Resistance to Bile Salts
MRS medium containing 0.2% sodium thiol acetate (MRS-THIO) was added to a 0.3% pig bile salt solution. Then, 5 mL of the activated strain was inoculated with 2% (v/v) inoculation volume into MRS-THIO media, followed by incubation at 37°C for 24 h. The optical density (OD) of the medium was determined at 600 nm. The tolerance to bile salts was calculated based on the following formula: bile salts tolerance (%) = (OD of 0.3% bile salts medium − OD of the blank medium)/(OD of 0.0% bile salts medium − OD of the blank medium) × 100.
Animal Experiments
After a 1-week acclimation, 40 C57BL/6J male mice (Chongqing Medical University, 6-week-old) were assigned to the normal group, the model group, the LP-ZS62 group (LP-ZS62), and the Lactobacillus delbrueckii subsp. bulgaricus (LB) group. There were 10 mice in each group, and all were served an adequate normal diet and drinking water. The mice in the LP-ZS62 and LB groups were, respectively, gavaged with 1.0 × 109 CFU/kg LP-ZS62 and 1.0 × 109 CFU/kg LB every day, and the other mice were administered intragastrically with 0.2 mL/10 g saline. After 3 h, the mice in the model, LP-ZS62 and LB groups were administered intragastrically with 56° liquor (0.13 mL/10 g), while the normal mice were treated 0.13 mL/10 g saline by intragastrical gavage. After 14 d, the mice were sacrificed after gavage of 56° liquor for 3 h. Blood was taken via the retro-orbital sinus, and gastric tissues were quickly dissected, photographed, and collected for subsequent experiments. To measure the inhibition rate of gastric injury, the following formula was used: gastric injury inhibition rate (%) = (1 − gastric injury area of sample treated mice/gastric injury area of injured group mice) × 100. The protocol for these experiments was approved by the Ethics Committee of Chongqing Collaborative Innovation Center for Functional Food (201903017b), Chongqing, China. At the same time comply with the 2010/63/EU directive.
Histological Analysis of the Gastric Tissue
The gastric tissue (~0.5 cm2) were fixed in 10% formalin solution for 48 h, followed by dehydration, embedding in paraffin, sectioning, and staining with hematoxylin and eosin. An optical microscope was used to examine the pathological changes in the gastric tissue (BX43 microscope, Olympus, Tokyo, Japan). Gastric histopathological examination rated the injury from mild to severe on a scale of 0 to 5 as previously described.15 The grading was performed based on the damage degree of gastric mucosal hyperemia, hemorrhage, degeneration of glands and epithelial cells in the whole mucosal layer.
Superoxide Dismutase (SOD), Glutathione (GSH), and Malondialdehyde (MDA) Levels in Gastric Tissue
Gastric tissue was ground in a 0.9% sodium chloride solution to afford a 10% tissue homogenate. The supernatant of the stomach homogenate was collected after centrifugation at 10,000 rpm for 15 min. Kits were employed to analyze the levels of SOD, GSH, and MDA in gastric tissue (Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu, China).
Serum Levels of Prostaglandin E2 (PGE2), Somatostatin (SS), Glutathione Peroxidase (GSH-Px), Tumor Necrosis Factor (TNF)-α, Interleukin (IL)-1β, IL-6, and IL-10
Serum was obtained by centrifuging the blood at 10,000 rpm for 20 min. The serum cytokine levels of PGE2, TNF-α, GSH-Px, SS, IL-1β, IL-6, and IL-10 were tested with corresponding kits (Beijing Chenglin Biotechnology Co., Ltd., Beijing, China).
Quantitative Polymerase Chain Reaction Assay
After grinding the gastric tissue, total RNA was extracted by adding 1 mL RNAzol reagent (Invitrogen, Carlsbad, CA, USA), which was diluted to 1 μg/μL. The cDNA template was synthesized by employing 1 μL diluted total RNA for reverse transcription according to the kit instructions (Tiangen Biotech Co., Ltd., Beijing, China). The reaction system was prepared by adding the upstream and downstream primers (1 μL, Table 1) to a solution of SYBR Green PCR Master Mix (10 μL) and the cDNA template (1 μL). After thoroughly mixing, the solution was reacted under the condition: 95°C for 90 s, 40 cycles of 95°C for 30 s, 60°C for 30 s, 72°C for 30 s, then, 95°C for 30 s, and 55°C for 35 s. Relative gene expression levels were obtained by the 2−ΔΔCt method, and glyceraldehyde-3-phosphate dehydrogenase was selected as the housekeeping gene.
 |
Table 1 Sequences of Primers Used in This Study |
Statistical Analysis
The experimental data were analyzed with SPSS 17.0 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism 7 statistical software (Graph Pad Software Inc., La Jolla, CA, USA). The results are expressed as mean ± SD. Comparisons among groups were made by Kruskal–Wallis ANOVA, post hoc Dunn’s multiple comparison test (non-parametric tests). A P-value < 0.05 was considered significant.
Results
Resistance of LP-ZS62 to Artificial Gastric Juice and Bile Salts
The survival rate of LP-ZS62 was 89.48 ± 2.31% in the artificial gastric juice at pH 3.0. The survival rate of LP-ZS62 in 0.3% bile salts was 11.2 ± 1.50%. Thus, the strain was highly resistant to artificial gastric juice.
Gastric Injury Inhibitory Effect
The mice gastric mucosa was intact, and there were no abnormalities, such as bleeding, ulcers, or erosion in the normal group (Figure 1). The mice gastric mucosa in the model group was hyperemic and erosive, with extensive mucosal injury. The mucosa in the LB and LP-ZS62 groups had less bleeding and erosional area compared with that in the model group. The gastric mucosal inhibition rate was calculated based on the injured gastric area. Table 2 shows that the model, LB, and LP-ZS62 groups had different inhibitory degrees of gastric injury, and LP-ZS62 was greater than LB.
 |
Table 2 The Gastric Injury Area and Gastric Injury Inhibitory Rate of Mice |
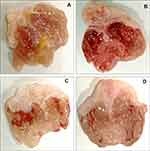 |
Figure 1 Images of stomach specimens from mice of each group. (A) normal group; (B) model group; (C) LB group; (D) LP-ZS62 group. |
Histopathological Examination of Gastric Tissue
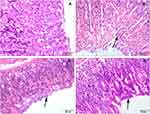
Figure 2 reveals that the structure of the gastric mucosa was complete in the normal group, and the cells were arranged neatly with clear morphology. No abnormal lesions were seen, such as tissue defects, bleeding (a score of 0). However, exfoliation of the mucosal cells was severe, and glandular disruption in the upper mucosa (black arrow) and numerous erythrocytes were seen in the mucosal layer of the model group (white arrow; a score of 4). The cells were arranged neatly and closely in the LB and LP-ZS62 groups. Few erythrocytes were observed. However, some mucosal epithelial cells were necrotic and shedding (black arrow) compared with that in the model group (a score of 1).
SOD, GSH, and MDA Levels in Gastric Tissue
Alcohol reduced SOD and GSH levels, and increased the MDA level in gastric tissue of the model group, compared with the normal mice (P < 0.05; Table 3). The increase in SOD and GSH levels and the decrease in MDA level were detected after treating with LP-ZS62 compared with the model group (P < 0.05). And the effect of LP-ZS62 was close to that of the normal group.
 |
Table 3 Gastric Tissue Levels of SOD, MDA and GSH in Mice of Each Groups |
Serum GSH-Px, PGE2, and SS Levels in Mice
Table 4 shows that serum GSH-Px, PGE2, and SS levels were lowest in the model group compared to all other groups, and those were significant differences compared with normal group (P < 0.05). Although there was no significant difference in PGE2 and GSH-Px levels between the treatment groups and the model group, but LP-ZS62 increased the levels of GSH-Px, PGE2, and SS, which was similar to the normal group (P > 0.05).
 |
Table 4 Serum Levels of GSH-Px, PGE2 and SS in Mice of Each Groups |
Serum TNF-α, IL-1β, IL-6, and IL-10 Levels in Mice
The levels of the mouse pro-inflammatory cytokines TNF-α, IL-6, and IL-1β were increased significantly in the model group compared with the normal mice, whereas IL-10 level was decreased (P < 0.05; Table 5), indicating the mice in the model group were in an inflamed state. The difference of inflammatory cytokines among LB and model group was not remarkable. LP-ZS62 decreased TNF-α, IL-1β, and IL-6 levels, and increased the IL-10 level compared with the model group (P < 0.05), the effect of which was better than LB and similar to the normal level.
 |
Table 5 Serum Levels of TNF-α, IL-1β, IL-6 and IL-10 in Mice of Each Groups |
Nuclear Factor E2-Related Factor 2 (Nrf2), Superoxide Dismutase (SOD)1, SOD2, Catalase (CAT), Glutathione Peroxidase (GSH-Px), and Gamma-Glutamylcysteine Synthetase (GSH1) mRNA Expression Levels in Gastric Tissue
The antioxidant-related genes were further investigated to explore the mechanism of LP-ZS62 for alleviating gastric injury induced by alcohol. Figure 3 shows that the Nrf2, SOD1, GSH-Px, SOD2, CAT, and GSH1 mRNA expression levels were highest in the normal group, which was opposite to the model group (P < 0.05). After LB and LP-ZS62 were employed to treat the mice, the mRNA expression of Nrf2, SOD1, CAT, SOD2, GSH-Px, and GSH1 in LB group had no significant differences compared with the model group, but the mRNA expression of those in LP-ZS62 group was enhanced at different degree (P < 0.05).
Discussion
Ethanol damages gastric tissue in many ways. It is usually a result of the gastric mucosal injury factor being stronger than the gastric mucosal protective factor, along with the inflammatory response and oxygen free radical damage.16 The core of protecting the gastric mucosa is to clear oxygen free radicals, inhibit inflammation, weaken gastric mucosal attack factors, and enhance mucosal defense factors. Studies have shown that a high concentration of ethanol damages the gastric mucosa epithelium and destroys the gastric mucosa epithelium barrier, leading to destruction of the gastric mucosa barrier.16 The present experiment showed that alcohol induced gastric mucosal bleeding, ulcers, erosion, and inflammation. The visual observations and histopathological examination of gastric tissue showed that LP-ZS62 has a protective effect on the gastric injury caused by alcohol.
Endogenous cytokines, such as epidermal growth factor, PGEs, and SS, occur in the gastric mucosa and surface mucus barrier where they participate in the protective response to injury. PGE2 is important for promoting the recovery of gastric mucosal blood flow, inhibiting gastric acid secretion, maintaining integrity of the gastric mucosa, and increasing self-repair of damaged gastric mucosa.17,18 SS reduces mucosal damage and the inflammatory response.19 The SS protective mechanism is generally believed to be related to its inhibition of gastric acid, pepsin, and other substances that stimulate mucosal injury. The anti-inflammatory effect of SS is achieved by inhibiting the production of pro-inflammatory cytokines.20,21 LP-ZS62 increased the levels of PGE2 and SS to some extent, which are protective factors in gastric mucosa, to enhance the gastric mucosal barrier and protect the gastric mucosa from alcohol-induced injury.
The gastric mucosal injury caused by alcohol is closely related to increased ROS.22 The gastric mucosa produces a large amount of oxygen free radicals stimulated by anhydrous ethanol and other chemicals, leading to lipid peroxidation in gastric tissue cells and injury of the gastric mucosa.22 SOD catalyzes disproportionation of superoxide anion radicals to produce oxygen and hydrogen peroxide.23 GSH-Px is the main enzyme that catalyzes the oxidation of reduced GSH in the GSH redox cycle to clear peroxide and hydroxyl radicals.23 MDA is a lipid peroxidation product caused by ROS, and its content reflects the peroxidation damage in gastric tissue.24 LP-ZS62 increased the activities of SOD, GSH-Px, and GSH to raise the ability of free radical scavenging and reduced MDA content to inhibit lipid peroxidation of gastric mucosa. The result showed that LP-ZS62 raised the antioxidant capacity to inhibit oxidative stress, which played a protective role in alcohol-induced gastric injury.
Inflammation is the most direct manifestation of gastric injury. Alcohol activates the immune system and causes changes in inflammatory cytokines, such as TNF-α, IL-10, and IL-6.25–27 TNF-α is mainly produced by mononuclear macrophage, which promotes the initiation and occurrence of the inflammatory response. Also, TNF-α is a stimulating factor that promotes the assembly of neutrophils at the injury site.28,29 In the process of inflammation, IL-6 activates neutrophils and lymphocytes at the inflammation site and enhances self-destructive inflammation.30 IL-1β could be rapidly produced and released by a variety of different immune cells and non-immune cells in response to inflammatory signals, and plays an important role in the regulation of acute and chronic inflammation as an immune response amplifier.31 IL-10 suppresses the immune response through several methods, including promoting the accumulation of tolerant macrophages and dendritic cells, inhibiting the Th1-mediated immune response, and facilitating the differentiation of immunosuppressive T cells.32 LP-ZS62 decreased the levels of the proinflammatory cytokines IL-6, IL-1β, and TNF-α, and increased the anti-inflammatory cytokine IL-10 to suppress inflammatory response, thereby alleviating alcohol-induced gastric injury.
Inflammation and oxidative stress are inseparable in the pathological process of alcohol-induced gastric injury. ROS promote the production of a large number of pro-inflammatory factors and enhance intracellular signaling cascades, thereby intensifying inflammation and causing tissue damage.33,34 Nrf2 is an important transcription factor that regulates oxidative stress response of cells, and also a central regulator that maintains intracellular redox homeostasis.35,36 The downstream antioxidant enzymes encoded by the Nrf2 signaling pathway include GSH1, CAT, SOD, and GSH-Px.35,36 LP-ZS62 enhanced the mRNA expression of the upstream gene Nrf2 and then upregulated mRNA expression of the downstream antioxidant genes SOD1, SOD2, GSH-Px, CAT, and GSH1, thereby protecting against alcohol-induced gastric mucosal injury via increasing the antioxidant capacity to inhibit oxidative stress and inflammation.
Conclusions
This study confirmed that LP-ZS62 increased the level of antioxidant and gastric mucosal defense factors, and inhibited inflammation to protect stomach tissue. The LP-ZS62 protected the stomach from alcohol-induced damage may be through an anti-oxidative mechanism. Therefore, LP-ZS62 possesses a protective effect on the occurrence and development of gastric injury induced by alcohol and has the potential to be developed as a supplementary treatment for alcoholic gastric injury.
Acknowledgments
This research was funded by the Chongqing University Innovation Research Group Project (CXQTP20033) and the Science and Technology Project of the Chongqing Education Commission (KJQN202001604), China.
Disclosure
The authors declare no conflicts of interest.
References
1. Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–2233. doi:10.1016/S0140-6736(09)60746-7
2. Bishehsari F, Magno E, Swanson G, et al. Alcohol and gut-derived inflammation. Alcohol Res. 2017;38(2):163–171.
3. Patel S, Behara R, Swanson GR, Forsyth CB, Voigt RM, Keshavarzian A. Alcohol and the intestine. Biomolecules. 2015;5(4):2573–2588. doi:10.3390/biom5042573
4. Zhang C, Gao F, Gan S, et al. Chemical characterization and gastroprotective effect of an isolated polysaccharide fraction from Bletilla striata against ethanol-induced acute gastric ulcer. Food Chem Toxicol. 2019;131:110539. doi:10.1016/j.fct.2019.05.047
5. Tramacere I, Negri E, Pelucchi C, et al. A meta-analysis on alcohol drinking and gastric cancer risk. Ann Oncol. 2012;23(1):28–36. doi:10.1093/annonc/mdr135
6. F.W.W. Group; Guidelines for the evaluation of probiotics in food. FAO/WHO. London, ON; 2002.
7. Coconnier MH, Liévin V, Bernet-Camard MF, Hudault S, Servin AL. Antibacterial effect of the adhering human Lactobacillus acidophilus strain LB. Antimicrob Agents Chemother. 1997;41(5):1046–1052. doi:10.1128/AAC.41.5.1046
8. Akama F, Nishino R, Makino S, et al. The effect of probiotics on gastric mucosal permeability in humans administered with aspirin. Scand J Gastroenterol. 2011;46(7–8):831–836. doi:10.3109/00365521.2011.574730
9. Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–323. doi:10.1038/nri2515
10. Mack DR, Ahrne S, Hyde L, Wei S, Hollingsworth MA. Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut. 2003;52(6):827–833. doi:10.1136/gut.52.6.827
11. Kemgang TS, Kapila S, Shanmugam VP, Kapila R. Cross-talk between probiotic lactobacilli and host immune system. J Appl Microbiol. 2014;117(2):303–319. doi:10.1111/jam.12521
12. Wang R, Zeng X, Liu B, et al. Prophylactic effect of Lactobacillus plantarum KSFY06 on HCl/ethanol-induced gastric injury in mice. Food Funct. 2020;11(3):2679–2692. doi:10.1039/c9fo02474c
13. Park H, Cho D, Huang E, et al. Amelioration of alcohol induced gastric ulcers through the administration of Lactobacillus plantarum APSulloc 331261 isolated from green tea. Front Microbiol. 2020;11:420. doi:10.3389/fmicb.2020.00420
14. Li F, Sun HL, Ran GJ, et al. Preventive effect of Lactobacillus plantarum HFY09 on HCl/ethanol-induced gastric injury in mice. Appl Biol Chem. 2020;63(1):49. doi:10.1186/s13765-020-00536-8
15. Masuda E, Kawano S, Nagano K, et al. Role of endogenous endothelin in pathogenesis of ethanol-induced gastric mucosal injury in rats. Am J Physiol. 1993;265(3 Pt 1):G474–G481. doi:10.1152/ajpgi.1993.265.3.G474
16. Zhao W, Zhu F, Shen W, et al. Protective effects of DIDS against ethanol-induced gastric mucosal injury in rats. Acta Biochim Biophys Sin (Shanghai). 2009;41(4):301–308. doi:10.1093/abbs/gmp014
17. Motilva V, Alarcón de la Lastra C, Bruseghini L, Manuel HJ, Sánchez-Fidalgo S. COX expression and PGE(2) and PGD(2) production in experimental acute and chronic gastric lesions. Int Immunopharmacol. 2005;5(2):369–379. doi:10.1016/j.intimp.2004.10.005
18. Takeuchi K. Prophylactic effects of prostaglandin E2 on NSAID-induced enteropathy-role of EP4 receptors in its protective and healing-promoting effects. Curr Opin Pharmacol. 2014;19:38–45. doi:10.1016/j.coph.2014.07.005
19. Weckbecker G, Lewis I, Albert R, Schmid HA, Hoyer D, Bruns C. Opportunities in somatostatin research: biological, chemical and therapeutic aspects. Nat Rev Drug Discov. 2003;2(12):999–1017. doi:10.1038/nrd1255
20. Chowers Y, Cahalon L, Lahav M, et al. Somatostatin through its specific receptor inhibits spontaneous and TNF-alpha- and bacteria-induced IL-8 and IL-1 beta secretion from intestinal epithelial cells. J Immunol. 2000;165(6):2955–2961. doi:10.4049/jimmunol.165.6.2955
21. Tang WF, Wang YG, Zhu L, et al. Effect of somatostatin on immune inflammatory response in patients with severe acute pancreatitis. J Dig Dis. 2007;8(2):96–102. doi:10.1111/j.1443-9573.2007.00293.x
22. Parlesak A, Billinger MH, Bode C, Bode JC. Gastric alcohol dehydrogenase activity in man: influence of gender, age, alcohol consumption and smoking in a Caucasian population. Alcohol Alcohol. 2002;37(4):388–393. doi:10.1093/alcalc/37.4.388
23. Birben E, Sahiner UM, Sackesen C, Erzurum S, Kalayci O. Oxidative stress and antioxidant defense. World Allergy Organ J. 2012;5(1):9–19. doi:10.1097/WOX.0b013e3182439613
24. Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43(2 Suppl 1):S63–74. doi:10.1002/hep.20957
25. Salga MS, Ali HM, Abdulla MA, Abdelwahab SI. Gastroprotective activity and mechanism of novel dichlorido-zinc (II)-4-(2-(5-methoxybenzylideneamino)ethyl) piperazin-1-iumphenolate complex on ethanol-induced gastric ulceration. Chem Biol Interact. 2012;95(2):144–153. doi:10.1016/j.cbi.2011.11.008
26. Mei X, Xu D, Xu S, Zheng Y, Xu S. Novel role of Zn(II)-curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine-mediated oxidative damage of ethanol-induced acute gastric ulcers. Chem Biol Interact. 2012;197(1):31–39. doi:10.1016/j.cbi.2012.03.006
27. Li CY, Xu HD, Zhao BT, Chang HI, Rhee HI. Gastroprotective effect of cyanidin 3-glucoside on ethanol-induced gastric lesions in rats. Alcohol. 2008;42(8):683–687. doi:10.1016/j.alcohol.2008.08.009
28. Ji H, Pettit A, Ohmura K, et al. Critical roles for interleukin 1 and tumor necrosis factor alpha in antibody-induced arthritis. J Exp Med. 2002;196(1):77–85. doi:10.1084/jem.20020439
29. Natalicchio A, De Stefano F, Orlando MR, et al. Exendin-4 prevents c-Jun N-terminal protein kinase activation by tumor necrosis factor-alpha (TNFalpha) and inhibits TNFalpha-induced apoptosis in insulin-secreting cells. Endocrinology. 2010;151(5):2019–2029. doi:10.1210/en.2009-1166
30. McLoughlin RM, Jenkins BJ, Grail D, et al. IL-6 trans-signaling via STAT3 directs T cell infiltration in acute inflammation. Proc Natl Acad Sci USA. 2005;102(27):9589–9594. doi:10.1073/pnas.0501794102
31. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. 2013;39(6):1003–10018. doi:10.1016/j.immuni.2013.11.010
32. Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi:10.1146/annurev.immunol.19.1.683
33. Chen SJ, Yen CH, Huang YC, Lee BJ, Hsia S, Lin PT. Relationships between inflammation, adiponectin, and oxidative stress in metabolic syndrome. PLoS One. 2012;7(9):e45693. doi:10.1371/journal.pone.0045693
34. Elmarakby AA, Sullivan JC. Relationship between oxidative stress and inflammatory cytokines in diabetic nephropathy. Cardiovasc Ther. 2012;30(1):49–59. doi:10.1111/j.1755-5922.2010.00218.x
35. Zhang H, Davies KJA, Forman HJ. Oxidative stress response and Nrf2 signaling in aging. Free Radic Biol Med. 2015;88:314–336. doi:10.1016/j.freeradbiomed.2015.05.036
36. Turpaev KT. Keap1-Nrf2 signaling pathway: mechanisms of regulation and role in protection of cells against toxicity caused by xenobiotics and electrophiles. Biochemistry. 2013;78(2):111–126. doi:10.1134/S0006297913020016
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.