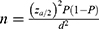Back to Journals » Journal of Multidisciplinary Healthcare » Volume 15
Knowledge of Drug–Food Interactions Among Healthcare Professionals Working in Public Hospitals in Ethiopia
Authors Degefu N , Getachew M , Amare F
Received 7 September 2022
Accepted for publication 28 October 2022
Published 15 November 2022 Volume 2022:15 Pages 2635—2645
DOI https://doi.org/10.2147/JMDH.S389068
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Natanim Degefu,1 Melaku Getachew,2 Firehiwot Amare3
1Department of Pharmaceutics, School of Pharmacy, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 2Department of Emergency Medicine and Critical Care, School of Medicine, College of Health and Medical Sciences, Haramaya University, Harar, Ethiopia; 3Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Firehiwot Amare, Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia, Tel +251 913183027, Email [email protected]
Background: Drug–food interactions can result in unfavorable outcomes during the treatment of patients. Healthcare professionals (HCPs) should advise patients on drug–food interactions. Knowledge of such interactions is crucial to avoid their occurrence. However, there is no information regarding the knowledge of HCPs about drug–food interactions in Harari Regional State.
Objective: To assess knowledge of drug–food interactions and associated factors among HCPs working in public hospitals in Harari Regional State, Eastern Ethiopia from April 15 to May 15, 2022.
Methods: A cross-sectional study was conducted in public hospitals in Harari Regional State, Eastern Ethiopia, among 251 HCPs. After stratification was done based on profession (pharmacists, nurses, and doctors), the sample size was proportionally allocated for the respective groups. Data were collected using a standardized self-administered questionnaire, entered into Epi-Data 3.1 and analyzed using Statistical Package for Social Sciences 26.0. Descriptive statistics were used to summarize variables. Multivariable logistic regression was done to determine factors associated with knowledge of drug–food interactions. P < 0.05 was used to declare significant association.
Results: Among the HCPs who completed the questionnaire, 56 (22.3%), 36 (14.3%), and 159 (63.3%) were doctors, pharmacists, and nurses, respectively. The majority of the HCPs were males (174 (69.3%)). The mean age of the HCPs was 27.6± 3.8. The mean knowledge score±SD of the HCPs was 28.6± 6.6 out of an overall score of 59. The HCPs poorly identified drug–food interactions and the correct administration time of drugs relative to meals. Being a pharmacist (AOR: 2.8, CI: 1.3– 6.4, p-value=0.012), and working at a tertiary hospital (AOR: 3.9, CI: 2.1– 7.3, p-value < 0.001), were associated with higher knowledge of drug-food interactions.
Conclusion: The HCPs in this study had inadequate knowledge of drug–food interactions. Thus, additional educational courses and training should be provided in order to improve knowledge regarding drug-food interaction.
Keywords: drug–food interaction, knowledge, healthcare professionals, public hospitals
Background
Food is one of the core components of patients’ therapeutic plans and healthy lifestyles. Many food can be used in the prevention and treatment of diseases.1–3 As a result, the use of healthy food is now increasing among patients.4 The concomitant use of food and drugs should be of great concern due to potential interactions between food products and drugs.1–3
Drug–food interaction is one of the constraints for the safe and effective delivery of pharmacotherapy. It can occur with prescription and non-prescription products. Drug–food interactions can result in a decrease in drug efficacy or an increase in drug toxicity.5–7 Clinically significant drug–food interactions can be classified into pharmaceutical, pharmacokinetic, and pharmacodynamics based on the mechanisms involved.8,9
Pharmaceutical interaction involves physicochemical reactions that occur within delivery devices, such as enteral feeding tubes. Pharmacokinetic interaction occurs when the processes related to absorption, distribution, metabolism, and excretion of drugs varied because of food presence. Divalent cations found in dairy products forming a chelate with ciprofloxacin, CYP3A4 metabolism of simvastatin inhibited by grapefruit juice, and lithium and sodium competing for tubular reabsorption in the kidney are examples of pharmacokinetic interactions. On the other hand, pharmacodynamic interaction happens when foods alter the clinical effect of a drug on the body. The result of the interaction can be additive, synergistic, or antagonistic. A diet containing high vitamin K antagonizing the therapeutic effects of warfarin is an example of pharmacodynamic interactions.8–13
The degree of interaction depends on the physical and chemical nature of the drug, the formulation, the type of meal, and the time interval between eating and dosing.14 Drugs achieve their intended purpose only if administered in the proper dosage, and at the appropriate time with the appropriate food.9
Drug–food interactions represent an essential and widely under-recognized source of medication errors, which predisposes to treatment failure, or an increased bioavailability, which increases the risk of adverse events and may even precipitate toxicities.9,15,16 The prevalence of potential drug–food interaction in different countries varies in the range from 6% to 70%.8,16,17 Elderly patients taking three or more medications for chronic diseases, patients with hypertension, diabetes, congestive heart failure, hyperlipidemia, or depression are high-risk patients for drug–food interactions. They should be specially monitored for drug–food interactions.18–20
Healthcare professionals (HCPs) play a pivotal role in preventing drug–food interaction. Their clear understanding of factors that affect the time course of drug action can lead to rational use of drugs.21 HCPs are required to identify efficacy influencing food and beverages and provide information on possible drug–food interactions including the recommended intervals of time between drugs and food. Therefore, their knowledge about drug–food interactions is crucial.22,23 However, studies done in the United States, Colombia, India, Palestine and Jordan reported that HCPs had inadequate knowledge about drug–food interaction.3,16,23–25 A study conducted in South Africa also revealed HCPs had poor knowledge on drug–food interactions.2 However, in Ethiopia, there is paucity of data with regard to knowledge of HCPs about drug–food interactions. Therefore, this study is particularly interested to identify HCPs’ knowledge about drug–food interactions and associated factors.
Methods
Study Design, Setting and Period
An institution-based cross-sectional study was conducted in public hospitals in Harari Regional State. Harari Regional State is 1 of the 11 regional states in Ethiopia.26 There is one federal police, two public and two private hospitals, nine health centers (five urban and four rural), 19 health posts, and 10 non-profit clinics in the Harari regional state. Two of the public hospitals are Hiwot Fana Comprehensive Specialized Hospital (HFCSH) and Jugal General Hospital (JGH). HFCSH is a teaching hospital of Haramaya University. It has 64 pharmacists, 300 nurse professionals, and 137 doctors. JGH is a general hospital. It has 26 pharmacists, 116 nurse professionals, and 44 doctors. In the two public hospitals, there are 687 HCPs. Data were collected from April 15 to May 15, 2022.
Study Participants, Sample Size and Sampling Procedure
All HCPs working in public hospitals in Harari Regional State for at least 6 months were considered for inclusion in the study. While HCPs on sick leave and annual leave were excluded from the study. The sample size of the study was calculated using the single population proportion formula as follows.
To calculate the sample size (n):1.96 was substituted for Z which is the standard normal value at 95% confidence level, p which is the proportion of knowledge about drug–food interaction among HCPs was taken as 50%, the value of q was taken as 1-p, d which is the margin of error was taken as 0.05. Then, a finite population correction and a 10% non-response rate were considered to calculate a final sample size of 271.
To select the study units from the HCPs working in the two public hospitals in the Harari regional state, a stratified random sampling technique with proportionate allocation was used to obtain a representative sample from each profession. Hence, the determined sample size was proportionately allocated to the three professions (pharmacists, nurses, and doctors) based on the total number of HCPs in each profession. The sampling frame was prepared after a list of all HCPs working in each hospital was obtained from each hospital’s human resource office. Finally, HCPs were selected from each profession by a simple random sampling method. As a result, 36 pharmacists, 71 doctors, and 164 nurses were included in the study.
Data Collection Methods
A self-administered paper-based questionnaire was used to collect data from the study participants. The questionnaire was adapted and modified from a previous tool used to assess knowledge of HCPs towards drug–food interactions in the published work by Osuala et al and Zawiah et al.2,3 The questionnaire incorporated questions for the HCPs pertaining to demographic characteristics such as age, sex, level of education, profession, years of work experience, and training on drug–food interactions as well as knowledge on drug–food interactions. The knowledge section consisted of 32 questions that assessed the general knowledge of drug–food interaction, knowledge of interactions of specific food and drugs, the timing of food intake relative to a drug, and the knowledge of antihypertensive and antiretroviral drugs and food interactions as these disease conditions are prevalent in the area. The data were collected by two well-trained HCPs.
Operational Definitions
- HCPs: HCPs comprise any pharmacist, nurses and doctors working in any clinical department.
- Low knowledge of drug–food interaction: HCPs who scored less than the mean knowledge score or within the exact mean cutoff were classified as having low knowledge of drug–food interaction.2
- High knowledge of drug–food interaction: HCPs who scored above the mean knowledge score were classified as having high drug–food interaction knowledge.2
Data Quality Control
Data collectors were trained before the data collection process. A pretest was done on 5% of the total study population in Haramaya General Hospital before the start of data collection. Any error found during the process of the pretest was corrected, and modification was made to the final version of the questionnaire. All collected data were examined for completeness and consistency during data management, storage, and analysis.
Methods of Data Processing and Analysis
The data were checked for consistency and completeness, entered into Epi-Data version 3.1, and exported to Statistical Package for Social Science (SPSS) version 26 for analysis. Sociodemographic characteristics were analyzed using descriptive statistics. Continuous variables were expressed as mean ± SD, while categorical variables were expressed as frequency and percentages. The knowledge of the HCPs was assessed based on their responses to questions on drug–food interactions. The knowledge questions were grouped into four categories: general knowledge of the HCPs about drug–food interactions, knowledge of interactions of specific food and drugs, knowledge of the timing of food intake relative to a drug, and knowledge of antihypertensive and antiretroviral drugs and food interactions; each of the categories had total scores of 8, 39, 7, and 5, respectively. The overall mean score percentage was calculated as the percentage of the overall mean score divided by the total score per category. Logistic regressions with a 95% confidence interval were done to identify factors associated with knowledge of HCPs. Binary logistic regression was done to determine the crude odds ratio. Variables with a p-value of 0.25 or less in the bivariate analysis were introduced to the multivariate logistic regression by using the forward selection method. P < 0.05 was used to declare a statistically significant association. The fitness of the logistic regression model was checked using Hosmer and Lemeshow test.
Results
Characteristics of Study Participants
The total number of HCPs involved in this study was 271. Complete response was obtained from 251 participants, with a response rate of 92.6%. A total of 56 (22.3%) doctors, 36 (14.3%) pharmacists, and 159 (63.3%) nurses participated in this study. The mean age of the HCPs was 27.6 ± 3.8, and the age category 25–29 years was the highest in frequency 145 (57.8%). Males were the predominant participants in the study, 174 (69.3%). One hundred forty-nine (59.4%) study participants had less than 3 years of work experience. Only seven participants attended previous training on drug-food interactions. The majority of the HCPs work at a tertiary hospital 180 (71.7%) (Table 1).
 |
Table 1 Characteristics of HCPs Working in Public Hospitals in Harari Regional State, Eastern Ethiopia, N=251 |
Knowledge of Healthcare Professionals About Drug–Food Interactions
In this study, the overall mean score ±SD of knowledge of HCPs about drug–food interactions was 28.6 ± 6.6 (48.4%) (doctors (29.6±6.8), pharmacists (31.5±6.4), and nurses (27.6±6.3)). HCPs had low knowledge about drug–food interactions, as more than half of the HCPs (52.2%) scored below the mean.
The mean score ± SD of general knowledge regarding drug–food interaction questions category (total score= 8) of doctors, pharmacists, and nurses was 5.1 ±1.7, 5.9 ±1.8, and 4.6 ±1.5, respectively, with an overall mean score ±SD of 4.9 ±1.6 (60.8%). Majority of the HCPs correctly answered that some of the food (90.0%) and drinks (85.7%) can interfere with the effectiveness of drugs. Majority of the participants also recognized that some food increase or decrease the action of a drug and can alter the nutritional status of a patient, which was 84.5% and 88.1%, respectively. Less than half (47.0%) of HCPs recognized that elderly patients were at higher risk for drug–food interactions. Only 31.5% (n = 79) discerned all four levels (absorption, distribution, metabolism, and excretion) in which food/beverages interact with drugs.
The overall mean score ± SD of HCPs’ knowledge on specific drug–food interaction was 18.5 ± 4.7 (47.4%) out of 39 total scores. The mean score ±SD of knowledge about specific drug–food interactions of doctors, pharmacists, and nurses was 18.6±4.8, 19.5± 4.3, and 18.1 ±4.7, respectively. More than half of the HCPs proved a knowledge score for some specific drug–food interaction questions. HCPs’ correct knowledge scores of the interaction of theophylline with a large amount of tea, theophylline with a large amount of coffee, antibiotics such as tetracycline and fluoroquinolones with milk, and a high-fat diet with albendazole were 129 (51.4%), 196 (78.1%), 160 (63.8%), and 149 (59.4%), respectively. Alternatively, less than half of HCPs recognized interaction between monoamine oxidase inhibitors (MAOIs) with cheese (99 (39.4%)), high-fat diet with griseofulvin (120 (47.8%)), warfarin with spinach (83 (33.1%)), warfarin with broccoli (100 (39.8%)) and warfarin with green leaf lettuce (104 (41.4%)). Concerning alcohol–drug interactions, more than half of HCPs answered correctly for diazepam (174 (69.3%)), metronidazole (144 (57.4%)), and antihistamines (126 (50.2%)) and less than half recognized interaction with isoniazid (105 (41.8%)) and metformin (103 (41.0%)) (Table 2).
 |
Table 2 Knowledge of Interactions Between Specific Drugs and Food Among HCPs Working in Public Hospitals in Harari Regional State, Eastern Ethiopia, N=251 |
Overall mean score±SD of HCPs’ knowledge regarding the timing of food intake relative to drugs was 2.9±1.4 (42.0%) out of a total score of 7. The mean score±SD of the knowledge of the timing of food intake relative to drugs of doctors, pharmacists, and nurses was 3.1±1.5, 3.4±1.8, and 2.8±1.3, respectively. Considering the timing of medication intake with regards to food, the majority of HCPs 177 (70.5%) correctly identified omeprazole’s administration. There was a poor knowledge for isoniazid, levothyroxine, and erythromycin stearate, with correct knowledge score of 92 (36.7%), 67 (26.7%), and 60 (23.9%), respectively (Figure 1).
 |
Figure 1 Knowledge about timing of drug intake with respect to food among HCPs working in public hospitals in Harari Regional State, Eastern Ethiopia, N=251. |
The overall mean score ± SD of HCPs’ knowledge regarding interaction of food with antihypertensive and antiretroviral drug was 2.3 ± 1.2 (45.8%) out of a total score of 5. The mean score ±SD of knowledge of interactions of food with antihypertensive and antiretroviral drugs of doctors, pharmacists, and nurses was 2.6±1.3, 2.8±1.3, and 2.1±1.1, respectively. Only 71 (28.3%) HCPs knew that propranolol immediate release and captopril must be taken on an empty stomach. One hundred thirty-five of HCPs (53.8%) identified that spironolactone must be avoided with potassium-rich food. Considering antiretroviral drugs, 140 (55.8%), 113 (45.0%), and 115 (45.8%) HCPs recognized that lopinavir/ritonavir syrup must be taken with food, efavirenz must be taken on an empty stomach, and zidovudine can be taken without relation to food intake, respectively.
Factors Associated with Knowledge of the Healthcare Professionals on Drug–Food Interactions
In the multivariate logistic regression analysis to identify factors associated with knowledge of HCPs on drug–food interaction, only profession and the type of hospital were statistically significant (Table 3). The odds of having high knowledge among pharmacists were 2.8 times higher as compared to nurses (adjusted odds ratio (AOR): 2.8, 95% confidence interval (CI): (1.3–6.0)). Additionally, the odds of having high knowledge among HCPs who practiced at a tertiary hospital were 3.9 times higher than those who practiced at a secondary hospital (AOR: 3.9, 95% CI: (2.1–7.3)).
 |
Table 3 Factors Associated with Knowledge About Drug–Food Interaction Among HCPs Working in Public Hospitals in Harari Regional State, Eastern Ethiopia, N=251 |
Discussion
This study evaluated the health care professionals (doctors, pharmacists, and nurses) knowledge of drug–food interactions and its associated factors. To the authors’ knowledge, this study is the first to evaluate knowledge of drug–food interactions among HCPs in public hospitals in Harari regional state, Ethiopia. To assess their knowledge, scores were calculated from the correctly answered questions. Overall, the HCPs had low knowledge of drug–food interactions, with a mean score of 28.6 (doctors (29.6), pharmacists (31.5), and nurses (27.6)) out of a maximum score of 59. Only seven (2.8%) of the HCPs were trained on drug–food interactions, which could result in low knowledge of drug–food interactions. Likewise, studies done in different areas showed that HCPs had insufficient knowledge about drug–food interactions.2,3,16,23,24 In this study, the knowledge score of the HCPs was lower than those reported in a study conducted in Palestine (61.7%)16 and Jordan (60%),3 the reason might be because the latter studies involved only pharmacists. The finding of the present study was comparable with a study conducted in eThekwini, KwaZulu-Nata, and South Africa (49.3%).2
Regarding general knowledge questions, as reported elsewhere,2 more than half of the HCPs knew that some food can alter the action of a drug and knew that some drugs can alter the nutritional status of a patient. Identifying the age group highly susceptible to drug–food interactions is sole to monitor any potential consequence of drug–food interaction and patient counseling. Elderly patients are more susceptible to drug–food interaction than adults and children because of comorbidities, polypharmacy, and reduced clearance of pharmacologically active compounds.27,28 Less than half (47.0%) of the HCPs who participated in this study recognized that elderly patients were at higher risk for drug–food interactions. However, it is slightly higher than the finding of a study conducted in eThekwini, KwaZulu-Nata, South Africa (37.5%)2 and slightly lower than reported by a study conducted in Palestine (63.7%).16
In the present study, HCPs demonstrated low knowledge in recognizing specific drug–food interactions. The absorption of tetracyclines and fluoroquinolones can be impaired in the presence of dairy products (such as milk and cheese).8 Chelation by dietary cations (like calcium and magnesium) found in milk and other dairy products decrease the absorption of the aformentioned antibiotics.29 Almost two-third of HCPs participated in this study were aware of tetracycline and fluoroquinolones interaction with milk, whereas less than half of HCPs knew about interaction with dairy products and iron-rich food. The study conducted among pharmacists in Jordan (87.3%)3 and in Palestine (94.21%)16 reflects comparatively higher knowledge about tetracycline interaction with milk and dairy products. This discrepancy might have resulted as the latter studies included only pharmacists.
Cheese, smoked meats, alcoholic beverages, fava beans, and fermented food contain high levels of tyramine; these food have the potential to cause a hypertensive crisis when ingested concurrently with MAOIs. This a result of a decrease in tyramine degradation that leads to accumulation in the systemic circulation to levels where it is taken up by adrenergic neurons, thereby precipitating a hypertensive crisis.8 In this study, only less than half of the HCPs correctly identified food that have interaction with MAOIs. Similarly, the finding of a study conducted in eThekwini, KwaZulu-Nata, South Africa revealed that the majority of HCPs could not identify food that interacts with MAOIs.2 This could be a result of MAOIs being no longer commonly used as antidepressants. However, a study conducted in Jordan (68.0%)3 and Palestine (71.8%)16 involving only pharmacists showed higher knowledge about the interaction between MAOI with cheese and fermented food.
Patients on warfarin should avoid consumption of vegetables that have high vitamin K contents such as spinach, broccoli, and green leaf lettuce because high vitamin K can increase the production of clotting factors that diminishes the therapeutic effect of warfarin.8 In this study, more than half of HCPs were unable to distinguish food that interact with warfarin. Likewise, a study conducted in eThekwini, KwaZulu-Nata, South Africa exposed that except for spinach majority of HCPs poorly identified a food that interacts with warfarin.2
Grapefruit can affect the safety and efficacy of medications such as atorvastatin and amiodarone. It can increase the risk of rhabdomyolysis in patients taking atorvastatin and torsade de pointes in patients taking amiodarone.3,30 In this study, more than half of the HCPs knew about the interaction between amiodarone and grapefruit while less than half were aware of the interaction between atorvastatin and grapefruit. Unlike this study finding, a study conducted in Jordan involving only pharmacists reflected that more than half of the participants were aware of the interaction of grapefruit with both amiodarone (59.7%) and atorvastatin (70.3%).3 On a similar note, levodopa competes with amino acids for absorption, hence co-administration of the drug with protein-rich food would result in reduced effectiveness.8,31 Similar to another study conducted in Jordan,3 more than half of the HCPs who participated in this study recognized that protein-rich food affects the efficacy of levodopa.
In this study, less than half of the HCPs knew the appropriate administration time of drugs relative to food intake. Comparable results were reported in a study from Palestine16 and eThekwini, KwaZulu-Nata, South Africa.2 Administration of drugs before, with, and after food intake can affect the absorption, distribution, metabolism, and excretion of the drugs. Thyroid supplements such as levothyroxine should be given 30 minutes to 1 hour before a meal. This is because dietary fiber can reduce the absorption of levothyroxine and result in sub- therapeutic serum levels.8 Food can raise gastric pH preventing dissolution and absorption of isoniazid, thus, it is recommended to take isoniazid on an empty stomach if tolerated.31 Knowing the correct administration time of medications is critical since inappropriate timing can lead to medication errors, and compromise patient safety and effective therapy.
In this study, the knowledge of HCPs’ on drug–food interaction was not significantly associated with age, sex, years of work experience, and level of education. However, it was significantly associated with the profession and type of hospital where they are practicing. The odds of having high knowledge about drug–food interaction were higher among pharmacists as compared to nurses. Likewise, a study conducted in eThekwini, KwaZulu-Nata, South Africa revealed that pharmacists had the highest knowledge score in all sections compared to the other HCPs.2 Since only 14.3% of the study participants were pharmacists, thus results of this study should be interpreted cautiously. In this study, HCPs who practiced at a tertiary hospital had higher knowledge regarding drug–food interaction. This might have resulted as the tertiary hospital is a teaching hospital and hence a better exposure to have an up-to-date knowledge. A study conducted in Jordan exposed that there was no significant difference in the overall knowledge score between pharmacists practicing in different setups.3
The study was limited to only one regional state in Ethiopia, and thus the study findings may not be generalizable to public hospitals in the country. Further, factors associated with knowledge of drug–food interactions were assessed using the cross-sectional study design, which might not show causal relationships with the potential risk factors.
Conclusion
In conclusion, this study showed that HCPs had inadequate knowledge of drug–food interactions. Being a pharmacist and working in a tertiary center is significantly associated with knowledge of drug–food interactions. Since HCPs’ knowledge has great implications for the delivery of safe and effective patient management, the hospital administration and the regional health bureau should give attention and efforts to improve HCPs’ knowledge of drug–food interactions by providing additional educational courses and training.
Abbreviations
HFCSH, Hiwot Fana Comprehensive Specialized Hospital; JGH, Jugal General Hospital; HCPs, healthcare professionals; SD, standard deviations; IHRERC, Institutional Health Research Ethics Review Committee; MAOIs, monoamine oxidase inhibitors.
Data Sharing Statement
All the data used for the study are contained within the manuscript.
Ethics Approval and Consent to Participate
Ethical approval was obtained from the Institutional Health Research Ethics Review Committee (IHRERC) of the College of Health and Medical Sciences, Haramaya University, with a reference number of IHRERC/050/2022. Permission was obtained from the Medical Directors of HFCSH and JGH to conduct the study. Informed, voluntary, written and signed consent was obtained from each participant. The information that the participants gave was kept confidential and there was no information that specifically identifies the participants.
Acknowledgments
We would like to thank all the HCPs who participated in this study and the management at HFCSH and JGH for their cooperation during data collection.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted, and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest.
References
1. Choi JH, Ko CM. Food and drug interactions. J Lifestyle Med. 2017;1:1. doi:10.15280/jlm.2017.7.1.1
2. Osuala EC, Tlou B, Ojewole EB. Assessment of knowledge of drug-food interactions among healthcare professionals in public sector hospitals in eThekwini, KwaZulu-Natal. PLoS One. 2021;16(11):e0259402. doi:10.1371/journal.pone.0259402
3. Zawiah M, Yousef AM, Khan AH, et al. Food-drug interactions: knowledge among pharmacists in Jordan. PLoS One. 2020;15:e0234779. doi:10.1371/journal.pone.0234779
4. Chiba T, Kobayashi E. アドバイザリースタッフの資格を有する薬剤師における医薬品と健康食品との併用に対する認識[Consulting pharmacists who have advisory staff license regarding the use of medicines and health foods]. Yakugaku Zasshi. 2020;140:723–728. doi:10.1248/yakushi.19-00256. Japanese.
5. Aman SF, Hassan F, Naqvi BS, Hasan SM. Studies of food drug interactions. Pak J Pharm Sci. 2010;23:313–320.
6. Gauthier I, Malone M. Drug-food interactions in hospitalised patients. Methods of prevention. Drug Safety. 1998;18:383–393. doi:10.2165/00002018-199818060-00001
7. Spanakis M, Spanakis EG, Kondylakis H, et al. Addressing drug-drug and drug-food interactions through personalized empowerment services for healthcare. Annu Int Conf IEEE Eng Med Biol Soc. 2016;2016:5640–5643. doi:10.1109/EMBC.2016.7592006
8. Ased S, Wells J, Morrow LE, Malesker MA. Morrow LE and Malesker MA. Clinically significant food-drug interactions. Consult Pharm. 2018;33(11):649–657. doi:10.4140/TCP.n.2018.649.
9. Bushra R. Aslam N and Khan AY. Food-drug interactions. Oman Med J. 2011;26:77. doi:10.5001/omj.2011.21
10. Almazrou SH, Alaujan SS. Knowledge and readiness for interprofessional learning among pharmacy and clinical nutrition students at King Saud University. J Multidiscip Healthc. 2022;15:1965–1970. doi:10.2147/JMDH.S360608
11. D’Alessandro C, Benedetti A, Di Paolo A. Giannese D and Cupisti A. Interactions between food and drugs, and nutritional status in renal patients: a narrative review. Nutrients. 2022;14:548.
12. Ötles S, Food SA. Drug interactions: a general review. Acta Sci Pol, Technol Aliment. 2014;13:89–102. doi:10.17306/J.AFS.2014.1.8
13. Spanakis M, Melissourgaki M, Lazopoulos G, Patelarou AE, Patelarou E. Prevalence and clinical significance of drug-drug and drug-dietary supplement interactions among patients admitted for cardiothoracic surgery in Greece. Pharmaceutics. 2021;14(1):13. doi:10.3390/pharmaceutics14010013
14. Welling PG. Interactions affecting drug absorption. Clin Pharmacokinet. 1984;9:404–434. doi:10.2165/00003088-198409050-00002
15. Genser D. Food and drug interaction: consequences for the nutrition/health status. Ann Nutr Metab. 2008;52:29–32. doi:10.1159/000115345
16. Radwan A, Sweileh A, Shraim W, Hroub A. Elaraj J and Shraim N. Evaluation of community pharmacists’ knowledge and awareness of food-drug interactions in Palestine. Int J Clin Pharm. 2018;40:668–675. doi:10.1007/s11096-018-0640-x
17. Koziolek M, Alcaro S, Augustijns P, Basit AW, Grimm M, Bea H. The mechanisms of pharmacokinetic food-drug interactions–A perspective from the UNGAP group. Eur J Pharmaceutical Sci. 2019;134:31–59. doi:10.1016/j.ejps.2019.04.003
18. Grabowsky JA. Drug interactions and the pharmacist: focus on everolimus. Ann Pharmacother. 2013;47(7–8):1055–1063. doi:10.1345/aph.1R769
19. Mohammad Ismail MY. Drug-food interactions and role of pharmacist. Asian J Pharm Clin Res. 2009;2:548.
20. Osuala EC, Tlou B, B Ojewole E. Tlou B and Ojewole EB. Knowledge, attitudes, and practices towards drug-food interactions among patients at public hospitals in eThekwini, KwaZulu-Natal, South Africa. Afr Health Sci. 2022;22(1):681–690. doi:10.4314/ahs.v22i1.79
21. Deng J, Zhu X, Chen Z, et al. A review of food–drug interactions on oral drug absorption. Drugs. 2017;77(17):1833–1855. doi:10.1007/s40265-017-0832-z
22. Debus JL, Bachmann P, Frahm N, et al. Associated factors of potential drug-drug interactions and drug-food interactions in patients with multiple sclerosis. Ther Adv Chronic Dis. 2022;13:20406223221108391. doi:10.1177/20406223221108391
23. Enwerem NM, Okunji P. Knowledge, Attitudes and Awareness of Food and Drug Interactions among Nurses with Different Levels of Experience. Int J Nurs. 2015;2:1–9.
24. Benni JM, Jayanthi MK. Basavaraj R and Renuka M. Knowledge and awareness of food and drug interactions (FDI): a survey among health care professionals. Int J Pharmacol Clin Sci. 2012;1:87.
25. Bertrand B, Livingston-Bowen C. Duffrin C and Mann A. ACE inhibitors and potassium foods–nurses’ knowledge. Int J Health Care Qual Assur. 2014;27:54–64. doi:10.1108/IJHCQA-06-2012-0057
26. Central Statistical Agency FDRoE. Population Projection of Ethiopia for All Regions At Wereda Level from. Geographical J. 2014;2017:2014.
27. Agbabiaka TB, Spencer NH. Khanom S and Goodman C. Prevalence of drug-herb and drug-supplement interactions in older adults: a cross-sectional survey. Br j General Practice. 2018;68:e711–e7. doi:10.3399/bjgp18X699101
28. Boullata JI, Armenti VT. Handbook of Drug-Nutrient Interactions. Totowa, NJ: Humana Press; 2004.
29. Leibovitch ER. Deamer RL and Sanderson LA. Food-drug interactions: careful drug selection and patient counseling can reduce the risk in older patients. Geriatrics. 2004;59:19–22, 32–3.
30. Huang SM, Lesko LJ. Drug-drug, drug-dietary supplement, and drug-citrus fruit and other food interactions: what have we learned? J Clin Pharmacol. 2004;44:559–569. doi:10.1177/0091270004265367
31. Ismail MYM, Yaheya M. Drug-food interactions and role of pharmacist. Asian J Pharm Clin Res. 2009;2:1–10.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.