Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Koji amazake Maintains Water Content in the Left Cheek Skin of Healthy Adults: A Randomized, Double-Blind, Placebo-Controlled, Parallel-Group, Comparative Trial
Authors Enomoto T, Kojima-Nakamura A, Kodaira K, Oguro Y, Kurahashi A
Received 18 March 2022
Accepted for publication 20 June 2022
Published 8 July 2022 Volume 2022:15 Pages 1283—1291
DOI https://doi.org/10.2147/CCID.S366979
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Toshihiko Enomoto, Ayana Kojima-Nakamura, Kazuya Kodaira, Yoshifumi Oguro, Atsushi Kurahashi
Hakkaisan Brewery Co., Ltd, Minamiuonuma, Niigata, Japan
Correspondence: Atsushi Kurahashi, Hakkaisan Brewery Co., Ltd, 1051 Nagamori, Minamiuonuma, Niigata, 949-7112, Japan, Tel +81 25-788-0910, Fax +81 25-788-0911, Email [email protected]
Purpose: Improvement in water content and skin barrier function on human skin is believed to be induced by koji amazake, a non-alcoholic beverage derived from rice fermented by Aspergillus oryzae (A. oryzae). In order to scientifically identify the effects of koji amazake on human skin, we performed a randomized, double-blind, placebo-controlled, parallel-group comparative trial and quantified the content of glucosylceramide (GlcCer) which would be responsible for the effects.
Participants and Methods: Healthy adults concerned with their skin dryness were divided into koji amazake (N = 30) or placebo group (N = 30). During this test, the test beverages were ingested at 118 g/day. Their water content and trans-epidermal water loss (TEWL) were measured at 0 week (baseline) and 8 weeks. The content of GlcCer in test beverages was quantified by HPLC-ELSD.
Results: In comparison with the placebo group, the water content in the left cheek of individuals in the koji amazake group was maintained for 8 weeks. In addition, changes in water content from the baseline to 8 weeks differed significantly between the koji amazake (0.19) and placebo groups (− 3.98). Unexpectedly, there was no significant difference in the TEWL between koji amazake and placebo group. We analyzed GlcCer in both koji amazake and placebo beverages, which were found to contain 1.35 ± 0.11 and 0.30 ± 0.07 mg/118 g, respectively. The amount of GlcCer in koji amazake was approximately equal to the dosage of plant-derived GlcCer which has the ability to improve water content and TEWL in humans.
Conclusion: Present study has shown that intake of koji amazake contributes to maintain the water content only on the left cheek. The content of GlcCer derived from koji amazake was adequate for maintenance of the water content compared to previous reports. Therefore, it was concluded that GlcCer in koji amazake acts as a functional ingredient.
Keywords: Aspergillus oryzae, glucosylceramide, koji amazake, water content
Introduction
Koji is a grain (eg, rice, wheat, and soybeans) fermented by Aspergillus oryzae (A. oryzae) and related molds. It is essential for the fermentation of various foods and beverages in Japanese cuisine, including sake, koji amazake, miso, and soy sauce.1 Koji amazake, which is made from rice-koji or rice-koji and steamed rice and water, is popular fermented beverage in Japan for a long time.2 During the koji amazake manufacturing process, various enzymes produced by A. oryzae break down starch, proteins, and lipids in rice into glucose, amino acids, and fatty acids, respectively. Therefore, koji amazake has a sweet taste. It has been widely recognized as a functional food with healthy and cosmetic effects, since 2016.3 Unfortunately, few scientific research studies on koji amazake have been published, although this beverage has been familiar to many Japanese people for a long time. To reveal the effects of the intake of koji amazake on those who drink it, we have identified over 300 compounds in koji amazake through metabolome analysis.4 Human clinical trials have shown that koji amazake contains compounds that suppress the postprandial increase in glucose and insulin levels in blood.5 Furthermore, the safety of drinking koji amazake has also been guaranteed through long-term and excessive intake tests.6,7
As the skin barrier plays important role in protecting the body from environmental pollutants, functional foods that enhance this barrier function have been investigated by many researchers.8 In addition, enhancement of the skin water content contributes to reduce fine lines and wrinkles.9,10 Recently, it was proposed that koji amazake also has beneficial effects on human skin. Ueda et al have reported that intake of koji amazake improves water content in the left upper arm and trans-epidermal water loss (TEWL) in the left cheek in women with high TEWL.11 Ueda et al also have guessed glucosylceramide (GlcCer) and N-acetylglucosamine (GlcNAc) as functional ingredients in koji amazake that improve water content and TEWL.
GlcCer in particular has been shown to be an important functional ingredient in previous reports.12,13 GlcCer consists of a sphingoid base with a fatty acid attached to a glucose molecule, and localized on fungi and plant plasma membranes.14 Improvements in skin water content and TEWL have been verified by the administration of GlcCer derived from some plants, including rice, corn, and konjac.15–17 Fermented foods contain rich GlcCer; approximately 200 mL of amazake has been proven to contain 1.31–4.15 mg GlcCer.13 Considering that GlcCer works efficiently at milli-gram quantities, koji amazake has the potential to improve water content and TEWL. However, the main GlcCer in koji amazake shows a unique structure in filamentous fungi, including a C9-methylated sphingoid base, derived from A. oryzae, and this structure differs from that of plant GlcCer.18,19 The differences in the sphingoid base between fungi and plants may result in changes in absorption dynamics. In fact, in vitro studies have shown that mammalian GlcCer is more easily absorbed into caco-2 cells than plant GlcCer.20,21 Thus, it is not known whether GlcCer in koji amazake exerts the same effects concerned with water content and TEWL as plant GlcCer. However, the administration of GlcCer derived from Candida utilis improves skin barrier function in human, as well as plant GlcCer.22 Furthermore, previous studies have shown that when A. oryzae-derived GlcCer is added to human epidermal keratinocytes, the expression of genes involved in tight junction formation is enhanced.23
Based on these reports, sphingoid backbones in GlcCer are considered to be slightly different due to the presence of original organisms, but A. oryzae-derived and rice-derived GlcCer in koji amazake are also expected to improve water content and TEWL. In this study, we examined the effects of koji amazake intake on human skin by performing a randomized, double-blind, placebo-controlled, parallel-group comparative trial. Moreover, the GlcCer content of koji amazake was quantified by HPLC-ELSD, and we discussed the validity of the effect of GlcCer in koji amazake on water content. To our knowledge, this study is the first to show that GlcCer in koji amazake maintains water content only in left cheek.
Materials and Methods
Composition of Koji Amazake and Placebo Beverages
Commercially available koji amazake (manufactured by Hakkaisan Brewery Co., Ltd., Minamiuonuma, Japan) and a placebo beverage, manufactured by mixing only steamed rice and purified enzymes without rice-koji, were used as test beverages (118 g/bottle). The nutritional components of both beverages are shown in Table 1. Energy, water, protein, lipid, dietary fiber, ash, and carbohydrate were measured by the Japan Food Research Laboratories (Tokyo, Japan).
 |
Table 1 Nutritional Components in Test Beverages |
Quantification of GlcCer in Koji Amazake
Based on the previous study,18 GlcCer was extracted from both beverages (koji amazake N = 15, placebo N = 3). The total GlcCer content in the beverage was quantified by HPLC with 2424 ELS detector (Waters Corporation, Milford, MA, USA). GlcCer was separated from other lipids using Inertsil SIL-100A column (4.6 mm × 150 mm, 5 μm) (GL Science Inc., Tokyo, Japan) with the following gradient conditions: 0 min 99% A, 1% B; 15 min 75% A, 25% B; 20 min 10% A, 90% B; 21–26 min 100% B; 28–36 min 99% A, 1% B. Buffer A was chloroform, and buffer B was 95% methanol. Other parameters were as follows: column temperature, 40 °C; flow rate 1 mL/min; and injection volume 20 μL. The GlcCer standard (NS370401) was purchased from Nagara Science, Gifu, Japan.
Participants
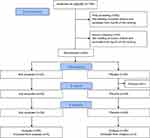
At the first screening, 168 participants concerned with skin dryness between the ages of 30 and 59 were screened. Physical and physiological parameters (eg, blood biochemistry and urea) of participants were measured, including body weight, body mass index, body fat percentage, blood pressure, pulse measurement, white blood cells, red blood cells, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, platelets, blood total proteins, creatinine, blood uric acid, aspartate of aminotransferase, alanine aminotransferase, γ-glutamyl transpeptidase, total cholesterol, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, blood glucose, HbA1c, urobilinogen, urine glucose, urine ketone bodies, urine protein, bilirubin, urine occult blood reaction, urine pH, and urine specific gravity. In addition to these parameters, the water content and TEWL in the left cheek were measured. Selection criteria were as follows: (1) individuals between the ages of 30 and 59 years; (2) healthy individuals without chronic diseases (including skin diseases); (3) individuals who indicated their skin dryness through questionnaires; (4) individuals with water content and TEWL in the left cheek ranging from 25 to below 55 and 15 to below 30 g/hm2, respectively; and (5) individuals identified as appropriate participants by an investigator who is responsible doctor for this test. Meeting selection criteria, 85 participants were selected from top of the ranking in descending order of TEWL.
The same parameters were measured in the second screening, and additional blood biochemical parameters were also included: albumin, urea nitrogen, alkaline phosphatase, lactate dehydrogenase, creatine kinase, C-reactive protein, total bilirubin, sodium, potassium, and chlorine. In addition, the water content and TEWL on the left cheek, left upper arm and left upper back were measured. After measurement, all participants meeting criteria (4) were ranked in descending order of TWEL and in ascending order of water content. On calculating the sum of both TEWL and water content rankings corresponding to each participant, they were then ranked again in ascending order of the sum value. Finally, 60 participants were selected from the top of the ranking for the sum values (Table S1). Based on the previous trials, the difference in water content between test beverage and placebo group was exploited to set sample size. Given that the difference after 16 weeks (intake period) is 1.9 with standard deviation 2.4, sample size was 27 according to 0.8 (1-β). Considering dropouts, 30 participants per one group was set for this study. Above blood biochemistry and urea parameters were measured to confirm safety for successive intake of test beverages during 16 weeks. However, this test was interrupted before 16 weeks because of influence of the spread of COVID-19 in Japan. Hence, these parameters were exploited only to confirm no difference between both groups before intake period.
Study Design
For 60 participants, a randomized, double-blind, placebo-controlled, parallel-group comparative trial was conducted between November 2019 and March 2020 at TES Holdings Co. Ltd (Tokyo, Japan). This test was planned to be performed for 16 weeks but, was interrupted at 8 weeks (February 2020) due to influence of the spread of COVID-19 in Japan. This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the ethics committee of Ueno-Asagao Clinic (Tokyo, Japan; approval number U-2019-32), which is the affiliated hospital of TES Holdings Co., LTD. This study was registered in the University Hospital Medical Information Network (UMIN) Clinical Trials Registry, Japan, under the code UMIN000038630. All participants were divided into either the koji amazake (N = 30) or a placebo group (N = 30) by stratified block randomization based on age, sex, water content, and TEWL. Computer-generated randomization numbers were used for above allocation. The marks were printed on the cardboard enclosing test beverages by personnel in Hakkaisan Brewery Co. LTD., and then both beverages were delivered to allocation manager, Kazunori Goto, who is personnel in TES Holdings Co. LTD. Allocation manager confirmed no difference between test beverages and marked key codes corresponding to each beverage for blinding. Test beverages were delivered to personnel managing test foods. Key codes and randomization list were not opened until key open to guarantee blinding. A series of randomization processes was carried out by allocation manager. The investigators, study participants, and all staff participating in test planning and operation were blinded to allocation. The test beverages were ingested at 118 g/day for 8 weeks (intake period). Water content and TEWL at 0 and 8 weeks of intake were measured as indices of skin hydration and skin barrier function, respectively. All participants agreed with the purpose of this test, and written informed consent was obtained.
During this test, all participants were required to observe the following rules: (1) avoidance of an irregular lifestyle (eg, overeating and poor sleep); (2) maintenance of the same diet, exercise, and sleep habits as before the test; (3) protection of certain anatomical sites from UV by wearing a hat and using sunscreen; (4) prohibition of the use of additional cosmetics or changes in cosmetic routines (if any such addition or change was made, information about these cosmetics and reasons for their use were recorded in a diary); (5) maintenance of the same face care routine as before the test and avoiding certain skin treatments; (6) avoiding the use of drugs, quasi-drugs, and Chinese medicine (if the participants used any drugs, information about the drugs and the reasons for their use were recorded in a diary); (7) prohibition of the intake of new health foods (if participants ingested health foods before the test, they could continue ingesting these health foods with no change in dosage and administration—on the other hand, if participants consumed any additional or new health foods, information regarding these foods and reasons for consuming them were recorded in a diary); (8) prohibition of blood drawing; (9) prohibition of participation in other human trials; and (10) avoidance of all activities that might impact test results. To ensure the accuracy of data collection, the following rules of measurement were specified: (1) prohibition of shaving in certain anatomical sites for one week before measurement; (2) prohibition of sauna and hot spring use for two days before measurement; (3) prohibition of excessive exercise and intake of alcohol and foods containing rich spices on the day before measurement; (4) requirement of taking a bath on the day before measurement, and subsequently not taking a bath until the completion of measurement; (5) avoidance of high sugar and lipid meals just before measurement; and (6) prohibition of eating for six hours before drawing blood.
Evaluation of Primary Outcomes (Water Content and TEWL)
Water content in the top of the left cheek, central part of the left upper arm, and left upper back were measured five times by Corneometer CM825® (Courage-Khazaka, Germany). The mean of the three measurements was used in this study, excluding the maximum and minimum values.
TEWL at the same anatomical positions which were selected in water content measurements was measured by Tewameter TM300 (Courage-Khazaka, Germany). The measurement per second was continued for over 60s. The most stable mean obtained 5s during 30s before completion of measurement was used as a single measurement. Both measurements were performed in specific conditions (room temperature: 21 °C± 1 °C; humidity: 50% ± 5%).
Statistics
The results in the present study were expressed as mean ± standard deviation using Microsoft Office Excel 2010 (Microsoft Corp., USA). Paired and unpaired t-tests were used for intra- and inter-group comparisons, respectively. All statistical analyses were performed using SAS (SS 9.4) or SPSS (Statistics 26). Tendency and statistical significance were set at p < 0.1 and p < 0.05, respectively.
Results
Selection of Participants and Their Baseline Characteristics
A total of 168 applicants were enrolled into the test in the course of three days, 18th, 20th and 21st November 2019. A randomized, double-blind, placebo-controlled, parallel-group comparative trial was performed with 60 participants who met the test criteria (Figure 1). The 60 participants were then divided into a koji amazake group (N = 30) and a placebo group (N = 30). Because one participant dropped out immediately before this test, a total of 59 participants started to ingest test beverages. Physical and physiological parameters for all participants are shown in Table S2. As 8 participants were excluded from analysis, a total of 51 participants (koji amazake group [N = 25 (aged 45.2 ± 6.9) (4 males, aged 48.0 ± 4.9, and 21 females, aged 44.7 ± 7.2)] and placebo group [N = 26 (aged 45.2 ± 6.9) (5 males, aged 44.6 ± 8.8, and 21 females, aged 45.3 ± 6.6)]) were included in the final analysis. This test was planned to be performed for 16 weeks. However, this test was interrupted regrettably at 8 weeks (February 2020) due to influence of the spread of COVID-19 in Japan.
Intake of Koji Amazake and Maintenance of Water Content
To confirm the effects of koji amazake intake on water content on human skin, water content at certain anatomical sites was measured. There was no significant difference in water content in the left cheek between 0 and 8 weeks in the koji amazake group (Table 2). Alternatively, the water content (38.71 ± 7.97) at 8 weeks in the placebo group was significantly lower in comparison to that at 0 weeks (42.70 ± 8.98). The change (∆ values) in water content from 0 to 8 weeks in the placebo group was significantly decreased when the value at 0 weeks was used for normalization. Furthermore, there was a significant difference in ∆ values between the koji amazake (0.19 ± 9.05) and placebo groups (−3.99 ± 5.16). In the placebo group, water content at other sites was slightly increased. However, the results obtained from the water content at other anatomical sites showed no significant difference in either the intra-group or the inter-group comparison (Table 2).
 |
Table 2 Absolute and ∆ Values for Water Content and TEWL (g/hm2) |
Reduction in TEWL Induced by Intake of Koji Amazake and Placebo
Next, we investigated the effect of koji amazake on TEWL, which is one of the representative indices of skin barrier function. TEWL in the upper back at 8 weeks in the koji amazake group was significantly lower compared to that at 0 weeks (Table 2). Similarity, the change (∆ value) in TEWL from 0 to 8 weeks in the koji amazake group (−1.66 ± 1.86 g/hm2) was significantly different from the baseline value. In the placebo group, the tendency for decreasing TEWL from the baseline value was shown in absolute and ∆ values (8.98 ± 1.72 and −0.87 ± 2.27 g/hm2) on the same sites and periods as koji amazake group. Unexpectedly, there was no significant change in TEWL between the koji amazake and placebo groups. Other TEWL data (obtained from cheek and upper arm sites) showed no statistical significance.
The Content of GlcCer in Both Test Beverages
To determine the effects of koji amazake on water content, the total GlcCer content in both beverages was quantified using HPLC-ELSD. Consequently, 1.35 ± 0.11 and 0.30 ± 0.07 mg/118 g GlcCer was detected in the koji amazake and placebo beverages, respectively. The main component of koji amazake is rice-koji, which is steamed rice fermented by A. oryzae. According to previous reports, GlcCer has been found to be abundant in fermented food with A. oryzae.13,24 This explains why the GlcCer content in koji amazake was higher than that in the placebo beverage.
Adverse Events
Present study showed no adverse events attributed to intake of test beverages.
Discussion
This study showed that koji amazake, the most popular Japanese fermentation food using koji, maintains water content only in left cheek. Specifically, the water content on the left cheek at 8 weeks in the koji amazake group was maintained at the same level as that at 0 weeks, but the water content in the placebo group showed a significant decrease in comparison with the baseline value. This study was conducted in winter, which is the driest and coldest climate in Japan. Therefore, it was considered that the decrease in water content of the upper arm and upper back covered with clothes was suppressed, and there was no difference in the water content between koji amazake and the placebo group. However, we required further studies for relationship between water content and measurement site covered with clothes in winter.
While absolute values and changes in TEWL in the upper back showed no significant inter-group differences, both values in the koji amazake group were found to have decreased significantly between 0 and 8 weeks. TEWL on other sites showed no difference in either the intra-group or the inter-group comparison. Ueda et al have shown that intake of koji amazake improved water content in left upper arm and TEWL in left cheek in humans.11 Ueda et al’s study was performed from summer to autumn. In addition, koji amazake exploited by Ueda et al has rich rice and low rice-koji. On the other hand, the material of koji amazake in this study is only rice-koji. Consequently, the difference in test beverages and season would be responsible for discordance between our study and previous reports. In future work, we need to detail how the component of koji amazake and season affect water content in humans. A reverse correlation between water content and TEWL was not observed in this study. Ohta et al have reported that oral administration of GlcCer improved TWEL, but not water content in mice.25 In addition, human clinical trial for intake of koji amazake has shown no reverse correlation between water content and TEWL.11 Future works are needed to examine whether such correlation was observed in humans.
In light of these results, it was concluded that koji amazake maintains water content in left cheek. As the raw material in the placebo beverage was only steamed rice, which is used to make the rice-koji contained in koji amazake, it was posited that the differences in the effects between the koji amazake and placebo beverages were attributable to GlcCer derived from A. oryzae. Previous studies have focused on the improvement of TEWL15–17 and water content17 through GlcCer intake in human trials. As a result, koji amazake and the placebo beverage contained 1.35 ± 0.11 mg/118 g GlcCer derived from rice-koji and 0.30 ± 0.07 mg/118 g GlcCer derived from rice, respectively. Koji amazake had some GlcCer originating from A. oryzae and rice, and the main GlcCer was derived from A. oryzae.18 Then, the difference in GlcCer between koji amazake and placebo is certainly attributable to A. oryzae. Furthermore, considering that plant-derived GlcCer works to improve TEWL15–17 and water content17 at mg order in human trials, we identified that koji amazake contained enough GlcCer for improvement.
Some studies have proposed mechanisms for skin barrier function enhanced by GlcCer. The previous in vivo study has shown that plant-derived GlcCer upregulates some genes involved in tight junction formation and the constitution of cornified envelopes in mice.26 Other in vitro studies have revealed that some genes involved in skin barrier function are upregulated in normal human epidermal keratinocyte (NHEK) cells by degradation products originating from animal and plant GlcCer.27,28 Reportedly, C9-methylated GlcCer in koji amazake originates from A. oryzae and has a unique structure.18 While the structure of A. oryzae-derived GlcCer differs from that of plant-derived GlcCer, one in vitro study has reported that the expression of occludin, which is involved in the formation of tight junctions, was enhanced in NHEK cells by A. oryzae-derived GlcCer as well as plant-derived GlcCer.23,26 It has been reported that intake of GlcCer up-regulated aquaporin 3 gene contributes to incorporating water into the cells in mice.29 GlcCer significantly inhibited dehydration of the stratum corneum and decreased aquaporin 3 mRNA expression. The findings of this research support our findings.
Conclusion
The present study confirmed that water content in the left cheek skin of participants in the koji amazake group was maintained for 8 weeks. However, water content in upper arm and back were not significantly different between koji amazake and placebo group. In conclusion, koji amazake maintains water content in left cheek though reverse correlation between water content and TEWL was not observed. Furthermore, the GlcCer content in koji amazake was 4.5 times greater than that in the placebo. To our knowledge, this study is the first to examine the relationship between maintenance of water content in left cheek and the effect of GlcCer in koji amazake. It is hoped that, in the future, scientific research on traditional Japanese fermented foods such as koji amazake will advance greatly.
Data Sharing Assessment
The data supporting the findings of this study are available from the corresponding author on request.
Acknowledgments
The authors are grateful to the Foundation for Promotion of Material Science and Technology (Tokyo, Japan) for their important contributions to analyzing the GlcCer.
Funding
The present study was funded by Hakkaisan Brewery Co., Ltd., a koji amazake manufacturing company.
Disclosure
A.K. is the managing director of production, and research and development for Hakkaisan brewery Co. Ltd. T.E., A. K-N., K.K. and Y.O. are employees of Hakkaisan brewery Co. Ltd. The authors report no other conflicts of interest in this work.
References
1. Yamashita H. Koji starter and koji world in Japan. J Fungi. 2021;7(7):569. doi:10.3390/jof7070569
2. Kurahashi A. Ingredients, functionality, and safety of the Japanese traditional sweet drink amazake. J Fungi. 2021;7(6):469. doi:10.3390/jof7060469
3. Fuji Keizai Co. Ltd. Foodstuff Marketing Handbook 2021. Fuji Keizai. 2020;4:342–345.
4. Oguro Y, Nishiwaki T, Shinada R, Kobayashi K, Kurahashi A. Metabolite profile of koji amazake and its lactic acid fermentation product by Lactobacillus sakei UONUMA. J Biosci Bioeng. 2017;124(2):178–183. doi:10.1016/j.jbiosc.2017.03.011
5. Kurahashi A, Nakamura A, Oguro Y, et al. Beneficial effects of koji amazake in suppressing the postprandial increase in blood glucose and insulin levels in healthy adults. J Brew Soc Jpn. 2020;115(1):43–53.
6. Kurahashi A, Nakamura A, Oguro Y, Yonei Y. Safety evaluation of a long-term intake of. J Brew Soc Jpn. 2020;115(3):159–172.
7. Kurahashi A, Yonei Y. Effects and safety of koji amazake: an excessive intake test. J Brew Soc Jpn. 2019;114(10):654–662.
8. Parke MA, Perez-Sanchez A, Zamil DH, Katta R. Diet and Skin Barrier: the role of dietary interventions on skin barrier function. Dermatol Pract Concept. 2021;11(1):e2021132. doi:10.5826/dpc.1101a132
9. Draelos ZD, Diaz I, Namkoong J, Wu J, Boyd T. Efficacy Evaluation of a Topical Hyaluronic Acid Serum in Facial Photoaging. Dermatol Ther. 2021;11(4):1385–1394. doi:10.1007/s13555-021-00566-0
10. Purnamawati S, Indrastuti N, Danarti R, Saefudin T. The Role of Moisturizers in Addressing Various Kinds of Dermatitis: a Review. Clin Med Res. 2017;15(3–4):75–87. doi:10.3121/cmr.2017.1363
11. Manami U, Manabu K, Shogo K, Tetsuro Y, Sumio K. Effect of intake of amazake on skin barrier functions in healthy adult women subjects-a randomized, double-blind, placebo-controlled study-. Jpn Pharmacol Ther. 2017;45(11):1811–1820.
12. Kitagaki H. Medical application of substances derived from non-pathogenic fungi Aspergillus oryzae and A. luchuensis-containing koji. J Fungi. 2021;7(4):243. doi:10.3390/jof7040243
13. Sakamoto M, Sakatani M, Ferdouse J, et al. Development of a quantitative method for the contents of glycosylceramide contained in Japanese foods brewed with koji and its application. J Bew Soc Jpn. 2017;112(9):655–662.
14. Fernandes CM, Goldman GH, Del Poeta M. Biological roles played by sphingolipids in dimorphic and filamentous fungi. mBio. 2018;9(3):e00642–18. doi:10.1128/mBio.00642-18
15. Hirakawa S, Sato A, Hattori Y, Matsumoto T, Yokoyama K, Kanai S. Dietary rice bran extract improves TEWL of whole body. Jpn Pharmacol Ther. 2013;41(11):1051–1059.
16. Asai S, Miyachi H. Evaluation of skin-moisturizing effects of oral or percutaneous use of plant ceramides. Jpn J Clinic Pathol. 2007;55(3):209–215.
17. Uchiyama T, Nakano Y, Ueda O, et al. Oral intake of glucosylceramide improves relatively higher level of transepidermal water loss in mice and healthy human subjects. J Health Sci. 2008;54(5):559–566. doi:10.1248/jhs.54.559
18. Kurahashi A, Enomoto T, Oguro Y, et al. Intake of koji amazake improves defecation frequency in healthy adults. J Fungi. 2021;7(9):782. doi:10.3390/jof7090782
19. Yasuhiko F, Masao O. Structure of cerebroside in Aspergillus oryzae. Biochim Biophys Acta. 1977;486(1):161–171. doi:10.1016/0005-2760(77)90080-7
20. Sugawara T, Kinoshita M, Ohnishi M, et al. Efflux of sphingoid bases by P-glycoprotein in human intestinal Caco-2 cells. Biosci Biotech Biochem. 2004;68(12):2541–2546. doi:10.1271/bbb.68.2541
21. Sugawara T, Kinoshita M, Ohnishi M, Nagata J, Saito M. Digestion of maize sphingolipids in rats and uptake of sphingadienine by Caco-2 cells. J Nutr. 2003;133(9):2777–2782. doi:10.1093/jn/133.9.2777
22. Fukunaga S, Wada S, Sato T, Hamaguchi M, Aoi W, Higashi A. Effect of torula yeast (Candida utilis)-derived glucosylceramide on skin dryness and other skin conditions in winter. J Nutr Sci Vitaminol. 2018;64(4):265–270. doi:10.3177/jnsv.64.265
23. Miyagawa M, Fujikawa A, Nagadome M, et al. Glycosylceramides purified from the Japanese traditional non-pathogenic fungus Aspergillus and koji increase the expression of genes involved in tight junctions and ceramide delivery in normal human epidermal keratinocytes. Fermentation. 2019;5(2):43. doi:10.3390/fermentation5020043
24. Hirata M, Tsuge K, Jayakody LN, et al. Structural determination of glucosylceramides in the distillation remnants of shochu, the Japanese traditional liquor, and its production by. J Agri Food Chem. 2012;60(46):11473–11482. doi:10.1021/jf303117e
25. Ohta K, Hiraki S, Miyanabe M, Ueki T, Manabe Y, Sugawara T. Dietary Ceramide Prepared from Soy Sauce Lees Improves Skin Barrier Function in Hairless Mice. J Oleo Sci. 2021;70(9):1325–1334. doi:10.5650/jos.ess21128
26. Ideta R, Sakuta T, Nakano Y, Uchiyama T. Orally administered glucosylceramide improves the skin barrier function by upregulating genes associated with the tight junction and cornified envelope formation. Biosci Biotech Biochem. 2011;75(8):1516–1523. doi:10.1271/bbb.110215
27. Kawada C, Hasegawa T, Watanabe M, Nomura Y. Dietary glucosylceramide enhances tight junction function in skin epidermis via induction of Claudin-1. Biosci Biotech Biochem. 2013;77(4):867–869. doi:10.1271/bbb.120874
28. Shirakura Y, Kikuchi K, Matsumura K, Mukai K, Mitsutake S, Igarashi Y. 4,8-Sphingadienine and 4-hydroxy-8-sphingenine activate ceramide production in the skin. Lipids Health Dis. 2012;108:11. doi:10.1186/1476-511X-11-108
29. Yeom M, Kim S-H, Lee B, et al. Oral administration of glucosylceramide ameliorates inflammatory dry-skin condition in chronic oxazolone-induced irritant contact dermatitis in the mouse ear. J Dermatol Sci. 2012;67(2):101–110. doi:10.1016/j.jdermsci.2012.05.009
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.