Back to Journals » Infection and Drug Resistance » Volume 13
Knowledge, Attitude, and Practices Towards Tuberculosis Among Clients Visiting Tepi General Hospital Outpatient Departments, 2019
Authors Angelo AT , Geltore TE , Asega T
Received 17 October 2020
Accepted for publication 9 December 2020
Published 21 December 2020 Volume 2020:13 Pages 4559—4568
DOI https://doi.org/10.2147/IDR.S287288
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Suresh Antony
Abiy Tadesse Angelo,1 Teketel Ermias Geltore,2 Tagay Asega3
1Department of Nursing, Mizan Tepi University, Mizan Aman, Ethiopia; 2Department of Midwifery, Wachamo University, Durame, Ethiopia; 3Nursing Department, Tepi General Hospital, Tepi, Ethiopia
Correspondence: Abiy Tadesse Angelo Email [email protected]
Background: Tuberculosis, which is an infectious disease, is one of the leading causes of morbidity and mortality in developing countries. Ethiopia is facing high tuberculosis burdens. Even if it is preventable and curable, individuals’ KAP towards the disease is one of the bottlenecks in decreasing the disease burdens. TGH, located in the Sheka zone, is one of the remote areas and the KAP towards TB is unknown. Therefore, the current study was undertaken in TGH to assess the KAP towards TB.
Materials and Methods: A cross-sectional study was conducted among 415 randomly selected participants. A structured questionnaire was used to collect the data by a face-to-face interview from May 23 to June 23/2019. Data were entered into Epidata 3.1 and exported to SPSS version 21 for descriptive analysis.
Results: A total of 345 (83%) respondents have heard about TB, while 76 (18%) respondents said persistence productive cough as symptoms of tuberculosis. Only 9.9% of participants mentioned bacteria as the cause of the diseases and 170 (41%) considered that the transmission is via air droplets. The majority (70%) of participants responded that its transmission is not preventable and overall 236 (56.9%) had high overall knowledge about TB. Thirty percent of the respondents considered that TB is serious to the area while 29% considered TB is not very serious for them. Fifty-three percent of the participants were having a favorable attitude towards tuberculosis. The majority (85%) did not cover their mouth while coughing, while 79.5% did not screen for tuberculosis and 82% of participants have not received any health education about TB. Overall, 44.6% practiced TB prevention.
Conclusion: The majority of the study participants had high overall knowledge and positive attitude towards tuberculosis prevention, which are not seen in the practice of tuberculosis. Effective educational programs should be implemented to overcome the problem.
Keywords: knowledge, attitude, practice, tuberculosis, Tepi
Introduction
Tuberculosis (TB) is a communicable disease caused mainly by Mycobacterium tuberculosis. Among infectious diseases, it is the leading cause of death by preceding HIV/AIDS.1 Tuberculosis (TB) is a global challenge affecting around 10 million people worldwide. It is not only limited to morbidity, but causing estimated 1.2 million deaths in HIV negative and 251, 000 deaths in HIV-positive patients in 2018.2 Its impact is also not limited to, health as it causes severe economic crisis in underdeveloped countries.3
In the world health organization (WHO) 2018 report; most of the cases were reported from South-East Asia (44%) and Africa (24%). Ethiopia located in eastern horn Africa is one of the top 30 high TB burden countries with a total annual estimated incidence of 165,000 HIV-positive tuberculosis incidences, 24,000 HIV-negative patient mortality, and 2200 HIV-positive tuberculosis mortality.2
Ending the TB epidemic by 2030 is one of the sustainable development goals. Countries, mostly high TB burden areas are not on the track to meet this sustainable development goal.2 Awareness of individual’s impacts TB transmission and early screenings for TB, which could help to end the TB.4
Factors contributing to the progression of latent TB to active TB were poor nutritional status, smoking, HIV infection, re-infection which could increase the load of the bacilli, and poor socio-economic status.5,6 Individual with the low economic class, having no access to health facilities and lack of knowledge about the disease as well as a mode of transmission were determinants for contracting the diseases.7–14 Individual attitude is also crucial in control of the tuberculosis transmission.15,16
Up-to-date there are no data about the knowledge, attitude, and practice of adults towards TB in the current study area. Therefore, the study was conducted to assess adults’ knowledge, attitude, and practice towards TB who were visiting outpatient departments.
Materials and Methods
Study Setting and Design
The study was conducted in Tepi general hospital, which is one of the government hospitals in Sheka zone, southwestern Ethiopia. It is located in Yeki woreda (an administration under zone), which is one of the woredas in the Sheka zone. It is about 611km away from the capital city of the country, Addis Ababa. The hospital has eight outpatients departments and four wards namely medical, surgical, pediatrics, and obstetrics/gynecology wards. This institutional-based quantitative cross-sectional study design was conducted from May 23 to June 23/2019.
Source Population
Adult clients visited outpatient departments of the Tepi general hospital in 2019.
Study Participants
Sampled adult clients visited outpatient departments of the Tepi general hospital during the time of data collection.
Inclusion and Exclusion Criteria
All adult clients visiting the outpatient department of a Tepi general hospital during the data collection time were included. Clients who were severely ill and age less than 18 years old were excluded from the study.
Sample Size and Sampling Technique
The sample size was determined by using a single population proportion formula. Based on the assumption of a 95% confidence interval, 5% margin of error, the proportion of awareness about TB 54.4%,17 and 10% non-response rate; a total sample of 419 was needed.
A systematic sampling technique was applied to collect data from participants. To determine the sampling interval (K-th), a one-month maximum number of clients who visited the outpatient department before the actual data collection were taken which were 4200. The sampling interval or K - th obtained for this study was 10. Throughout 1 to 10, number 2 was randomly selected and this was the starting point to collect the data. On each day of the data collection period, the second comer patient was taken as a first sample and then every tenth patient was included in the sample until the desired sample was completed. This procedure was applied to all outpatient departments in the study area.
Data Collection Tool, Quality Control and Procedure
The questionnaire for this study purpose was developed based on the previous relevant studies which were conducted in another part of Ethiopia for the similar purpose,17,18 and WHO TB guideline to develop knowledge, attitude, and practice surveys.19 The structured questionnaire which was first designed in the English version was translated to the Amharic version, which was the local language to make it clearer for easy understanding purposes. The questionnaire had four essential components related to participants’ socio-demographic characteristics, knowledge-related questions, attitude related questions, and practice-related questions about TB. One day orientation on data collection was given to eight data collector diploma nurses. Before actual data collection, the questionnaire was pretested on 21 clients attending at Mizan Tepi university teaching hospital which is 50 km away from the current study site and necessary corrections were made before using it for the actual study. The face to face interview was done by eight diploma nurses who collected the data.
Knowledge about tuberculosis was measured by 8 item questions assessing the cause of TB, signs/symptoms of TB, the transmissibility of TB, the correct mode of transmission of TB, the treatability of TB, knowledge on effective treatment for TB, knowledge on the preventability of TB and knowledge of effective prevention methods. The responses of participants’ on questions were rated by giving 1 for all correct answers and 0 for incorrect answers, including “I don”t know responses’ in each question. Participants were allowed to select all possible correct answers for those items having more than one possible answer. These items have 14 possible correct answers. The sum of knowledge answers was dichotomized based on the mean which was 9.4. By using mean as cut –off value, participants’ responses were coded to ‘1ʹ indicated having high overall TB knowledge, if the knowledge responses were greater or equal to mean value and to ‘0ʹ indicated having low overall TB knowledge, if the knowledge responses were below the mean.
The attitude was measured by 4 items focusing on the seriousness of the disease, seriousness of the disease in the area, the chance of contracting the disease, and attitude towards the disease concerning religion. The attitude responses were measured by a rating scale having 1 to 5 values and the total responses were ranged from 4 to 20. The mean value (12.6) of attitude response was taken as a cutoff value to code attitude response to ‘1ʹ which indicated having a favorable attitude regarding TB if the response sum was greater or equal to men. Similarly attitude response was coded to ‘0ʹ indicated having unfavorable attitude, if the response sum was below the mean.
The practice was measured by 6 items assessing on having a window at home or not, opening window regularly, opening the car window during traveling, screening for TB, having health education about TB, and care taken during having TB. Participants’ response was rated by giving 1 point for “yes” responses and 0 for no responses. The response of participants’ were ranged from 0 to 6 and the mean which was 1.79 computed to code participants’ response to ‘1ʹ indicating having good practice towards TB if the response was greater or equal to mean otherwise having poor practice.
Data Processing and Analysis
Data were entered into Epidata 3.1 version after a manual check for completeness, skip pattern and wrong coding which was corrected at the study site. The entered data were exported to SPSS version 21 for analysis. Descriptive statistics using a table of frequency distribution were used to summarize the results such as socio-demographic characteristics, knowledge, attitudes, and practices towards TB. Then the data were presented by using sentences, graphs, tables, frequencies, percentages.
Ethical Considerations
This study was conducted in accordance with Declaration of Helsinki and a formal letter was obtained to conduct the study from Mizan Tepi University, Nursing Department. The Tepi General Hospital administration office was informed about the purpose of the study to get permission. Confidentiality of the respondents was secured by excluding respondent’s identifiers like a name from the data collection format. Informed written consent was obtained from the respondents before conducting the study.
Results
Socio-Demographic Characteristics
Out of 419 participants, 415 were involved in the study and made a response rate of 99.04%. The mean age of the participants was 32 years with the majority (53.5%) between the age group of 18 to 35 years old. The majority (67%) of respondents were Orthodox by religion while 205 (49%) participants were kaffa by ethnicity and about 120 (28.9%) participants attended their secondary school (Table 1).
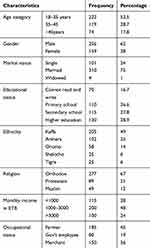 |
Table 1 Socio-Demographic Characteristic of the Respondents in Tepi General Hospital, Southwest Ethiopia, May – June 2019 (N=415) |
Knowledge of Participants Towards Cause, Symptoms, Transmissibility, Mode of Transmission, Prevention and Treatment of TB
Among the participants, 345 (83%) had heard information about TB. The main source of information for participants was mass media 236 (68.4%) followed by health-care workers 100 (29%) (Table 2). Regarding each knowledge item, only 41 (9.9%) study participants mentioned bacteria as the cause of the diseases (Figure 1), while 205 (49%) did not know the sign and symptoms of TB. One hundred seventy (41%) of participants responded the mode of transmission as through the air when a person with TB sneezes or cough, the majority (82%) said TB can be transmitted from person to person. The majority (70%) of participants responded that its transmission is not preventable, while 92 (22.4%) replayed that covering the mouth and nose while coughing or sneezing is a possible method to prevent the transmissions, 330 (79.5%) said it is curable, and majority (51%) mentioned the modern drugs as the means for curability (Table 3). The mean knowledge score was 9.4 and 236 (56.9%) of participants had scored above the mean and were considered as having high overall knowledge about TB (Figure 2).
 |
Table 2 Showing Participants’ Source of Information About TB in Tepi General Hospital, Southwestern Ethiopia, May –June 2019 (N=415) |
 |
Figure 1 Participants’ response to the causes of tuberculosis in Tepi general hospital, southwestern Ethiopia, May to June 2019 (N=415). |
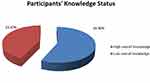 |
Figure 2 Participants’ knowledge status regarding tuberculosis in Tepi general hospital, southwestern Ethiopia, May - June 2019 (N=415). |
Attitude of Participants’ Towards Tuberculosis
The majority of participants (29%) considered TB as not very serious, 125 (30%) believed that TB is serious to their area, while the majority (45%) considered that they can get TB, and 146 (35%) were neutral for the disease is whether by God as a punishment (Table 4). In the present study, the mean attitude score was 12.6 with the minimum and maximum attitude scores of 5 and 20, respectively. Based on the mean attitude score, 221 (53.3%) of study participants were scored an attitude score above the mean and considered as having a favorable attitude towards TB (Figure 3).
 |
Table 4 Showing Participants’ Attitude Towards Tuberculosis in Tepi General Hospital, Southwestern Ethiopia, May–June 2019 (N=415) |
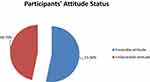 |
Figure 3 Participants’ attitude status regarding tuberculosis in Tepi general hospital, southwestern Ethiopia, May – June 2019 (N=415). |
Practice Towards in the Prevention of TB
The majority of study participants (71%) had a window at their home, amazingly from those having windows only 87 (30%) opened their window regularly, while 280 (67.5%) did not open car window during traveling and 330 (79.5%) were not screened for TB. More than three-fourth (82%) of participants were have not received any health education about TB and 352 (85%) respondents did not take care during coughing or sneezing (Table 5). In this study, the mean practice score was 1.79 with a minimum of 0 and a maximum of 5 practice scores. Two hundred thirty (55.4%) of study participants was scored below the mean practice score and were considered as having a poor practice about TB prevention (Figure 4).
 |
Table 5 Showing Participants’ Practice Towards Tuberculosis Prevention in Tepi General Hospital, Southwestern Ethiopia, May–June 2019 (N=415) |
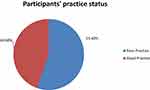 |
Figure 4 Participants’ practice status regarding tuberculosis in Tepi general hospital, south western Ethiopia, May – June 2019 (N=415). |
Discussion
The present study assessed knowledge, attitude, and practice towards TB. In this study majority (83%) of the participants had heard information about TB. As it was expected that, having information may raise the level of knowledge among participants, however, in this study about 56.9% of the participants had high overall knowledge about TB. The finding of this study regarding information about TB is nearly in line with studies done in the Mecha district20 where 87.8% of participants had information about TB. However, the present finding is lower than study done in Shinile town in which 94.2% had heard information about TB17 and lower Awash Valley of Afar region where 95.6% of participants had information about TB.21
In this study, 56.9% of study participants had high overall knowledge about TB. This finding is similar to studies from Mecha district (54%) and Shinile town (54.4%).20,21 This finding is also better than a study done in Thailand, where 25.8% had good knowledge regarding TB. However, this finding is lower than a study done in Iran in which 62% of the participants had good knowledge.22 The finding revealed that only 9.9% of participants knew the etiology of the diseases as Bacteria with the majority (25.1%) stating dust as the cause of TB. Knowing the exact cause of the disease is the baseline for having a positive attitude as well as for applying effective prevention methods. However, in this study, participants who mentioned the exact cause of TB are lower than the study conducted in Pakistan and Shinile town.21,23 The finding also depicted that 50.5% of participants answered the correct sign and symptoms of TB, more specifically 18.3% replayed sign and symptoms as cough more than 2 weeks, 19.5% stating sputum with blood while 10.8% mentioned as weight loss, 1.4% mentioned as loss of appetite and only 0.5% stated as fever and night sweat. However, the finding is not consistent with studies done in Shinile, northeast Ethiopia and Philippines.17,21,24 The possible explanation for this variation may be a lack of basic knowledge and information about the sign and symptoms of TB. Having information about the sign and symptoms of the disease is crucial for early treatment-seeking which could decrease the transmission in the community.
The study also founded that participants’ awareness about treatment and modern drugs as an option of treatment was good. This finding is in line with studies performed in Pakistan, and other parts of Ethiopia.12,17,23 However, participants’ preference for visiting traditional healers and praying as the option for treatment is considerable and it is in line with studies performed in rural and urban communities in Ethiopia.25 This should keep in mind that these practices are still bottlenecking for the prevention and control of TB in many parts of Ethiopia, like in the study area.
The study noted that 29% of study participants considered that TB is not very serious; however, 30% considered TB is serious to their area and 31% were neutral for their causality in getting TB. This misconception of TB, which is not very serious and neutral in being getting TB is dangerous and may alter their attitude in prevention practices and early getting treatment. These findings are contrary with studies done in another part of Ethiopia and Pakistan.17,23 Overall, participants with a favorable attitude towards TB in this study were 53.3% which were more or less similar to studies done in Thailand26 in which 47.9% had a high attitude level and below than study done in Mecha district, where 68% had high levels of attitude.20
Regarding the practice, from those who had a window in their home (71%), only 30% opened their window regularly and only 32.5% opened car window during traveling. These practices are bad in the area where TB is prevalent like in Ethiopia and particularly in the study area as these practices facilitate the transmission of the TB. The study also pointed out that only 20.5% had screened for TB. Such practice is not only limited to the study area as it was previously reported from studies done in the Mecha district (19.4%) and Thailand (18.6%).20,26 In the present study respondents who received health education was only 17.5% and who covered their mouth and nose during coughing were 15%. The finding regarding covering mouth and nose while sneezing or coughing is much lower than studies done in the Mecha district20 in which 65.5% practiced such behavior and Mongolia in which 42.9% covered their mouth and nose during coughing and sneezing.27 Overall, participants with good practice in the prevention of TB were 44.4%, which is in line with studies done in Iran in which 42.6% had a good practice.22 However, this finding is below than studies conducted in Mecha20 district where 48% had good preventive practices and Thailand in which 55.5% had good preventive practices.26
The study identified that the majority (82.5%) of the participants did not receive any health education about TB during their visit to the health facility. This could affect the participants’ habit in the prevention and control of TB and this finding is not supported by CDC as it suggests providing training and education for patients, community groups, and the general public is on of strategy in the prevention and control of TB.28 Again, not receiving health education could affect the treatment-seeking behavior of participants as the study suggests providing health education strategies like delivering basic information about signs/symptoms of TB to individual or group can shorten the diagnosis and delayed treatment which decreases the transmission rate in the community.29,30 Continuous education regarding some aspects of TB to clients visiting the health facility which may be in the morning before the beginning of routine hospital work is one of educational strategy in the prevention and control of TB.29,30,31
Limitations
The descriptive nature of the study could not explain the determinants which are affecting the knowledge, attitude, and practices towards TB prevention in study participants. The study is also conducted on clients who are visiting the health facility and it is not at the community level. The study also lacks in triangulating the deep insight of participants, especially in attitude and practices as the study is an only a quantitative part.
Conclusion
The study concluded that study participants had good knowledge and attitude towards TB. But this knowledge is not effectively seen in explaining the cause of TB and effective preventive mechanisms of TB. Hospital administrates and the health office of the town should strengthen the routine health education programs for clients visiting the hospital.
Abbreviations
ETB, Ethiopian Birr; CDC, Center for Disease Control; HIV, human immune deficiency virus; KAP, knowledge attitude practice; SPSS, statistical package for social sciences; TB, tuberculosis; TGH, Tepi General Hospital; WHO, World Health Organization.
Acknowledgments
We are very grateful to all study participants for their commitment in responding to questionnaires and all data collectors for their collaboration up to the end of the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Moscow Declaration to End TB. First WHO global ministerial conference on ending TB in the sustainable development era: a multispectral response. Geneva: World Health Organization and the Ministry of Health of the Russian Federation; 2017. Available from: https://www.who.int/tb/features_archive/Moscow_Declaration_to_End_TB_final_ENGLISH.pdf?ua=1.
2. Global tuberculosis report 2019. Geneva: World Health Organization; 2019.
3. World Health Organization: Global tuberculosis control: epidemiology, strategy, financing. WHO report. Geneva; 2009. Available from: http://whqlibdoc.who.int/publications/2009/9789241563802_eng_doc.pdf.
4. World Health Organization. Global tuberculosis report. Geneva: World Health Organization; 2016.
5. Hassmiller KM. The association between smoking and tuberculosis. Salud Publica Mex. 2006;48(1):201–216. doi:10.1590/S0036-36342006000700024
6. Cegielski JP, McMurray DN. The relationship between malnutrition and tuberculosis: evidences from studies in humans and experimental animals. Int J Tuberc Lung Dis. 2004;8:286–298.
7. Lienhardt C. From exposure to disease: the role of environmental factors in susceptibility to and development of tuberculosis. Epidemiol Rev. 2001;23:288–301. doi:10.1093/oxfordjournals.epirev.a000807
8. Cambanis A, Yassin AM, Ramsay A, Squire BS, Arbide I, Cuevas EL. Rural poverty and delayed presentation to tuberculosis services in Ethiopia. Trop Med Int Health. 2005;10(4):330–335. doi:10.1111/j.1365-3156.2005.01393.x
9. Demissie M, Lindtjorn B, Berhane Y. Patient and health service delay in the diagnosis of pulmonary tuberculosis in Ethiopia. BMC Public Health. 2002;2:23. doi:10.1186/1471-2458-2-23
10. Gele AA, Bjune G, Abebe F. Pastoralism and delay in diagnosis of TB in Ethiopia. BMC Public Health. 2009;9:5. doi:10.1186/1471-2458-9-5
11. Yimer S, Bjune G, Alene G. Diagnostic and treatment delay among pulmonary tuberculosis patients in Ethiopia, a cross sectional study. BMC Infect Dis. 2005;5:112. doi:10.1186/1471-2334-5-112
12. Melaku S, Sharma RH, Alemie AG. Pastoralist community’s perception of tuberculosis, a quantitative study from Shinille area of Ethiopia. Tuberc Res Treat. 2013.
13. Kaona FA, Tuba M, Siziya S, Sikaona L. An assessment of factors contributing to treatment adherence and knowledge of TB transmission among patients on TB treatment. BMC Public Health. 2004;29:68. doi:10.1186/1471-2458-4-68
14. Hill PC, Stevens W, Hill S. Risk factors for defaulting from tuberculosis treatment: a prospective cohort study of 301 cases in the Gambia. Int J Lung Dis. 2005;9:1349–1354.
15. Niehter M. Illness semantics and international health: the weak lungs/TB complex in the Philippines. Soc Sci Med. 1994;38:649–663. doi:10.1016/0277-9536(94)90456-1
16. Shimao T. Drug resistance in tuberculosis control. PUBMED Tubercle. 1987;68:5–15. doi:10.1016/S0041-3879(87)80014-4
17. Tolossa D, Medhin G, Legesse M. Community knowledge, attitude, and practices towards tuberculosis in Shinile town, Somali regional state, eastern Ethiopia: a cross-sectional study. BMC Public Health. 2014;14(804):1–13. doi:10.1186/1471-2458-14-804
18. Abebe G, Deribew A, Apers L, et al. Knowledge, health seeking behaviour and perceived stigma towards tuberculosis among tuberculosis suspects in a rural community in southwest Ethiopia. PLoS One. 2010;5:10. doi:10.1371/journal.pone.0013339
19. World Health Organization. Advocacy, communication and social mobilization for TB control: a guide to developing knowledge, attitude and practice surveys. Geneva: World Health Organization; 2008.
20. Ayele S, Alebachew M, Getasew M. Knowledge, attitude and preventive practice towards tuberculosis among clients visiting public health facilities. BMC Res Notes. 2019;12:276. doi:10.1186/s13104-019-4292-2
21. Legesse M, Ameni G, Mamo G, et al. Knowledge and perception of pulmonary tuberculosis in pastoral communities in the middle and lower Awash valley of Afar region, Ethiopia. BMC Public Health. 2010;10:187. doi:10.1186/1471-2458-10-187
22. Amiri FB, Doosti-Irani A, Sedaghat A, Fahimfar N, Mostafavi E. Original article knowledge, attitude, and practices regarding HIV and TB among homeless people in Tehran, Iran. Int J Health Policy Manag. 2017;6(x):1–7.
23. Khan AJ, Irfan M, Zaki A, Beg M, Hussain FS, Rizvi N. Knowledge, attitude and misconception regarding tuberculosis in Pakistani patients. J Pak Med Assoc. 2006;56:211.
24. Christina M, Domingo B, Lisa A. A descriptive study of the knowledge, attitude and practices on tuberculosis among treatment partners of pediatric patients in Tarlac city. PIDSP J. 2009;10:1.
25. Deribew A, Abebe G, Apers L, et al. Prejudice and misconceptions about tuberculosis and HIV in rural and urban communities in Ethiopia: a challenge for the TB/HIV control program. BMC Public Health. 2010;10:400. doi:10.1186/1471-2458-10-400
26. Sreechat S. Assessment of knowledge, attitude and preventive behavior of pulmonary tuberculosis among Myanmar refugees in Ban Mai Nai Soi temporary shelter, Mae Hong Son, Thailand. J Health Res. 2013;27(6):391–398.
27. Davaalkham D, Naranzul D, Chimedsuren O, et al. Knowledge, attitudes and practices on tuberculosis among general population: the nationwide study report. Ulaanbaatar; 2012.
28. Cole B, Nilsen DM, Will L, Etkind SC, Burgos M, Chorba T. Essential components of a public health tuberculosis prevention, control, and elimination program. Recommendations of the advisory council for the elimination of tuberculosis and the national tuberculosis controllers association. MMWR Recomm Rep. 2020;69(7):1–27. doi:10.15585/mmwr.rr6907a1
29. Verhagen LM, Kapinga R, Rosmalen-Nooijens KAWLV. Detection delay of pulmonary tuberculosis patients among migrants in China: a cross-sectional study. Infection. 2010;38:4436.
30. Sreramareddy CT, Panduru KV, Menten J, Ende JVD. Time delays in diagnosis pulmonary tuberculosis: a systematic review of literature. BMC Infect Dis. 2009;9:91. doi:10.1186/1471-2334-9-91
31. Ferreira V, Brito C, Portela M, Escosteguy C, Lima S. DOTS in primary care units in the city of Rio de Janeiro, Southeastern Brazil. Revista De Saúde Pública. 2011;45(1):40–48. doi:10.1590/S0034-89102010005000055
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

