Back to Journals » Cancer Management and Research » Volume 12
KRT17 Functions as a Tumor Promoter and Regulates Proliferation, Migration and Invasion in Pancreatic Cancer via mTOR/S6k1 Pathway
Authors Li D , Ni XF , Tang H, Zhang J, Zheng C , Lin J, Wang C , Sun L , Chen B
Received 20 December 2019
Accepted for publication 14 February 2020
Published 19 March 2020 Volume 2020:12 Pages 2087—2095
DOI https://doi.org/10.2147/CMAR.S243129
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Antonella D'Anneo
Ding Li,1,* Xiao-Feng Ni,1,* Hengjie Tang,1,* Jiecheng Zhang,1 Chenlei Zheng,1 Jianhu Lin,1 Cheng Wang,1 Linxiao Sun,1 Bicheng Chen1,2
1Key Laboratory of Diagnosis and Treatment of Severe Hepato-Pancreatic Diseases of Zhejiang Province, Zhejiang Provincial Top Key Discipline in Surgery, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, People’s Republic of China; 2Department of Clinical Laboratory, The First Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang Province, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Bicheng Chen; Linxiao Sun Email [email protected]; [email protected]
Background: Pancreatic cancer (PC) is one of the most well-known malignancies with high mortality, but the underlying mechanism of PC remains unknown. Keratin17 (KRT17) expression has been reported in many malignancies, but its functions in PC are not clear. The aim of our study was to evaluate KRT17 expression and its potential role in PC.
Methods: The online databases GEPIA and THPA were used to identify KRT17 expression in tissues. Quantitative real-time PCR (qRT-PCR) was used to determine KRT17 expression in cell lines. Ki67 and ROS levels were detected by immunofluorescence assay and a 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (DCFH-DA) probe. KRT17 downregulation was induced by the small interfering RNA (siRNA) technique. Proliferation function was evaluated by colony formation assay and RTCA. Migration and invasion were evaluated by transwell migration assay. A Western blot assay was used to detect protein levels.
Results: KRT17 was overexpressed in PC tissues compared to that in normal tissues. The results showed that Ki67 and ROS levels were decreased in pancreatic cancer cells after transfection with siKRT17. After KRT17 downregulation in PC cell lines, cell viability functions, including proliferation, migration and invasion, and mTOR/S6K1 phosphorylation levels were attenuated.
Conclusion: KRT17 knockdown significantly inhibited proliferation, migration and invasion in pancreatic cancer cells.
Keywords: KRT17, knockdown, proliferation, migration, invasion, pancreatic cancer, mTOR/S6K1
Introduction
Pancreatic cancer (PC), with its low incidence but high mortality, is one of the most fatal malignant neoplasms globally.1,2 The molecular mechanism and therapeutic strategies of PC have been continuously studied.3,4 Therefore, it is of great significance for us to determine the molecular mechanism of PC and find more novel biomarkers for PC to contribute to the developmental, preventive and therapeutic strategies for PC.
The mammalian target of rapamycin (mTOR) pathway is an important regulator of cell proliferation that is activated by amino acids, insulin, and growth factors.5,6 mTOR is overexpressed in breast cancer,7 gallbladder cancer8 and other cancers.9,10 S6K1 encodes a member of the ribosomal S6 kinase family of serine/threonine kinases and responds to mTOR signaling to promote protein synthesis, cell growth, and cell proliferation.11 Increasing evidence suggests that mTOR pathway deregulation is associated with human diseases, including cancer and diabetes. Results from clinical trials indicate that mTOR inhibitors may be used for oncology therapy and may be useful for treating subsets of patients with certain types of cancer.12,13
Keratin (KRT) is a protein family that is critical for hair formation and is abundant in the outer layer of the skin, thus protecting epithelial cells from damage. Depianto et al14 first reported that KRT17 promoted epithelial proliferation and tumor growth in skin. Previous studies illustrated that KRT17 is overexpressed in many cancers, including cervical cancer,15 gastric cancer,16 and lung cancer.17 However, the relationship between KRT17 and pancreatic cancer is not exactly clear.
Thus, we explored the expression and effect of KRT17 in PC. We hypothesize that KRT17 stimulates the proliferation, migration and invasion of PC cells, and it may be achieved by activating the mTOR/S6K1 pathway.
Materials and Methods
Materials
Trypsin (0.25%) and Dulbecco’s modified Eagle’s medium (DMEM) were purchased from Gibco (Gibco Life Technologies, Grand Island, NY, USA). Fetal bovine serum (FBS) was purchased from BI (Biological Industries Israel Beit Haemek LTD., Israel). Lipofectamine 3000 transfection reagent was purchased from Invitrogen (Thermo Fisher Scientific, USA). siRNAs were synthesized by GenePhama (Shanghai, China). Reactive oxygen species assay kit (ROS Assay Kit, NO. S0033) was purchased from Beyotime Biotechnology (Shanghai, China).
Online Databases
Gene expression profiling interactive analysis (GEPIA, http://gepia.cancer-pku.cn/index.html) provided sequencing expression data including 9736 tumors and 8,587 normal samples from The Cancer Genome Atlas (TCGA) and Genotype-Tissue Expression (GTEx) projects.37 The Human Protein Atlas (THPA, https://www.proteinatlas.org/) provided protein expression from the Tissue Atlas and Pathology Atlas.38
Cell Culture
PANC-1, CFPAC-1, MIA Paca-2 and BXPC3 cells were obtained from the Shanghai Cell Bank of the Institute of the Chinese Academy of Sciences (Shanghai, China) and HPNE, L3.6 and Patu8988 were obtained from ATCC(USA). All cells were cultured in DMEM containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin (Pen Strep, Gibco, USA). The cells were incubated in an incubator at 37°C with an atmosphere containing 5% carbon dioxide (CO2).
Cell Transfection
BXPC3 and L3.6 cells were transfected with the transfection reagent as follows. Approximately 1×105 cells were plated in 6-well plates 24 h before transfection. KRT17 was silenced by siRNA for 24 h or 48 h. The cells were transfected using 5 μL siRNA (20 μM) and 3.75 μL Lipofectamine 3000 according to the manufacturer’s instructions. The final concentration of siRNA was 5nM.The KRT17 siRNA sequences were as follows: siRNA1(Si-1), sense-1 5‘-GCCAGUACUACAGGACAAUTT-3ʹ and antisense-1 5ʹ-AUUGUCCUGUAGUACUGGCTT-3ʹ; siRNA2(Si-2), sense-2 5‘-CCUGCUACAGAUUGACAAUTT-3ʹ and antisense-2 5ʹ-AUUGUCAAUCUGUAGCAGGTT-3ʹ; siRNA3(Si-3), sense-3 5ʹ-GGUGGGUGGUGAGAUCAAUTT-3ʹ and antisense-3 5ʹ-AUUGAUCUCACCACCCACCTT-3ʹ.siNegative(NC),with no target sequence, was also introduced in our expriment.
Colony Formation Assay
After transfected 48 h, a total of 700 BXPC3 cells and 500 L3.6 cells were plated in a 12-well plate and incubated in media for 10 days. Colony numbers were calculated by Visionworks 8.20. All P values were determined using one-way ANOVA.
Real-Time Cellular Analysis (RTCA)
In this assay, 2×104 cells were plated in E-plate (ACEA Biosciences, Inc., a division of Agilent, USA) with 150 μL DMEM containing 10% FBS. The E-plate was placed in an RTCA instrument (ACEA Biosciences, Inc., a division of Agilent, USA) in a standard CO2 cell culture incubator for 100 or 60 h.
Immunofluorescence Assay
After transfection for 1 h and 24 h, cells were washed 3 times with PBS and then fixed with 4% paraformaldehyde (Solarbio, Beijing Solarbio Science & Technology Co. Ltd. China) for 30 min at 4°C. Then, 0.5% Triton-X100(Biotech, Shanghai boyun biotech Co. Ltd. China) was used to permeabilize the cells for 10 min, and 5% bovine serum albumin (BAS, sigma-aldrich life science & technology CO. LTD., Wuxi, China) was used to block the cells for 1 h at regular temperature. After that, the cells were incubated with a primary antibody against Ki67 (ab16667, abcam, USA) overnight at 4°C. Next, a 488-conjugated goat anti-rabbit IgG antibody (Biosharp Technology Inc. China) was incubated with the cells for 1 h at 37°C. Then, DAPI(Prolong, Invitrogen by Thermo Fisher Scientific, USA) was used to stain the nuclei. Photos were captured by immunofluorescence microscopy (Leica Microsystems, CMS GmbH, Wetzlar, Germany).
Detection of Intracellular Reactive Oxygen Species (ROS)
Intracellular ROS were detected with the molecular probe 2ʹ,7ʹ-dichlorodihydrofluorescein diacetate (DCFH-DA). After transfection, the cells were incubated with DCFH-DA (20 μM) for 20 min and washed three times with DMEM. Then, immunofluorescence microscopy was used to capture the ROS reaction.
RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
Total RNA was extracted using an RNAsimple Total RNA Kit (TianGen, Beijing, China) in accordance with the manufacturer’s protocol and analyzed for quality control. Reverse transcription was conducted with a Thermal Cycler (BioRad, USA). qRT-PCR reactions were performed and analyzed using a 7500 Fast System (Applied Biosystems, USA). The data were calculated by the comparative cycle threshold (Ct)(2−ΔΔCt) method, which set GAPDH as the endogenous control. Generay (Shanghai Generay Biotech Co., Ltd., Shanghai, China) provided sequences of the primers used as follows: KRT17 forward 5ʹ-GGTGGGTGGTGAGATCAATGT-3ʹ and reverse 5ʹ-CGCGGTTCAGTTCCTCTGTC-3ʹ; GAPDH forward 5ʹ-GGACCTGACCTGCCGTCTAG-3ʹ and reverse 5ʹ-GTAGCCCAGGATGCCCTTGA-3ʹ.
Western Blot Analyses
Cells lysates were prepared in protein extraction buffers composed of radioimmunoprecipitation assay buffer (Beyotime Biotechnology, Jiangsu, China), 10% phosphatase inhibitor (Roche Diagnostics GmbH, Mannheim, Germany) and 1% phenylmethylsulfonyl fluoride (Beyotime Biotechnology, Jiangsu, China) for 30 min. Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and then transferred to polyvinylidene fluoride (PVDF) membranes (Merck Millipore Ltd, Tullareen Carrigtwohill, Co, Cork IRL Rev.) in a Mini Trans-Blot Electrophoretic Transfer System (BIO-RAD, Hercules, CA, USA). After that, the membranes were blocked in 5% non-fat milk for 1 h at room temperature and incubated overnight with primary antibodies at 4°C. The following primary antibodies were used: mTOR (2983, Cell Signaling Technology, Danvers, MA, USA) and p-mTOR (2971, Cell Signaling Technology, Danvers, MA, USA), S6K1(14485, Proteintech Group, Inc., IL, USA) and p-S6k1 (ab32525, abcam, USA), and GAPDH (ab-p-r001,Goodhere Biotechnology CO., LTD, Hangzhou, China). The dilution ratio of antibodies above were 1:1000.
Cell Migration and Invasion Assays
For these assays, a total of 5×104 transfected cells (~300 μL) were placed into the upper chamber in medium with 10% FBS, and 600 μL medium with 20% FBS was added to the bottom chamber. Matrigel (BD Bioscience, NJ, USA) was used for the invasion assay. After diluted 8 times, 50 μL Matrigel was subpackaged into upper chamber before invasion assay. After 48 h for migration and 96 h for invasion in standard atmosphere conditions, the membranes were removed carefully, fixed with 4% paraformaldehyde for 15 min and stained with a 0.4% crystal violet solution(C0121, Beyotime Biotechnology,Shanghai, China) for 15 min at room temperature. The images were captured under a light microscope at 200× magnification (Leica DMi1, Shanghai, China), and five random fields were selected and counted.
Statistical Analysis
All statistical data were analyzed with SPSS 23.0 software (IBM Corporation, Armonk, NY, USA) and are shown as the mean ± standard error. One-way ANOVA or Student’s t-test was used for the analyses. P<0.05 was considered to be statistically significant.
Results
KRT17 Was Upregulated in Pancreatic Cancer
To determine the relationship between KRT17 and PC, we investigated the expression of KRT17 and the relationship of KRT17 expression with clinicopathological features in online databases. Our study found that KRT17 was upregulated in PC compared to that in normal samples according to GEPIA (Figure 1A); KRT17 was also associated with worse overall survival (Figure 1E). THPA showed that KRT17 could be detected in PC tissues but not in normal tissues (Figure 1C). Additionally, the expression levels of KRT17 were investigated in PC cell lines using qRT-PCR. We found that KRT17 was significantly higher in the CFPAC-1, BXPC3 and L3.6 cell lines than in the HPNE cell line (Figure 1B).
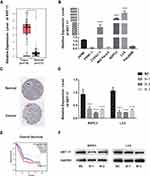 |
Figure 1 KRT17 expression in PC in validated cohort and GEPIA cohort. Notes: (A) GEPIA cohort shows KRT17 was upregulated in PC (*P<0.05). (|log2FC| cutoff >1, p-value cutoff <0.01). Picture was downloaded from GEPIA, Copyright © 2017 Zefang Tang, Chenwei Li, Boxi Kang. Zhang's Lab. GEPIA: http://gepia.cancer-pku.cn/index.html.37 (B) KRT17 relative expression was examined by qRT-PCR in normal and cancer cell lines. Compared to HPNEC, KRT17 was expressed at a higher level in CFPANC-1, BXPC3 and L3.6. ****P<0.0001. (C) THPA shows the pathology of KRT17 expression in PC tissure. KRT17 was detected in cytoplasmic and membranous, which had high staining and strong intensity (arrow indicators). Whereas, it was not detected in normal tissue. Image credit: Human Protein Atlas; pictures were obtained from https://images.proteinatlas.org/452/1886_A_3_3.jpg and https://images.proteinatlas.org/452/1936_B_6_1.jpg. Indicators were added by author.38 (D) The relative expression of KRT17 using qRT-PCR (compared with the GAPDH) in the BXPC3 and L3.6 cell lines. Compared to the negative control, the expression of KRT17 was lower in the siRNA groups. ****P<0.0001. (E) PC patients with high KRT17 levels had worse overall survival. Picture was downloaded from GEPIA, Copyright © 2017 Zefang Tang, Chenwei Li, Boxi Kang. Zhang's Lab. GEPIA: http://gepia.cancer-pku.cn/index.html. (F) The protein level of KRT17 in BXPC3 and L3.6, compared with the corresponding control group, was lower in the siRNA groups.Abbreviations: PC, pancreatic cancer; qRT-PCR, quantitative reverse transcription polymerase chain reaction; KRT17, keratin17; GEPIA, gene expression profiling interactive analysis; THPA, the human protein atlas. |
KRT17 Regulates Proliferation, Migration and Invasion of PC Cell Lines
To confirm the role of KRT17 in PC and according to the results of qRT-PCR above, we selected BXPC3 and L3.6 cells for further study. To determine whether KRT17 affects PC biological properties, we knocked down KRT17 expression in BXPC3 and L3.6 cells using siRNA. Figure 1D and F show that both mRNA expression and protein levels of KRT17 were significantly lower than those in the control samples.
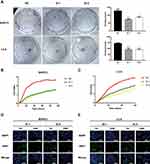
As demonstrated in Figure 2A (**P<0.01, ***P<0.001, ****P<0.000), PC cell proliferation was significantly inhibited in BXPC3 and L3.6 cells by knocking down KRT17 expression (transfection with Si-1 and Si-2) compared with that in NC cells (transfected with Si-NC). Results of RTCA also demonstrated that BXPC3 and L3.6 cells transfected with siKRT17 had significantly lower proliferation ability than NC cell lines (Figure 2B and C). Moreover, we examined Ki67 protein expression, which is a biomarker of cellular proliferation. Ki67 protein was more abundant in the nuclei after 1 h of treatment with siKRT17 than after 24 h of treatment with siKRT17 in the BXPC3 and L3.6 cell lines (Figure 2D and E). The above results showed that KRT17 indeed stimulated PC cell proliferation.
According to GEPIA, KRT17 was highly-expressed. Therefore, we investigated the role of KRT17 in PC through migration and invasion assays. Our study showed that downregulation of KRT17 could reduce the migration capacity in BXPC3 and L3.6 cells compared with the NC conditions (****P<0.000, Figure 3A and B). The invasion assay also demonstrated that the downregulation of KRT17 expression effectively reduced the invasion capacity of BXPC3 and L3.6 cells (*P<0.05, **P<0.01, ****P<0.000, Figure 3C and D).
Downregulation of KRT17 Suppressed ROS Activation
Precious studies showed that ROS generation was associated with the proliferation of cancer cells.18–20 To determine whether KRT17 affects ROS activation, we used DCFH-DA to detect intracellular ROS. As shown in Figure 4, after transfection with siKRT17 for 1 and 24 h, ROS activation increased over time. The results illustrated that downregulation of KRT17 suppressed intracellular ROS accumulation. Then, the proliferation of PC cell lines was inhibited.
KRT17 Affected Proliferation, Migration and Invasion via the mTOR Signaling Pathway
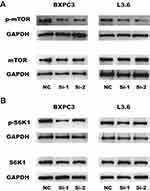
The mTOR pathway could play a role in cancer cell metastasis. In previous studies, the mTOR signaling pathway was found to regulate proliferation,21 migration22 and invasion.23 To determine whether KRT17 regulates proliferation, migration and invasion through the mTOR pathway, we used WB. We found that knockdown of KRT17 could significantly decrease phosphorylation levels of mTOR in PC cell lines (Figure 5A). S6K1 was also downstream of mTOR,24 and phosphorylation levels of S6K1 were attenuated in our study (Figure 5B). Collectively, these results suggested that downregulation of KRT17 attenuated the phosphorylation levels of mTOR to alleviate PC metastasis.
Discussion
Although much research progress has been made regarding pancreatic cancer,25,26 there are still many unknown aspects of the molecular mechanisms of PC. KRT17 is expressed in nail tissue,27 and seems to be a certain type of “stem cells” in complex epithelia.28 Studies have shown that the KRT17 gene plays a role in many tumors. Antonia et at29 reported that KRT17 was overexpressed in oral carcinogenesis. KRT17 was also one of promotors in breast cancer.30 Hobbs found that KRT17-dependent amplification in skin tumors.31 However, little is known about its function in PC.
Our study aimed to identify the endogenous function of KRT17 in PC. We found that KRT17 was upregulated in pancreatic cancer samples compared to that in normal samples according to GEPIA and THPA. The results were further proven by measuring mRNA expression in cell lines using qRT-PCR. In our study, KRT17 was expressed at a higher level in CFPAC1, BXPC3 and L3.6 than that in other pancreatic cancer cell lines. Then, cellular and molecular technology was used to show that knockdown of KRT17 led to the attenuation of ROS levels and proliferation, migration and invasion abilities in BXPC3 and L3.6 cells, which is consistent with KRT17 being associated with pancreatic cancer.
In recent years, the mTOR signaling pathway has been increasingly reported to promote tumor processes. Nandini et al32 inhibited the mTOR pathway in a clinical trial for breast cancers. Inhibiting the mTOR pathway can also have protective effects against colorectal cancer.33 The relationship between KRT17 and mTOR pathway has been reported in many studies. Chivu-Economescu et al34 demonstrated that downregulated of KRT17 induced effects in gastric cancer mainly via mTOR pathway. Khanom et al35 also reported KRT17 stimulated mTOR pathway which facilitating oral cancer growth.
Our study proves that knocking down KRT17 could decrease the phosphorylation levels of mTOR in PC cell lines. As a downstream target of the mTOR complex, S6K1 plays an extensive role in growth factor signaling, amino acids, energy levels and hypoxia.36 We found that phosphorylation levels of S6K1 were reduced after KRT17 knockdown. As shown above, these results indicate that knockdown of KRT17 attenuates the biological behavior of pancreatic cancer cell lines via the mTOR/S6K pathway.
However, this study has many deficiencies. First, gain-of-function assays need to be carried out for further verification of the results. After knockout of KRT17 in BXPC3 and L3.6 cell lines, gain-of-function assays should be verified the cell function. Additionally, the role of KRT17 in PC requires validation in animal experiments. Overall, other omics strategies could be conducted to provide reliable clinicopathological data.
Conclusion
Using online available gene expression data, KRT17 was overexpressed in PC compared to normal pancreatic tissue and its expression was associated with inferior survival of PC patients. Then our study used several cell culture based approaches to show that KRT17 expression is associated with malignant traits in PC cell lines, including higher proliferation, production of ROS and tumor cell invasion. Finally, our research claimed that there was an association between KRT17 expression and mTOR pathway activation, which presented as explanation for the increased malignancy in KRT17 expressing PC.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22(44):9694–9705. doi:10.3748/wjg.v22.i44.9694
2. Srivastava S, Koay EJ, Borowsky AD, et al. Cancer overdiagnosis: a biological challenge and clinical dilemma. Nat Rev Cancer. 2019;19(6):349–358. doi:10.1038/s41568-019-0142-8
3. Yiming M, Wei Y, Shrivastava A, et al. Inhibition of pancreatic cancer stem cell characteristics by α㎝angostin: molecular mechanisms involving Sonic hedgehog and Nanog. J Cell Mol Med. 2019;23:4.
4. Cao J, Jie L, Sun L, et al. Hypoxia‐driven paracrine osteopontin/integrin αvβ3 signaling promotes pancreatic cancer cell epithelial–mesenchymal transition and cancer stem cell‐like properties by modulating forkhead box protein M1. Mol oncol. 2019;13(2):228–245.
5. Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi:10.1016/j.ceb.2005.09.009
6. Wang X, Proud CG. The mTOR pathway in the control of protein synthesis. J Physiol. 2006;21(5):362. doi:10.1152/physiol.00024.2006
7. Lee C, Jaspreet Dhillon MY, Wang C, et al. Targeting YB-1 in HER-2 overexpressing breast cancer cells induces apoptosis via the mTOR/STAT3 pathway and suppresses tumor growth in mice. Cancer Res. 2015;68(21):8661.
8. Cao Y, Liu X, Lu W, et al. Fibronectin promotes cell proliferation and invasion through mTOR signaling pathway activation in gallbladder cancer. Cancer lett. 2015;360(2):141–150.
9. Gulhati P, Cai Q, Li J, et al. Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin Cancer Res. 2009;15(23):7207–7216. doi:10.1158/1078-0432.CCR-09-1249
10. Specenier PM, Vermorken JB. Targeted therapies in head and neck cancer. J Target Oncol. 2007;2(2):73–88. doi:10.1007/s11523-007-0048-3
11. Richardson CJ, Broenstrup M, Fingar DC, et al. SKAR is a specific target of S6 kinase 1 in cell growth control. Curr Biol. 2004;14(17):1540–1549. doi:10.1016/j.cub.2004.08.061
12. Easton JB, Houghton PJ. mTOR and cancer therapy. J Oncogene. 2006;25(48):6436–6446. doi:10.1038/sj.onc.1209886
13. Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. J Cancer Cell. 2007;12(1):9–22. doi:10.1016/j.ccr.2007.05.008
14. Depianto D, Kerns ML, Dlugosz AA, et al. Keratin 17 promotes epithelial proliferation and tumor growth by polarizing the immune response in skin. Nat Genet. 2010;42(10):910.
15. Li J, Chen Q, Deng Z, et al. KRT17 confers paclitaxel-induced resistance and migration to cervical cancer cells. Life Sci. 2019;224:255–262. doi:10.1016/j.lfs.2019.03.065
16. Chivu-Economescu M, Dragu DL, Necula LG, et al. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer. 2017;20(6):948–959. doi:10.1007/s10120-017-0712-y
17. Liu J, Liu L, Cao L, et al. Keratin 17 promotes lung adenocarcinoma progression by enhancing cell proliferation and invasion. Clin Res. 2018;24(Med Sci Monit):4782–4790.
18. Lei Z, Yuan F, Xu XF, et al. Moscatilin induces apoptosis of pancreatic cancer cells via reactive oxygen species and the JNK/SAPK pathway. Mol Med Rep. 2017;15(3):1195–1203.
19. Lu QB, Wan MY, Wang PY, et al. Chicoric acid prevents PDGF-BB-induced VSMC dedifferentiation, proliferation and migration by suppressing ROS/NFκB/mTOR/P70S6K signaling cascade. Redox Biol. 2018;14:656–668.
20. Luo J, Xiang Y, Xu X, et al. High glucose-induced ROS production stimulates proliferation of pancreatic cancer via inactivating the JNK pathway. Oxid Med Cell Longev. 2018;2018(undefined):6917206.
21. Hentges KE, Sirry B, Gingeras AC, et al. FRAP/mTOR is required for proliferation and patterning during embryonic development in the mouse. Proc Natl Acad Sci U S A. 2001;98(24):13796–13801. doi:10.1073/pnas.241184198
22. Zong H, Yin B, Zhou H, et al. Inhibition of mTOR pathway attenuates migration and invasion of gallbladder cancer via EMT inhibition. Mol Biol Rep. 2014;41(7):4507–4512. doi:10.1007/s11033-014-3321-4
23. Chen J, Wang Q, Huang X, et al. Involvement of PI3K/PTEN/AKT/mTOR pathway in invasion and metastasis in hepatocellular carcinoma: association with MMP-9. Hepatol Res. 2010;39(2):177–186.
24. Attila Garami FJ, Zwartkruis T, Nobukuni T, et al. Insulin activation of Rheb, a mediator of mTOR/S6K/4E-BP signaling, is inhibited by TSC1 and 2. Mol Cell. 2003;11(6):1457–1466. doi:10.1016/s1097-2765(03)00220-x
25. Hagiwara Y, Ohashi Y, Uesaka K, et al. Health-related quality of life of adjuvant chemotherapy with S-1 versus gemcitabine for resected pancreatic cancer: results from a randomised Phase III trial (JASPAC 01). Eur J Cancer. 2018;93:79–88. doi:10.1016/j.ejca.2018.01.081
26. Hu ZI, Shia J, Stadler ZK, et al. Evaluating mismatch repair deficiency in pancreatic adenocarcinoma: challenges and recommendations. Clin Cancer Res. 2018;24(6):1326–1336. doi:10.1158/1078-0432.CCR-17-3099
27. Tong X, Coulombe PA. A novel mouse type I intermediate filament gene, keratin 17n (K17n), exhibits preferred expression in nail tissue. J Investig Dermatol. 2004;122(4):965–970. doi:10.1111/j.0022-202X.2004.22422.x
28. Troyanovsky SM, Leube RE, Franke WW. Characterization of the human gene encoding cytokeratin 17 and its expression pattern. Eur J Cell Biol. 1992;59(1):127–137.
29. Kolokythas A, Schwartz JL, Pytynia KB, et al. Analysis of RNA from brush cytology detects changes in B2M, CYP1B1 and KRT17 levels with OSCC in tobacco users. Oral Oncol. 2011;47(6):532–536. doi:10.1016/j.oraloncology.2011.03.029
30. Gorski JJ, James CR, Quinn JE, et al. BRCA1 transcriptionally regulates genes associated with the basal-like phenotype in breast cancer. Breast Cancer Res Treat. 2010;122(3):721–731. doi:10.1007/s10549-009-0565-0
31. Hobbs RP, Depianto DJ, Jacob JT, et al. Keratin-dependent regulation of Aire and gene expression in skin tumor keratinocytes. Nat Genet. 2015;47(8):933–938. doi:10.1038/ng.3355
32. Nandini D, De Pradip L, Brian J, et al. PI3K-AKT-mTOR inhibitors in breast cancers: from tumor cell signaling to clinical trials. J Pharmacol. 2017;175:91.
33. Karki R, Man SM, Malireddi RS, et al. NLRC3 is an inhibitory sensor of PI3K–mTOR pathways in cancer. Nature. 2017;540(7634):583–587.
34. Chivu-Economescu M, Dragu DL, Necula LG, et al. Knockdown of KRT17 by siRNA induces antitumoral effects on gastric cancer cells. Gastric Cancer. 2017;20(6):1–12.
35. Khanom R, Nguyen CT, Kayamori K, et al. Keratin 17 is induced in oral cancer and facilitates tumor growth. PLoS One. 2016;11(8):e0161163.
36. Holz MK. The role of S6K1 in ER-positive breast cancer. J Cell Cycle. 2012;11(17):3159–3165. doi:10.4161/cc.21194
37. Tang ZLi C, Kang B. Zhang's Lab. GEPIA, Gene Expression Profiling Interactive Analysis. Available at:http://gepia.cancer-pku.cn/index.html. Accessed March 1, 2020.
38. Uhlen MZhang C, Lee S, et al. A pathology atlas of the human cancer transcriptome. Science. 2017;357:(6352)
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.