Back to Journals » Neuropsychiatric Disease and Treatment » Volume 18
KXS Balances the Tryptophan Metabolism in Mild to Moderate Depressed Patients and Chronic Restraint Stress Induced Depressive Rats
Authors Wang Y , Li X , Jing R , Yang W , Wang Y, Wang C , Yao L, Cui X, Hu Y
Received 20 June 2022
Accepted for publication 8 October 2022
Published 1 November 2022 Volume 2022:18 Pages 2485—2496
DOI https://doi.org/10.2147/NDT.S377982
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Yuanbo Wang1,2 *, Xia Li1,2 *, Rui Jing,2 Wenshan Yang,1,2 Yichen Wang,1,2 Chaochen Wang,1,2 Lei Yao,1,2 Xiaoming Cui,3 Yuan Hu2
1Graduate School of PLA General Hospital, Beijing, 100853, People’s Republic of China; 2Department of Pharmacy, Medical Supplies Center of PLA General Hospital, Beijing, 100853, People’s Republic of China; 3Department of Health Medicine, The Third Medical Center of PLA General Hospital, Beijing, 100039, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoming Cui, Department of Health Medicine, The Third Medical Center of PLA General Hospital, Beijing, 100039, People’s Republic of China, Email [email protected] Yuan Hu, Department of Pharmacy, Medical Supplies Center of PLA General Hospital, No. 28 FuXing Road, Beijing, 100853, People’s Republic of China, Email [email protected]
Purpose: Tryptophan metabolism is involved in the etiology and exacerbation of depressive disorders. Kai-Xin-San (KXS), a traditional Chinese medicine formula, has been widely used to treat depression and modulate serotonin simultaneously, but how it regulates depressive-like behavior by shifting the balance of the tryptophan-serotonin metabolism and kynurenine pathway remains vague.
Patients and Methods: Ten participants with mild to moderate depression treated with KXS (KXS preparation) were analyzed in this study. Depression rating scale score and the concentration of serum tryptophan, 5-hydroxytryptophan and kynurenine was measured at baseline and the endpoint of KXS treatment. To explore the specific regulatory mechanism of KXS in tryptophan metabolism, the chronic restraint stress (CRS) was used to induce depressive-like syndrome in rats and the hippocampus level of tryptophan, 5-hydroxytryptophan, kynurenine with downstream metabolites (kynurenic acid, quinolinic acid) and key enzymes (indoleamine 2,3-dioxygenase, kynurenine 3-monooxygenase, kynurenine aminotransferase) were analyzed by liquid chromatography–electros pray ionization tandem mass spectrometry, high performance liquid chromatography and enzyme-linked immunosorbent assay respectively.
Results: KXS significantly decreased depression rating scale scores and increased the serum tryptophan and kynurenine concentration in depressive patients compared to baseline. Also, it alleviated the depressive behavior in CRS rats obviously. Comparing with CRS group, KXS increased tryptophan, 5-hydroxytryptophan, kynurenine level in rat hippocampus. Furthermore, in kynurenine pathway, KXS decreased the expression of indoleamine 2,3-dioxygenase, increased kynurenic acid by upregulating the expression of kynurenine aminotransferase while decreased quinolinic acid level in hippocampus, which suggested that KXS more favored improving serotonin pathway, and neuroprotective kynurenic acid branch in the tryptophan metabolism.
Conclusion: This is the first tryptophan metabolomic study of patients with depression undergoing KXS treatment. Combining these clinical results with CRS induced rat model studies, it verified that KXS achieves an excellent antidepressant effect and balances tryptophan-kynurenine metabolic pathways by regulating some key metabolic products and enzymes.
Keywords: depression, amino acids, TCM, KaiXinSan
Introduction
Depression is a deleterious mental disorder, ranking the single largest contributor to non-fatal health loss worldwide (7.5% of all Years Lived with Disability).1,2 The current relevant studies range from serotonergic neurotransmitter deficiency hypothesis to immune and inflammation system.3–6 Although monoamine transmitter deficiency in the brain played a vital role for over the past few decades, more than 30% of depressive patients do not response to typical monoaminergic antidepressant treatments.7 The urge need for more efficient antidepressant strategies is calling for further exploration in monoamine transmitter mechanism. As the sole precursor of 5-hydroxytryptamine (serotonin), tryptophan has been paid attention in mental disorders research.8,9 The main catabolic route of tryptophan is the kynurenine pathway, which is responsible for approximately 95–99% of tryptophan metabolism in the body. Kynurenine final metabolic product, including neurotoxic quinolinic acid and neuroprotective kynurenic acid, have impacts on central nervous system partly by affecting N-methyl-D-aspartic acid receptor (NMDAR) function and inducing neural functional disturbance subsequently. Thus, kynurenine pathway is hypothesized to be another key link between tryptophan metabolism and emotional disorders, which may hold promise as a novel treatment target.10
KXS, a TCM formula, has been used to treat depression for a considerably long history with unique advantage.11,12 KXS’s anti-depressive efficacy and safety in the treatment of mild to moderate depression has been proved in a randomized, double-blind, and positive-drug-controlled clinical trial in recent years.13,14 Pharmacology studies indicate that the therapeutic effects of KXS may be associated with the function of neurotransmitter system, especially 5-HT system. KXS could significantly increase the expression of tryptophan hydroxylase, the key enzyme during the 5-HT synthesis process in the hippocampus and prefrontal cortex of the depressed rats and suppress the expression of 5-HT transporter.15 A metabolic study of Alzheimer rats indicated that KXS exerted regulatory effects in tryptophan metabolism by reducing indole sulfate, xanthurenic acid, and kynurenic acid.16 But there is no systematic study elaborates the mechanism of tryptophan-serotonin pathway and kynurenine pathway involved in the anti-depressive effects of KXS.
Here, it is hypothesized that KXS ameliorates depressive-like behavior by modifying tryptophan metabolism. First, we measured the serum concentration of tryptophan as well as 5-hydroxytryptophan and kynurenine in patients with mild to moderate depression treated with KXS. Then, to make the underlying mechanism clearer, we conducted chronic restraint stress (CRS) induced depressive rats and measured the concentrations of tryptophan, 5-hydroxytryptophan, kynurenine, kynurenic acid, quinolinic acid levels and the expressions of indoleamine 2,3-dioxygenase (IDO), kynurenine aminotransferase (KAT), kynurenine 3-monooxygenase (KMO) in rat hippocampus to clarify the metabolic characteristic alternations after KXS treatment.
Materials and Methods
Ethics Statements and Sample Collection
Experimental protocols relating human subjects have been reviewed and approved by the Ethics Committee of Xijing Hospital of The Fourth Military Medical University of the Chinese People’s Liberation Army (Xi’an China, Ethical approval No. YS201505074). The present study complies with the Declaration of Helsinki. All the participants have signed written inform consent.
Ten participants with mild to moderate depression treated with KXS and ten participants treated with fluoxetine were recruited randomly among the 156 patients treated in the Department of Psychosomatic Xijing Hospital of the Fourth Military Medical University and the 261 Hospital of the Chinese People’s Liberation Army (PLA Mental Health Center) (Table 1).14 The blood samples collected at baseline and after eight-week treatment were stored at −80 ℃ for further measurement.
 |
Table 1 The Diagnostic and Inclusive Criteria for the Patients |
The serum parameters of human blood samples were quantified by a targeted ultra-high performance liquid chromatography multiple reaction monitoring mass spectrometry (UHPLC-MRM-MS/MS) approach. An aliquot of each serum sample was precisely weighted and transferred into an Eppendorf tube. Triple-volume of precooled acetonitrile was added to the centrifuge tube. The samples were vortexed for 5 min and then incubated for 30 min at 4 ℃, followed by centrifugation for 15 min at 12,000 rpm at 4℃. After that, 300 μL filtered supernatant was transferred to an auto-sampler vial. After vacuum drying, 500 μL methanol solvent was added into each vial for reconstitution. The mobile phase A consists of 1% formic acid and 10mM ammonium acetate in water. The mobile phase B was 0.1% formic acid in acetonitrile. The column temperature was set at 40 ℃ and the injection volume was 2 μL, delivered at the speed of 0.4 mL/min. An AB SCIEX QTRAP 5500 ion trap mass spectrometer equipped with LC system (SHIMADZU 30AD) was used for assay development. Analyst TF 1.7 and Master View 1.0 software were used for data collection and processing. Multi Quant 3.0.2 software was applied for quantification.
Drug Preparation
KXS preparation was administrated to patients as KXS pills as previously reported.14 Ginseng Radix, Polygala Radix, Acorus Rhizoma and Poria (Lvye Pharmaceutical Co., Ltd., Beijing, China) were mixed at the ratio of 3:2:3:2 and processed as described formerly.17 KXS powder was obtained after soaking, circumfluence extraction and evaporation. 1g yield powder is equivalent to 4.83g total crude herbs.
Chemicals and Reagents
L-tryptophan, 5-hydroxy-L-tryptophan and L-kynurenine were purchased from Sigma-Aldrich (St. Louis, MO, USA). Methanol (AR grade) was purchased from Sinopharm Chemical Reagent Co., Ltd. Potassium dihydrogen phosphate (AR grade) was purchased from XiLONG SCIENTIFIC. Perchloric acid (AR grade) was obtained from Shanghai Jinlu Chemical Co., Ltd. All solutions were prepared with ultrapure water. IDO ELISA kit and KMO ELISA kit were purchased from Shanghai Enzyme-linked Biotechnology Co., Ltd.
Animal Ethics Evaluation
Male Sprague-Dawley rats (4 weeks old, weighing 100 ± 15 g) purchased from Animal Breeding Center of PLA General Hospital were housed under standard controlled conditions (lights on 08:00–20:00, room temperature 22 ± 1 ℃, humidity 65%) with food and water ad libitum. All the procedures were approved by the Animal Experimentation Ethics Committee of PLA General Hospital. The present research followed the Instructive Notions with Respect to Caring for Laboratory Animals. Rats were weighted and anesthetized with pentobarbital sodium (40 mg/kg) before sacrifice.
Modeling and Drug Administration
Rats were screened by sucrose preference test before recruitment. Eligible individuals were kept for follow-up management. The animals were placed individually into a stainless cylindrical tube (5cm in internal diameter and 20cm in length) with adjustable length for 4 hours every day for consecutive 28 days. A groove on the trunk of the tube assured the respiration and excretion of rats. Animals distributed to control group (n = 12) were housed in cages undisturbed. Each cage contains three rats with free access to chow and tap water. The rats were screened by sucrose preference test, tail suspension test and open-field test when the modeling procedure accomplished to assure successful modeling.
The depressed rats were randomly distributed into CRS group (n = 12) and KXS treated group (n = 12). Rats in CRS group were administrated with distilled water orally and individuals in KXS group were administrated with KXS at the dose of 365.4 mg/kg/day orally between 8:00 and 9:00 AM daily for consecutive 14 days. Restraint stress was continuously applied during the treatment. Sucrose preference test, tail suspension test and open-field test were carried out once the drug administration phase was accomplished.
Sucrose Preference Test
The SPT was conducted to assess the anhedonia behavior of rats over a 4-day period. After the foreshadowing procedures finished, two bottles filled with 1% sucrose solution and distilled water separately were presented to animals on the fourth day. The positions of two bottles were exchanged in the middle of the 2-hour duration and then the bottles were removed. The consumption of sucrose solution and distilled water was recorded.
Tail Suspension Test
The TST was conducted to appraise the desperation extent of rats. Animals were taken into testing room 30 min before the test for adaptation. Experimenters suspended the rats by adhibiting the tails to a metal rack with adhesive tape. Each test last for 5 min and the accumulated immobility time was calculated by behavioral test software automatically.
Open-Field Test
The OFT was carried out in an open squared arena (100 cm×100 m) to assess the locomotor activity of rats. The open field square was divided into 25 sections (20 cm in length and 20 cm in width). The behavior of animals during 5 min was recorded digitally by tracking software for further analysis. Between each assessment, the floor would be cleaned by 75% ethanol to get rid of olfactory interference. The travelled distance, times of crossing central areas and movement tracking of rats were recorded and calculated automatically.
Tissue Preparation
Rats were anesthetized and decapitated quickly after behavioral tests. The hippocampus tissues were stripped off with sterilized pre-cooled tools on ice, placed in cryopreservation tubes and preserved in a −80℃ refrigerator for later use. The hippocampus tissue was rinsed with pre-chilled PBS (0.01M, pH = 7.4) to remove residual blood and chopped after weighing. The shredded tissue was mixed with the corresponding volume of PBS at a weight-to-volume ratio of 1:9 and transferred into a glass homogenizer and triturated well on ice. The homogenate was centrifuged at 5000 × g for 10 minutes, and the supernatant was collected for detection.
Tryptophan, 5-Hydroxytryptophan and Kynurenine Determination in Rat Hippocampus by High Performance Liquid Chromatography
The HPLC system consists of an Agilent 1200 separation module equipped with an Agilent G1322A degasser, an Agilent G1329A Automatic temperature control sampler, an Agilent G1316A column oven, an Agilent G1315B diode array detector. The system was run isocratically using a C18 column (Agilent 5μm, 250×4.6 mm). A mobile phase with 0.05 mol/L potassium dihydrogen phosphate buffer: methanol (80:20, v/v) was used at a flow rate of 1.0 mL/min at 25 ℃. Rat hippocampus sample supernatants (20 μL) were autoinjected into the column. The detection was performed at 225 nm wavelength for tryptophan and kynurenine and 278 nm wavelength for 5-hydroxytryptophan.
Indoleamine 2.3-Dioxygenase, Kynurenine Aminotransferase, Kynurenine 3-Monooxygenase, Kynurenic Acid and Quinolinic Acid Determination in Rat Hippocampus by ELISA
With commercial ELISA kits, the level of indoleamine 2,3-dioxygenase (MLBIO, Shanghai, China), kynurenine aminotransferase (MLBIO, Shanghai, China), kynurenine 3-monooxygenase (MLBIO, Shanghai, China), kynurenic acid (MLBIO, Shanghai, China) and quinolinic acid (MLBIO, Shanghai, China) in rat hippocampus supernatants was performed according to manufacturer’s instructions. The mean optical density was detected at a wavelength of 450 nm and the content was calculated according to the standard curves and relative regression equations.
Data Analysis and Statistical Methods
All statistical analyses were performed with GraphPad Prism version 9.2.0 (GraphPad Software, San Diego, CA, USA). Results were reported as mean ± standard deviation. One-way analysis of variance (ANOVA) was employed to compare the differences among groups. A value of p < 0.05 was considered as statistically significant.
Results
Effects of KXS on Depressive Symptoms and Tryptophan Metabolism
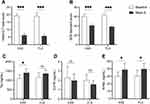
Patients in KXS group showed a statistically significant improvement in HAM-D17 total score and SDS standard score after 8 weeks of treatment (19.20 ± 1.48 vs 6.20 ± 1.32, p < 0.001; 60.50 ± 4.95 vs 41 ± 2.91, p < 0.001) (Figure 1A and B). The reductions in HAM-D17 score at 8 weeks in KXS and FLX groups were comparable, which suggested improvement in the clinical features. Compared to baseline, eight-week treatment with KXS induced increase in serum tryptophan (1401.00 ± 217.60 vs 1103.00 ± 190.20, p = 0.0149) (Figure 1C), and kynurenine (38.78 ± 8.84 vs 30.10 ± 5.72, p = 0.0352) (Figure 1E), but without significant change in 5-hydroxytryptophan serum level (1.98 ± 0.72 vs 2.28 ± 1.23, p = 0.56) (Figure 1D). Fluoxetine group displayed similar results as it increased serum tryptophan and kynurenine concentration (1257.00 ± 239.00 vs 1157.00 ± 246.90, p = 0.4237; 37.51 ± 7.97 vs 31.55 ± 8.20, p = 0.1496), while decreased serum 5-hydroxytryptophan level (1.14±0.53 vs 1.53±0.66, p = 0.16) after an eight-week treatment.
The Effects of KXS on Depressive-Like Behavior in Rats Exposed CRS
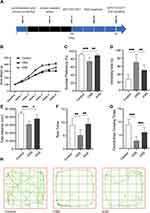
Comparing with control group, CRS rats showed lower sucrose consumption (74.56 ± 9.43 vs 91.87 ± 2.92, p < 0.001) (Figure 2C), prolonged TST immobility time (69.92 ± 19.80 vs 28.25 ± 10.52, p < 0.001) (Figure 2D), but less body weight gain (Figure 2B). CRS induced weakened locomotor activity reflected by less total distance (30,864 ± 3753 vs 44,606 ± 2251, p < 0.001), less rear times and central area crossing times in open-field test (Figure 2E–H). KXS reversed all the abnormal behaviors induced by CRS, as the rats showed increased sucrose consumption (89.33 ± 3.43 vs. 74.56 ± 9.43, p < 0.01), increased total moving distance (37,721 ± 5503 vs 30,864 ± 3753, p < 0.05), increased rear times (9.42 ± 1.98 vs 5.17 ± 2.04, p < 0.01) and central area crossing times and decreased TST immobility time (50.42 ± 14.02 vs 69.92 ± 19.80, p = 0.01), approving that KXS exerted efficacy in anti-depression as it significantly alleviated the anhedonia symptoms.
The Regulatory Effects of KXS on Tryptophan Metabolism in CRS Rats
The hippocampus concentrations of tryptophan and 5-hydroxytryptophan were detected to profile the changes in tryptophan metabolism. As shown in Figure 3 (Part 1), CRS rats presented decreased tryptophan level (15.40 ± 3.431 vs. 23.33 ± 1.643, p < 0.001) (Figure 3A) and decreased 5-hydroxytryptophan level (25.66 ± 2.917 vs 38.12±6.685, p = 0.0015) (Figure 3B). Two-week treatment with KXS significantly increased tryptophan concentration (23.97 ± 3.363 vs 15.40 ± 3.431, p < 0.001) and 5-hydroxytryptophan concentration (33.46 ± 6.767 vs 25.66 ± 2.917, p = 0.0455).
The Effects of KXS on Kynurenine Pathway
The levels of kynurenine, kynurenic acid, quinolinic acid, IDO, KAT and KMO were detected with ELISA kits. As shown in Figure 3 (Part 2), comparing with control group, CRS rats displayed decreased kynurenine level (0.656 ± 0.278 vs 1.239 ± 0.343, p = 0.0272) (Figure 3C), decreased kynurenic acid level (1.479 ± 0.157 vs 1.702 ± 0.054, p = 0.0145) (Figure 3D), and upregulated IDO level in hippocampus (245.8 ± 29 vs 166.5 ± 55.31, p = 0.03) (Figure 3F). There was not obvious change in quinolinic acid level (Figure 3E). We observed a tendency of increase for KMO, but not statistically significant (Figure 3G). CRS also induced the decrease of KAT concentration in model rat hippocampus (1246 ± 76.48 vs 1337 ± 36.56, p = 0.0492) (Figure 3H). Two-week treatment with KXS increased the level of kynurenine (1.243 ± 0.383 vs 0.656 ± 0.278, p = 0.0314) and kynurenic acid (1.737 ± 0.094 vs 1.479 ± 0.157, p = 0.0052). The level of quinolinic acid in hippocampus was downregulated after KXS treatment (2.595 ± 0.191 vs 2.852 ± 0.124, p = 0.0239). KXS reversed the alternations of IDO and KAT levels (182.6 ± 50.61 vs 245.8 ± 29, p = 0.0368; 1334 ± 66.67 vs 1246 ± 76.48, p = 0.0454) significantly. There was a trend of decrease in KMO level, but not statistically significant.
Discussion
To our knowledge, this is the first metabolomic study that systematically assessed the tryptophan metabolic abnormalities in the hippocampus in CRS induced rodent depression model combining with human serum analysis. Eight-week treatment with KXS preparation reduce patients’ depression scale scores, alleviate depressive symptoms and regulate the key indicators in tryptophan metabolism simultaneously. Two-week treatment with KXS improves the depressive behaviors of CRS rats, more clearly balances the tryptophan metabolism and the kynurenine pathway.
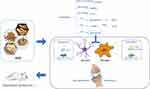
Tryptophan exerts important biological functions as the sole precursor of serotonin and the substrate for numerous bioactive metabolites with two metabolic pathways, the tryptophan-serotonin metabolism and the kynurenine pathways (Figure 4). 5-hydroxytryptophan is derived from tryptophan by the catalysis of tryptophan hydroxylase (TPH). As the intermediate in tryptophan-serotonin metabolism, 5-hydroxytryptophan is degraded into serotonin by the catalysis of aromatic L-amino acid decarboxylase (AADC), which is on behalf of the rate-limiting pivotal in serotonin biosynthesis. Thus, disturbed tryptophan availability can influence peripheral serotonin synthesis.18 Under physiological conditions, only a small proportion of tryptophan was utilized for serotonin synthesis. The majority flux of tryptophan is converted into kynurenine by tryptophan 2,3-dioxygenase (TDO) in liver tissue and IDO in extrahepatic tissue. In kynurenine pathway, kynurenine converting into quinolinic acid and kynurenic acid by different enzymes KMO and KAT have been widely researched in the aspects of their impacts on CNS function.19,20 Quinolinic acid is characterized by neurotoxicity by interacting with glutamatergic system and kynurenic acid exerts neuroprotective effects as an antagonist of NMDA receptors.21
Many studies have put insight into the alternations of tryptophan metabolites profiles in depression patients. It’s demonstrated that the plasm concentrations of tryptophan and kynurenine are significantly lower in major depressive disorder patients than those in healthy controls.22 Metabolic factors of kynurenine play a crucial role in the occurrence and development of major depressive disorder. Kynurenine or tryptophan would be a potential biomarker in diagnosing depression patients and a crucial role in depression remission.23–25 A systematic review suggested that treatment-resistant depression (TRD) was also associated with the activation of kynurenine pathway.24 Only relatively few studies have investigated the impact of antidepressant treatment on the tryptophan metabolism and kynurenine pathway without entirely consistent conclusions. In a study by Halaris et al escitalopram treatment in 20 depressed individuals showed a reduction of kynurenine pathway neurotoxic metabolites as well as in the kynurenine/tryptophan ratio.26 While interferon-alpha treatment in patients was associated with decreased tryptophan levels and increased levels of kynurenine and kynurenine/tryptophan ratio.27
Based on the previously randomized, double-blind, parallel-group study in 156 patients with mild to moderate depression, we investigated the serum changes in tryptophan metabolism in 10 patients, it was found that tryptophan and kynurenine level increased after KXS treatment. To verify whether the central metabolic characteristics were consistent with those in peripheral serum, the CRS rats were used. Two-week treatment with KXS significantly enriched the abundance of tryptophan followed, and an increase in 5-HTP was observed. Thus, KXS might promote the synthesis of the pivotal neurotransmitter 5-HT. KXS treatment exerted certain ability to downregulate the level of IDO. While an obvious increase in KYNU level was still observed. This might be explained by the enrichment of central tryptophan. Moreover, the out of balance state between neurotoxic QUIN and neuroprotective KYNA was modified by KXS treatment. The consistent results shown by CRS induced depressive rat model suggested KXS also improved the tryptophan and kynurenine level in hippocampus, but without increasing IDO, and the antidepressant-like efficacy could partly be attributed to the modification of KYNA/QUIN. Both preclinical and clinical studies indicated that KXS might favor the tryptophan-serotonin metabolism, by higher 5-HT catalyzed.
Over-activation of the kynurenine pathway can not only result in less tryptophan abundance for serotonin synthesis, but also cause imbalance of neuroactive metabolites like kynurenic acid and quinolinic acid, the dysregulation of which have hence been linked to depressive disorders.28–30 Both preclinical and clinical studies have reported that the dysfunction in tryptophan metabolism and kynurenine pathway is likely to be normalized by antidepressant treatment.31 Eskelund et al found that vortioxetine reduced quinolinic acid levels in various brain regions in both Flinders Sensitive Line rats, a genetic rat model, and lupus mice, a model of increased depression-like behavior associated with inflammation.32 As a model used to evaluate the efficacy of antidepressants in rodents, chronic unpredictable mild stress (CUMS) induces long-term behavioral disturbances that resemble symptoms of clinical depression. Tryptophan metabolism and kynurenine pathway may have an associated abnormality with changes in kynurenine, kynurenic acid, quinolinic acid, especially metabolized into neurotoxic 3-hydroxycaninuric acid (3-HK, the precursor of quinolinic acid) branch in the cortex and hippocampus under CUMS.33,34 We observed the process of depression is accompanied by changes in the concentrations of kynurenine, kynurenic acid and quinolinic acid, and the kynurenine pathway is over-activated by induction of CRS with or without IDO, KAT and KMO in rat experiments.
In recent two decades, studies have demonstrated KXS’s pleiotropic anti-depression effects in vitro and in vivo. Two-week treatment with KXS balanced kynurenine pathway by upregulating the expression of KAT and modulating the level of kynurenic acid and quinolinic acid. A possible explanation of this might be that KXS possesses the ability of modifying the hypothalamic-pituitary-adrenal (HPA) axis,35,36 regulating the neurogenesis and astrocytes dysfunction,37,38 impacting the expression of proinflammatory cytokines,39 which might all influences the kynurenine metabolism differently in the periphery and CNS.
Conclusion
Targeting tryptophan metabolism offers a wide range of potential therapeutic options which are particularly applicable for the treatment of depression. The present study has demonstrated that KXS shed light on new targets for the treatment of depressive disorder by modulating the tryptophan metabolism and kynurenine pathway. It helps increase the serotonin synthesis by enhancing the tryptophan-serotonin metabolism, Meanwhile, it reversed the abnormalities of the kynurenine pathway by shifting the degradation of kynurenine into kynurenic acid branch.
Statement
The present study was partly based on the clinical trial “A randomized, double-blind, placebo-controlled clinical trial on the efficacy and safety of Shen-Zhi-Ling tablets in the treatment of depression. (heart and spleen deficiency syndrome)”. The Chinese PLA General Hospital was the sponsor of the trial. The patients who provided the serum samples in the paper were enrolled and treated in Xijing Hospital of The Fourth Military Medical University of the Chinese People’s Liberation Army. The protocol was approved by the Ethics Committee of Xijing Hospital.
Ethics Approval and Informed Consent
Experimental protocols relating human subjects have been reviewed and approved by the Ethics Committee of Xijing Hospital of The Fourth Military Medical University of the Chinese People’s Liberation Army (Xi’an China, Ethical approval No. YS201505074). All the participants have signed written inform consent. The animal experimental procedures were approved by the Animal Experimentation Ethics Committee of PLA General Hospital. The present research followed the Instructive Notions with Respect to Caring for Laboratory Animals, the Ministry of Science and Technology of China (13 September 2006).
Acknowledgments
The present study was supported by National Natural Science Foundation of China (No. 81973502).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. World Health O. Depression and other common mental disorders: global health estimates; 2017. Available from: https://apps.who.int/iris/handle/10665/254610.
2. Baldessarini R, Forte A, Selle V, et al. Morbidity in depressive disorders. Psychother Psychosom. 2017;86(2):65–72. doi:10.1159/000448661
3. Ménard C, Hodes GE, Russo SJ. Pathogenesis of depression: insights from human and rodent studies. Neuroscience. 2016;321:138–162. doi:10.1016/j.neuroscience.2015.05.053
4. Delgado PL. Depression: the case for a monoamine deficiency. J Clin Psychiatry. 2000;61(Suppl 6):7–11.
5. Schildkraut JJ. The catecholamine hypothesis of affective disorders: a review of supporting evidence. J Neuropsychiatry Clin Neurosci. 1995;7(4):524–533. doi:10.1176/jnp.7.4.524
6. van Praag HM, Korf J. A pilot study of some kinetic aspects of the metabolism of 5-hydroxytryptamine in depressive patients. Biol Psychiatry. 1971;3(2):105–112.
7. Berlim M, Turecki G. What is the meaning of treatment resistant/refractory major depression (TRD)? A systematic review of current randomized trials. Eur neuropsychopharmacol. 2007;17(11):696–707. doi:10.1016/j.euroneuro.2007.03.009
8. Wigner P, Czarny P, Galecki P, Su KP, Sliwinski T. The molecular aspects of oxidative & nitrosative stress and the tryptophan catabolites pathway (TRYCATs) as potential causes of depression. Psychiatry Res. 2018;262:566–574. doi:10.1016/j.psychres.2017.09.045
9. Shaw K, Turner J, Del Mar C. Tryptophan and 5-hydroxytryptophan for depression. Cochrane Database Syst Rev. 2001;(3):Cd003198. doi:10.1002/14651858.Cd003198
10. Réus GZ, Jansen K, Titus S, Carvalho AF, Gabbay V, Quevedo J. Kynurenine pathway dysfunction in the pathophysiology and treatment of depression: evidences from animal and human studies. J Psychiatr Res. 2015;68:316–328. doi:10.1016/j.jpsychires.2015.05.007
11. Fu H, Xu Z, Zhang XL, Zheng GQ. Kaixinsan, a well-known Chinese herbal prescription, for alzheimer’s disease and depression: a preclinical systematic review. Front Neurosci. 2019;13:1421. doi:10.3389/fnins.2019.01421
12. Feng DD, Tang T, Lin XP, et al. Nine traditional Chinese herbal formulas for the treatment of depression: an ethnopharmacology, phytochemistry, and pharmacology review. Neuropsychiatr Dis Treat. 2016;12:2387–2402. doi:10.2147/ndt.S114560
13. Chen C, Hu Y, Dong X, Zhou X, Mu L, Liu P. Proteomic analysis of the antidepressant effects of shen-zhi-ling in depressed patients: identification of proteins associated with platelet activation and lipid metabolism. Cell Mol Neurobiol. 2018;38(5):1123–1135. doi:10.1007/s10571-018-0582-9
14. Hu Y, Wang Y, Chen C, et al. A randomized, placebo-controlled, double-blind study on the effects of SZL on patients with mild to moderate depressive disorder with comparison to fluoxetine. J Ethnopharmacol. 2021;281:114549. doi:10.1016/j.jep.2021.114549
15. Dong XZ, Li ZL, Zheng XL, Mu LH, Zhang GQ, Liu P. A representative prescription for emotional disease, Ding-Zhi-Xiao-Wan restores 5-HT system deficit through interfering the synthesis and transshipment in chronic mild stress-induced depressive rats. J Ethnopharmacol. 2013;150(3):1053–1061. doi:10.1016/j.jep.2013.10.018
16. He Y, Wang Y, Liu S, et al. A metabolomic study of the urine of rats with Alzheimer’s disease and the efficacy of Ding-Zhi-Xiao-Wan on the afflicted rats. J Sep Sci. 2020;43(8):1458–1465. doi:10.1002/jssc.201900944
17. Dong XZ, Wang DX, Zhang TY, Liu X, Liu P, Hu Y. Identification of protein targets for the antidepressant effects of Kai-Xin-San in Chinese medicine using isobaric tags for relative and absolute quantitation. Neural Regen Res. 2020;15(2):302–310. doi:10.4103/1673-5374.265555
18. Matthes S, Bader M. Peripheral serotonin synthesis as a new drug target. Trends Pharmacol Sci. 2018;39(6):560–572. doi:10.1016/j.tips.2018.03.004
19. Schwarcz R, Du F. Quinolinic acid and kynurenic acid in the mammalian brain. Adv Exp Med Biol. 1991;294:185–199. doi:10.1007/978-1-4684-5952-4_17
20. Heyes MP. Metabolism and neuropathologic significance of quinolinic acid and kynurenic acid. Biochem Soc Trans. 1993;21(1):83–89. doi:10.1042/bst0210083
21. Cervenka I, Agudelo LZ, Ruas JL. Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science. 2017;357(6349). doi:10.1126/science.aaf9794
22. Kuwano N, Kato TA, Setoyama D, et al. Tryptophan-kynurenine and lipid related metabolites as blood biomarkers for first-episode drug-naïve patients with major depressive disorder: an exploratory pilot case-control study. J Affect Disord. 2018;231:74–82. doi:10.1016/j.jad.2018.01.014
23. Liu H, Ding L, Zhang H, et al. The metabolic factor kynurenic acid of kynurenine pathway predicts major depressive disorder. Front Psychiatry. 2018;9:552. doi:10.3389/fpsyt.2018.00552
24. Wigner P, Czarny P, Galecki P, Sliwinski T. Oxidative and nitrosative stress as well as the tryptophan catabolites pathway in depressive disorders. Psychiatr Danub. 2017;29(4):394–400. doi:10.24869/psyd.2017.394
25. Benatti C, Blom JM, Rigillo G, et al. Disease-induced neuroinflammation and depression. CNS Neurol Disord Drug Targets. 2016;15(4):414–433. doi:10.2174/1871527315666160321104749
26. Halaris A, Myint AM, Savant V, et al. Does escitalopram reduce neurotoxicity in major depression? J Psychiatr Res. 2015;66–67:118–126. doi:10.1016/j.jpsychires.2015.04.026
27. Hunt C, Macedo ECT, Suchting R, et al. Effect of immune activation on the kynurenine pathway and depression symptoms - A systematic review and meta-analysis. Neurosci Biobehav Rev. 2020;118:514–523. doi:10.1016/j.neubiorev.2020.08.010
28. Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12(11):988–1000. doi:10.1038/sj.mp.4002006
29. Chen Y, Guillemin GJ. Kynurenine pathway metabolites in humans: disease and healthy states. Int J Tryptophan Res. 2009;2:1–19. doi:10.4137/ijtr.s2097
30. Müller N, Schwarz MJ. A psychoneuroimmunological perspective to Emil Kraepelins dichotomy: schizophrenia and major depression as inflammatory CNS disorders. Eur Arch Psychiatry Clin Neurosci. 2008;258(Suppl 2):97–106. doi:10.1007/s00406-008-2012-3
31. Ogyu K, Kubo K, Noda Y, et al. Kynurenine pathway in depression: a systematic review and meta-analysis. Neurosci Biobehav Rev. 2018;90:16–25. doi:10.1016/j.neubiorev.2018.03.023
32. Eskelund A, Li Y, Budac DP, et al. Drugs with antidepressant properties affect tryptophan metabolites differently in rodent models with depression-like behavior. J Neurochem. 2017;142(1):118–131. doi:10.1111/jnc.14043
33. Li CC, Jiang N, Gan L, et al. Peripheral and cerebral abnormalities of the tryptophan metabolism in the depression-like rats induced by chronic unpredicted mild stress. Neurochem Int. 2020;138:104771. doi:10.1016/j.neuint.2020.104771
34. Wang J, Li X, He S, et al. Regulation of the kynurenine metabolism pathway by Xiaoyao San and the underlying effect in the hippocampus of the depressed rat. J Ethnopharmacol. 2018;214:13–21. doi:10.1016/j.jep.2017.11.037
35. Dang H, Sun L, Liu X, et al. Preventive action of Kai Xin San aqueous extract on depressive-like symptoms and cognition deficit induced by chronic mild stress. Exp Biol Med. 2009;234(7):785–793. doi:10.3181/0812-rm-354
36. Zhang J, Wang D, Zhou J, Mao-Xing LI, Jia ZP, Zhang RX. Mechanism and effects of kaixin powder, danggui shaoyao powder and hypericum perforatum L. on the behavior of high fat rats with chronic stress. China J Tradit Chin Med Pharm. 2016;31:4230–4235.
37. Yan L, Hu Q, Mak MS, et al. A Chinese herbal decoction, reformulated from Kai-Xin-San, relieves the depression-like symptoms in stressed rats and induces neurogenesis in cultured neurons. Sci Rep. 2016;6:30014. doi:10.1038/srep30014
38. Zhu KY, Xu SL, Choi RC, Yan AL, Dong TT, Tsim KW. Kai-xin-san, a Chinese herbal decoction containing ginseng radix et rhizoma, polygalae radix, acori tatarinowii rhizoma, and poria, stimulates the expression and secretion of neurotrophic factors in cultured astrocytes. Evid Based Complement Alternat Med. 2013;2013:731385. doi:10.1155/2013/731385
39. Dong XZ, Wang DX, Lu YP, Yuan S, Liu P, Hu Y. Antidepressant effects of Kai-Xin-San in fluoxetine-resistant depression rats. Braz J Med Biol Res. 2017;50(10):e6161. doi:10.1590/1414-431x20176161
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.