Back to Journals » Cancer Management and Research » Volume 11
Lack of Efficacy: When Opioids Do Not Achieve Analgesia from the Beginning of Treatment in Cancer Patients
Authors Corli O, Damia G, Galli F, Verrastro C , Broggini M
Received 10 April 2019
Accepted for publication 22 October 2019
Published 10 December 2019 Volume 2019:11 Pages 10337—10344
DOI https://doi.org/10.2147/CMAR.S211818
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Alexandra R. Fernandes
Oscar Corli,1 Giovanna Damia,2 Francesca Galli,3 Carmen Verrastro,4 Massimo Broggini2
1Unit of Pain and Palliative Care Research, Laboratory of Methodology for Clinical Research, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milan, Italy; 2Laboratory of Molecular Pharmacology, Department of Oncology, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milan, Italy; 3Laboratory of Methodology for Clinical Research, Department of Oncology, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Milan, Italy; 4Day Hospital of Rheumatology, ASST Gaetano Pini CTO, Milan, Italy
Correspondence: Oscar Corli
Unit of Pain and Palliative Care Research, Laboratory of Methodology for Clinical Research, Department of Oncology, Istituto di Ricerche Farmacologiche Mario Negri IRCCS, Via G. La Masa 19, Milan 20156, Italy
Tel +39 02390141
Fax +39 023546277
Email [email protected]
Introduction: Opioids are often used to relieve moderate to severe pain, but their analgesic response may vary. We focused on the absolute lack of analgesic response immediately after beginning opioid treatment, quantifying the proportion of patients with unchanged or worse pain on day 3 (defined as early non-responders (ENRs)) and day 7.
Methods: This is a post-hoc analysis from a randomized controlled trial involving 498 cancer patients with pain, starting to receive WHO step III opioids. On days 1, 3 and 7 pain intensity (PI) was measured.
Results: On day 3, 68 (13.7%) patients were ENRs, 53 no change and 15 greater PI compared to baseline. The relationships between pain and clinical characteristics showed no significant differences between ENRs and Early responders (ERs), except for PI at baseline, which was significantly lower in ENRs. ENRs on day 3 were re-assessed on day 7 to explore the patterns of analgesic response: 31.7% of patients remained NRs, 48.3% had become responders, and 20.0% were poor responders. Adverse drug reactions were similar in ERs and ENRs at each visit.
Discussion: The complete lack of early response to opioids in cancer patients is clinically important and more frequent than expected. Better definition of the mechanism will allow better pain management in cancer and non-cancer patients.
Keywords: opioids, lack of analgesia, cancer pain, non-responders
Introduction
Cancer pain affects 39% to 64% of the patients depending on the disease stage.1 The pharmacological management of cancer pain is mainly based on non-steroidal-anti-inflammatory-drugs, opioids and analgesic adjuvants, these latter being largely aimed at relief of neuropathic pain.2 Opioids are often used for moderate to severe pain. Opioids can give different analgesic responses and side effects that can sometimes make continuation of the therapy problematic.
The efficacy of opioid analgesia is generally evaluated by comparing the pain intensity (PI) before and after the treatment. The condition of non-responders (NRs) corresponds to failure to obtain at least a 30% reduction of PI3 or not to reach pain rated ≤4 points on a Numeric Rating Scale (NRS) of 0–10.4 NRs are not uncommon, as they amount to 20% of the cancer patients treated with opioids.5 The response, positive or negative, is generally evaluated after weeks to months treatment with opioids, as their long-term efficacy is a necessary condition when treating chronic pain, including cancer pain.
However, the response over time is not always steady, and an initial good response can turn into a poor response. Especially in cancer patients, the analgesic response is influenced by numerous factors, the most important being the type of pain, the disease progression and the possible onset of tolerance.6 For instance, neuropathic pain was originally shown to be poorly responsive to usually effective doses of opioids,7 although this has not been univocally accepted. Recently, the Neuropathic Pain Special Interest Group of the International Association for the Study of Pain8 specified that the number of patients needed to treat (NTT) with strong opioids to obtain a clinically relevant neuropathic pain reduction treatment was 4.3 (95% confidence interval 3.4–5.8), showing the limited power of opioid response. Furthermore, cancer is a progressive disease causing over-activation in nociceptive pathways that can explain the declining analgesic effects during opioid treatment.6 This is coherent with the pattern of pain worsening in advanced disease.1
Finally, the repeated, protracted treatment with opioids can lead to tolerance. The need for rapid increase in opioid dose to maintain analgesia predicts this development which tends to vary widely among individuals.9
An alternative to dose escalation when analgesia fails is the shift from weak (WHO step II) to strong (WHO step III) opioids, or switching to another strong opioid. Generally, these therapeutic changes do give initial pain relief.
The lack of early analgesic effect is unusual and is probably due to mechanisms different from the late onset of tolerance. It can be considered an absence rather than a loss of analgesic action and raises several questions: what mechanisms cause the lack of analgesia? Why does it affect only a minority of patients? Do these patients present any particular features?
We here report a post hoc analysis in cancer patients participating in a phase IV clinical trial,5 aimed at quantifying and describing the absence of analgesic activity. Patients with unchanged or worse pain after 3 days’ opioid treatment – defined as early non-responders (ENRs) – were carefully examined. The first aim of the analysis was to determine the proportion of ENRs. Potential relations between the baseline pain characteristics, sites of primary tumor and metastases, performance status, presence and types of comorbidities, prevalence and severity of opioid-related adverse drug reactions (ADRs) were compared in the ENRs and Early Responders (ERs). Finally, the analgesic responses of the ENRs were re-evaluated after 7 days of therapy to establish the pattern of the response.
Materials and Methods
Patients and Procedures
This analysis follows a randomized, open-label, longitudinal, phase IV clinical trial on cancer patients experiencing moderate to severe pain. Forty-four Italian centers recruited and randomized 520 patients (1:1:1:1 ratio) to receive one WHO Step III opioid out of oral morphine (active comparator), transdermal buprenorphine, oral oxycodone, and transdermal fentanyl.5 Study approval, as stated in the original manuscript,5 was obtained by the review boards of each center and patients gave their written informed consent. The Clinical Trial registration number of the original trial from which the data were analysed was NCT01809106.5 The trial was conducted in compliance with the Declaration of Helsinki.
Patients with diagnostic evidence of locally advanced or metastatic tumor with pain needing a strong opioid, never previously administered, and age ≥18 years, were included. Patients with cerebral tumors or leukemia, concurrent radiotherapy or first-line chemotherapy, non-pharmacological analgesic therapy and renal failure were excluded.
The initial opioid doses followed the recommendations of the European Association for Palliative Care,10 starting with 30 to 60 mg daily of morphine-equivalent, on the basis of the patient’s previous analgesic therapy and general clinical condition. The original study5 was planned to define the baseline opioids by randomization and the starting dose following the EAPC recommendations. During follow-up, physicians were allowed to change dose, add other opioids or adjuvant drugs or change the opioid (switch) or discontinue the treatment based on his/her choice/experience and patients’ clinical needs, in line with the principle of the “real-life” research, as expressed in the title of the original study. No difference in the aggressive trend of treatment was observed among the different participating centres.
During 28 days of follow-up, six visits were planned: day 1 (baseline) and days 3, 7, 14, 21 and 28. At baseline, the following clinical features were recorded: primary tumor site, location of metastases, cancer treatments, concomitant diseases and co-treatments, and Karnofsky Performance Status (KPS). During the visit, the presence of neuropathic pain (NP) or breakthrough pain (BTP) was recorded using the DN4 questionnaire11 and the Davies algorithm,12 respectively. BTP episodes were recorded and treated based on the decision of the physicians. Drug use and dosages were evaluated independently from background daily dose of opioids.
Average pain intensity (API) and worst pain intensity (WPI) in the 24 hrs before the visits were measured using a numerical rating scale (NRS) from 0 (no pain) to 10 (worst imaginable pain). The difference in pain intensity (PID), between the first and subsequent visits, served to classify patients as non-responders (NRs), in case of no improvement or worsening of pain, partial responders (PRs) with a <30% decrease of PI, and responders (Rs) with a ≥30% pain reduction, based on Farrar criteria.3
The main opioid-induced ADRs were recorded at each visit, using the Therapy Impact Questionnaire,13 where patients self-reported the presence and degree of the ADRs.
Statistical Analysis
All the patients in the original randomized study without major violations of the eligibility criteria and with at least a second pain evaluation after baseline were included in this post hoc analysis. Patients’ characteristics were depicted as mean and standard deviation (SD), median and interquartile range (Q1–Q3), and minimum-maximum range or absolute and relative frequencies.
To illustrate the lack of analgesic response after 3 days of treatment, the PID from baseline was calculated. Patients with unchanged or worse pain were classified as ENRs. The ENRs on day 3 were evaluated again at day 7 to check for changes in the analgesic response. The PID between baseline and day 7 was used for classification as NR, PR, and R, previously described.
The frequency and severity of ADRs among the ENRs were compared with ERs. To investigate the relations between analgesic response and clinical features, pain characteristics and frequency of ADRs (absent or present), Wilcoxon and chi-square tests were used, respectively, for continuous variables and categorical variables. Statistical significance was set at p<0.05 for a bilateral test. Analyses were done with SAS Software, version 9.4 (SAS Institute, Cary, NC).
Results
Analysis included 498 patients, with the eligibility criteria of the CERP study and evaluation at least up to the second visit. Their demographic and main clinical features are shown in Table 1. The main sites of the primary tumor were lung, digestive, genitourinary and reproductive system. Metastases were present in 85.1% of the patients and 38.4% of them were under anticancer therapy. KPS was ≤40 in 11.5% of the cases and nearly two-thirds had at least one concomitant disease. Three-quarters of patients had already received WHO II opioids. At baseline, the API was 6.0 and WPI 8.0 and, oral morphine or oxycodone, transdermal fentanyl or buprenorphine were administered in equal proportions. The average initial dose, given to all the patients, was 49.7 mg/day of morphine equivalent.
 |
Table 1 Demographic and Main Clinical Characteristics of 498 Cancer Patients at Baseline |
Our primary aim was to report the proportion of ENRs in this well-characterized cohort of patients. On day 3, ENRs, patients with unchanged or worse pain, were 68 (13.7%); specifically, 53 patients had no change in API and 15 experienced greater pain intensity (Table 2). The remaining 430 patients (86.3%) reached a mean pain reduction of more than 50% and were considered ERs (Table 2).
 |
Table 2 Early (3 Days) Responder and Non-responder Patients and Changes of PID |
The relationships between pain characteristics and the analgesic responses in ERs and ENRs are outlined in Table 3. No differences were found between groups, except for the API at baseline, which was significantly lower in ENRs. In addition, there were no significant differences in the clinical characteristics of the patients and the analgesic response on day 3 (Table S1). The new doses of opioids in ENRs were in average 74.0 mg/daily, to indicate a noteworthy increase (48.9%) due to the previous lack of response.
 |
Table 3 Pain Characteristics in Early Responder and Non-Responder Patients |
The relationship between the severity of the main opioid-related ADRs and analgesic responses on day 3 is depicted in Table 4. The spectrum of ADRs was similar, with no important differences between ERs and ENRs.
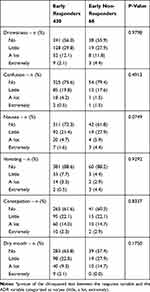 |
Table 4 Opioid ADRs Prevalence and Severity on Day 3 |
Patients classified as ENRs on day 3 were re-assessed on day 7 of treatment to explore the patterns of the analgesic responses. Of the 68 patients, eight were no longer evaluated because of premature withdrawal from the study due to inadequate analgesia (two cases), and other causes, while the remaining 60 patients could be re-assessed for pain (Table 5). Of the 60 NRs patients evaluated at day 7, 19 patients (31%) remained NRs with a further mean API worsening of 14%, 12 patients (20%) achieved PRs condition (API slightly decreasing but not reaching 30%), and 29 patients (48.3%) achieved Rs status. Overall, as compared to baseline, after 7 days of treatment 3.8% patients remained NR, 2.4% patients became PR and 5.8% reached the status of R. On day 7, the main opioid-related ADRs were again evaluated and no significant difference among NRs, PRs, and Rs in terms of prevalence and severity were observed (data not shown).
 |
Table 5 Analgesic Responses on Day 7 in the 60 Evaluable Patients Classified as NRs on Day 3 |
Discussion
Opioids are a cornerstone in the management of moderate/severe cancer pain. However, wide inter-patients variability in the response to opioids has been reported and 20–30% are defined as NRs.5 Up to 20% of the patients have persistent or refractory pain despite rapid and aggressive opioid titration, or develop refractory pain after long-term opioid use. A recent meta-analysis of the risk factors for clinical response to opioids14 suggests that young age, lung and gastrointestinal tumors, neuropathic or breakthrough pain, anxiety, and sleep disturbances were related to a low response to opioids.
Our post hoc analysis was carried out in advanced, metastatic cancer patients treated with opioids (basal mean API 6.0) with the aim to better quantify the proportion of non-responders to opioid treatment, assessing the response after 3 and 7 days of opioids treatment, out of the 498 patients, 430 achieved an immediate reduction of pain, while 68 were defined as non-responders, corresponding to a 13.6%. These findings corroborate published data showing that most patients experienced pain relief after starting opioid treatment.15,16 In our analysis, the majority of the ERs halved their PI from baseline, but 14% did not respond at all. When we looked for possible markers of response, we found that interestingly, basal API was significantly higher (P=0.001) in ERs than in ENRs (6.1±1.4 vs 5.5±1.2). The finding that lower baseline API is significantly associated with the risk of a negative response is quite intriguing. In a recent multivariate analysis, we found that the risk of negative response was halved in patients with a baseline API≥6 (OR= 0.49; 95% CI = 0.26 to 0.91; P= 0.024), comparing initial and final (4 weeks) API in the entire cohort of patients.17 The present analysis confirms this trend in a shorter time frame, 3 days of opioid treatment. Interestingly, the correlation between PI and response refers only to API and not to WPI. We do not have a clear cut explanation for these results; it could be that more severe pain reflects a more complex involvement of different signaling pathways and the corresponding ability of the opioid treatment to target them. In addition, the type of pain was irrelevant since neuropathic pain and BTP number of episodes were similar between the two groups. We assessed whether ENRs at day 3 were treated with a strong dose of therapy of opioids, but at baseline, the dose was in average 49.7 mg/daily that after 3 days was homogeneously increased about 50% in ENRs.
The presence and degree of opioid-induced ADRs can cast some light on understanding the analgesic response. Although we do not have any data on opioid plasma levels, as not planned in the original study, it is reasonable to assume that the lack of response is unlikely to be due to low drug availability (by reduced absorption or faster clearance) as the observed ADRs were similar in the two patient groups. In addition, considering that patients were treated with different opioid formulations (transdermal fentanyl, buprenorphine, and oral morphine, oxycodone) one could speculate that pharmacokinetic properties and time needed to reach the plasmatic steady state could be different at the beginning of treatment with oral or transdermal opioids and could be partially responsible for the results. However, the clinical results, reported in the original paper,5 suggest that independently of the type of the formulation used, the average and worst pain intensity after 72 hrs of treatment were similar in patients receiving oral morphine, oral oxycodone, transdermal fentanyl, and transdermal buprenorphine.
The types of response changed after 7 days of treatment, with 31.7% of the patients who remained NRs, experiencing a worsening of API; 48.3% switched and became Rs, with a drastic API reduction (about 60%), and 20.0% experienced only modest pain-relief, reaching the intermediate position of PRs. The possible mechanisms of opioid early non-response might hypothetically concern the onset of acute opioid tolerance, already observed after intra-operative use of remifentanil.18,19 In this case, opioids elicit a reaction that neutralizes the analgesic effect through desensitization, internalization, and down-regulation of opioid receptors. This occurs in a few minutes after agonist exposure, especially after short-acting opioids, and can persist. The worsening of pain intensity might also be due to a pro-nociceptive process, reportedly activated by certain opioids and/or their metabolites engaging N-methyl-D-aspartate (NMDA) receptors,20 which activate the central glutaminergic system, increasing neuronal excitability. This mechanism is also thought to generate opioid-induced-hyperalgesia (OIH). Both these mechanisms are consistent with the data described here.
A further possibility is that the intrinsic genetic characteristics of the patients could have affected the response. Pharmacogenomics offers insight into the variability in drug absorption, distribution, metabolism, and excretion that could affect efficacy and toxicity. Genes encoding for proteins involved in the transport or metabolism of opioids (such as ABC1 transporter or CYP enzymes) are present in different forms (polymorphisms) which could potentially alter the availability of the drugs.21–26 Several studies have tried to correlate the different genetic variants to the analgesic response, often with contrasting results.27–32
Similar considerations relating to the pharmacokinetics of the opioids in this cohort of patients can be applied for the pharmacogenetics: reduced transport or increased metabolism would in parallel reduce the efficacy and toxicity, while this is not the case. Indeed, variants in genes encoding for the different opioid receptors potentially affect the efficacy and adverse effects of these drugs.33–35
Recently the involvement of neuro-inflammation in chronic pain as well as in opioid analgesia has been put forward.36,37 It has been proposed that activation of the glia during illness and inflammation lead to the release of pro-inflammatory cytokines that could contribute to the response to pain. This neuro-inflammation has also been involved in opioid tolerance and the increase in pain upon opioid withdrawal.16 While these data could partially explain the tolerance to opioids, to our knowledge, no role of neuro-inflammation has been reported in the complete lack of opioid analgesic effect.
Conclusion
This analysis describes the clinical aspects of the lack of early response to morphine and similar opioids in cancer pain patients. Complete lack of response to opioid treatment is more common than expected and we are to publishing these data to spread information among physicians on the measure of this phenomenon. A better definition of its mechanisms is however needed both in cancer and non-cancer-related patient populations.
Ethics Approval and Informed Consent
This study was registered with ClinicalTrials.gov number NCT 018 09106. Its protocol was firstly approved by Ethical Committee of National Cancer Institute of Milan and subsequently by all Ethical Committees (ECs) of participating hospitals. The CEs names of the participating hospitals are listed as follows: P.O. di Piacenza; P. O. di Salemi-ASP 9, Trapani; Fondazione IRCCS Istituto Nazionale dei Tumori, Milan; Azienda Policlinico Umberto I, Rome; Ospedale degli Infermi, Rimini; Ospedale Buzzi Milan; Azienda Ospedaliera Valtellina e Valchiavenna; Policlinico Universitario Umberto I, Rome; Ospedale SS Trinità, Sora (FR); Ospedale di Mirano-ASL 13, Regione Veneto; Ospedale Monaldi, Napoli; Ospedale di Modena; Policlinico Universitario Tor Vergata, Rome; P.O. Spirito Santo, Pescara; Ospedale Oncologico Businco, Cagliari; Ospedale San Martino di Mede, Fondazione S. Maugeri; A. O. Universitaria di Parma; A.O.U. San Martino, Genova; Azienda Ospedaliera San Paolo, Milan; Ospedale di Macerata; Ospedale Civile Umberto I, Lugo; A.O. Ospedale di Circolo di Busto Arsizio; ASL 3 Genovese, Genova; Azienda Ospedaliera Carlo Poma, Mantova; E.O. Ospedali Galliera, Genova; Opedale Sacro Cuore di Gesù – Fatebenefratelli, Benevento; Fondazione Salvatore Maugeri, Pavia; IRCCS Cà Granda Ospedale Maggiore Policlinico, Milan; Ospedale San Raffaele IRCCS, Milan.
All patients provided written informed consent. All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and national research committees and with the 1964 Declaration of Helsinki and its later amendments.
Data Sharing Statement
Data will be accessible following a request to the authors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. van den Beuken-van Everdingen MHJ, Hochstenbach LMJ, Joosten EAJ, Tjan-Heijnen VCG, Janssen DJA. Update on prevalence of pain in patients with cancer: systematic review and meta-analysis. J Pain Symptom Manage. 2016;51(6):1070–1090.e9. doi:10.1016/j.jpainsymman.2015.12.340
2. World Health Organization. Cancer Pain Relief.
3. Farrar JT, Portenoy RK, Berlin JA, Kinman JL, Strom BL. Defining the clinically important difference in pain outcome measures. Pain. 2000;88(3):287–294. doi:10.1016/S0304-3959(00)00339-0
4. Corli O, Montanari M, Greco MT, et al. How to evaluate the effect of pain treatments in cancer patients: results from a longitudinal outcomes and endpoint Italian cohort study. Eur J Pain. 2013;17(6):858–866. doi:10.1002/j.1532-2149.2012.00257.x
5. Corli O, Floriani I, Roberto A, et al. Are strong opioids equally effective and safe in the treatment of chronic cancer pain? A multicenter randomized phase IV “real life” trial on the variability of response to opioids. Ann Oncol. 2016;27(6):1107–1115. doi:10.1093/annonc/mdw097
6. Mercadante S, Portenoy RK. Opioid poorly-responsive cancer pain. Part 1: clinical considerations. J Pain Symptom Manage. 2001;21(2):144–150. doi:10.1016/S0885-3924(00)00228-1
7. Hanks GW, O’Neill WM, Fallon MT. Paradoxical pain. BMJ. 1993;306(6880):793. doi:10.1136/bmj.306.6880.793-b
8. Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173. doi:10.1016/S1474-4422(14)70251-0
9. Portenoy RK. Pharmacologic management of cancer pain. Semin Oncol. 1995;22(2 Suppl 3):112–120.
10. Caraceni A, Hanks G, Kaasa S, et al. Use of opioid analgesics in the treatment of cancer pain: evidence-based recommendations from the EAPC. Lancet Oncol. 2012;13(2):e58–e68. doi:10.1016/S1470-2045(12)70040-2
11. Bouhassira D, Attal N, Alchaar H, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. 2005;114(1–2):29–36. doi:10.1016/j.pain.2004.12.010
12. Davies AN, Dickman A, Reid C, Stevens A-M, Zeppetella G. Science committee of the association for palliative medicine of Great Britain and Ireland. The management of cancer-related breakthrough pain: recommendations of a task group of the science committee of the association for palliative medicine of Great Britain and Ireland. Eur J Pain. 2009;13(4):331–338. doi:10.1016/j.ejpain.2008.06.014
13. Tamburini M, Rosso S, Gamba A, Mencaglia E, De Conno F, Ventafridda V. Original article: a therapy impact questionnaire for quality-of-life assessment in advanced cancer research. Ann Oncol. 1992;3(7):565–570. doi:10.1093/oxfordjournals.annonc.a058263
14. Lucenteforte E, Vagnoli L, Pugi A, et al. A systematic review of the risk factors for clinical response to opioids for all-age patients with cancer-related pain and presentation of the paediatric STOP pain study. BMC Cancer. 2018;18(1):568. doi:10.1186/s12885-018-4478-3
15. Apolone G, Corli O, Caraceni A, et al. Pattern and quality of care of cancer pain management. Results from the cancer pain outcome research study group. Br J Cancer. 2009;100(10):1566–1574. doi:10.1038/sj.bjc.6605053
16. Pasternak GW. Opiate pharmacology and relief of pain. J Clin Oncol. 2014;32(16):1655–1661. doi:10.1200/JCO.2013.53.1079
17. Corli O, Roberto A, Bennett MI, et al. Nonresponsiveness and susceptibility of opioid side effects related to cancer patients’ clinical characteristics: a post-hoc analysis. Pain Pract. 2018;18(6):748–757. doi:10.1111/papr.12669
18. Angst MS. Intraoperative use of remifentanil for TIVA: postoperative pain, acute tolerance, and opioid-induced hyperalgesia. J Cardiothorac Vasc Anesth. 2015;29(Suppl 1):S16–S22. doi:10.1053/j.jvca.2015.01.026
19. Yu EHY, Tran DHD, Lam SW, Irwin MG. Remifentanil tolerance and hyperalgesia: short-term gain, long-term pain? Anaesthesia. 2016;71(11):1347–1362. doi:10.1111/anae.13602
20. South SM, Smith SM. Analgesic tolerance to opioids. Pain Clin Updates. 2001;IX(5):1–4.
21. Miotto K, Cho AK, Khalil MA, Blanco K, Sasaki JD, Rawson R. Trends in tramadol: pharmacology, metabolism, and misuse. Anesth Analg. 2017;124(1):44–51. doi:10.1213/ANE.0000000000001683
22. Saba R, Kaye AD, Urman RD. Pharmacogenomics in pain management. Anesthesiol Clin. 2017;35(2):295–304. doi:10.1016/j.anclin.2017.01.015
23. Tanaka N, Naito T, Yagi T, Doi M, Sato S, Kawakami J. Impact of CYP3A5*3 on plasma exposure and urinary excretion of fentanyl and norfentanyl in the early postsurgical period. Ther Drug Monit. 2014;36(3):345–352. doi:10.1097/FTD.0000000000000029
24. Luo R, Li X, Qin S, et al. Impact of SNP-SNP interaction among ABCB1, ARRB2, DRD1 and OPRD1 on methadone dosage requirement in Han Chinese patients. Pharmacogenomics. 2017;18(18):1659–1670. doi:10.2217/pgs-2017-0072
25. Venkatasubramanian R, Fukuda T, Niu J, et al. ABCC3 and OCT1 genotypes influence pharmacokinetics of morphine in children. Pharmacogenomics. 2014;15(10):1297–1309. doi:10.2217/pgs.14.99
26. Dennis BB, Bawor M, Thabane L, Sohani Z, Samaan Z. Impact of ABCB1 and CYP2B6 genetic polymorphisms on methadone metabolism, dose and treatment response in patients with opioid addiction: a systematic review and meta-analysis. PLoS One. 2014;9(1):e86114. doi:10.1371/journal.pone.0086114
27. Olesen AE, Grønlund D, Gram M, Skorpen F, Drewes AM, Klepstad P. Prediction of opioid dose in cancer pain patients using genetic profiling: not yet an option with support vector machine learning. BMC Res Notes. 2018;11(1):78. doi:10.1186/s13104-018-3194-z
28. Ladebo L, Olesen AE. Do genes affect morphine response? Pharmacogenomics. 2017;18(17):1553–1555. doi:10.2217/pgs-2017-0138
29. Fonseca F, Torrens M. Pharmacogenetics of methadone response. Mol Diagn Ther. 2018;22(1):57–78. doi:10.1007/s40291-017-0311-y
30. Owusu Obeng A, Hamadeh I, Smith M. Review of opioid pharmacogenetics and considerations for pain management. Pharmacotherapy. 2017;37(9):1105–1121. doi:10.1002/phar.1986
31. Ren Z-Y, Xu X-Q, Bao Y-P, et al. The impact of genetic variation on sensitivity to opioid analgesics in patients with postoperative pain: a systematic review and meta-analysis. Pain Physician. 2015;18(2):131–152.
32. Currow DC, Quinn S, Ekstrom M, et al. Can variability in the effect of opioids on refractory breathlessness be explained by genetic factors? BMJ Open. 2015;5(5):e006818. doi:10.1136/bmjopen-2014-006818
33. Bauer IE, Soares JC, Nielsen DA. The role of opioidergic genes in the treatment outcome of drug addiction pharmacotherapy: a systematic review. Am J Addict. 2015;24(1):15–23. doi:10.1111/ajad.12172
34. Darcq E, Kieffer BL. Opioid receptors: drivers to addiction? Nat Rev Neurosci. 2018;19(8):499–514. doi:10.1038/s41583-018-0028-x
35. Shang Y, Filizola M. Opioid receptors: structural and mechanistic insights into pharmacology and signaling. Eur J Pharmacol. 2015;763(Pt B):206–213. doi:10.1016/j.ejphar.2015.05.012
36. Giron SE, Bjurstrom MF, Griffis CA, et al. Increased central nervous system interleukin-8 in a majority postlaminectomy syndrome chronic pain population. Pain Med. 2018;19(5):1033–1043. doi:10.1093/pm/pnx126
37. Giron SE, Griffis CA, Burkard JF. Chronic pain and decreased opioid efficacy: an inflammatory link. Pain Manag Nurs. 2015;16(5):819–831. doi:10.1016/j.pmn.2015.04.001
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
