Back to Journals » Open Access Emergency Medicine » Volume 12
Knowledge and Acceptability of Human Papillomavirus Vaccination and Text Message Reminders for Adolescents in Urban Emergency Departments: A Pilot Study
Authors Allison WE, Rubin A, Melhado TV, Choi A , Levine DA
Received 8 January 2020
Accepted for publication 25 March 2020
Published 2 June 2020 Volume 2020:12 Pages 145—153
DOI https://doi.org/10.2147/OAEM.S245221
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Waridibo E Allison,1 Ada Rubin,2 Trisha V Melhado,1 Aro Choi,1 Deborah A Levine2
1University of Texas Health Science Center San Antonio, Department of Medicine, Division of Infectious Disease, San Antonio, TX, USA; 2New York University, Ronald O. Perelman Department of Emergency Services, New York, NY, USA
Correspondence: Waridibo E Allison
Department of Medicine, Division of Infectious Disease, University of Texas Health Science Center San Antonio, 7703 Floyd Curl Drive, Mail Code 7881, San Antonio, TX 78229, USA
Tel +1 210 567 0939
Fax +1 210 567 4670
Email [email protected]
Purpose: Cervical, oropharyngeal and anogenital cancers are vaccine-preventable diseases, but human papillomavirus (HPV) vaccination coverage in the US remains poor overall with regional variations in vaccination rates. We explore the acceptability by adolescents and their parents of HPV vaccination and text message reminders in the non-traditional setting of the emergency department (ED).
Patients and Methods: The modified validated Carolina HPV Attitudes and Beliefs Scale (CHIAS) survey was administered at two urban EDs to adolescents aged 13– 18 years and their parents. Demographic information was collected for each participating adolescent. Recruitment occurred with consecutive eligible participants on the ED census list approached within 4-hour blocks from 8am to 8pm.
Results: Ninety-six adolescents completed the survey. The mean adolescent and parental knowledge scores were 63% (SD=29.7) and 60% (SD=22.1), respectively. The higher the HPV knowledge score among both adolescents and parents, the more likely they were to accept HPV vaccine in ED. Among the 10 cases where the parents disagreed to the HPV vaccine and the adolescents agreed to the HPV vaccine, the mean knowledge score among parents disagreeing was 47 compared to 62 among the remaining parents (p=0.04). Sixty-seven percent of adolescents and 68% of parents were agreeable to the adolescent receiving vaccination in the ED (kappa = 0.24). Seventy-five percent of adolescents and 71% of parents reported being agreeable to receiving text reminders for HPV vaccines (kappa = 0.20). Adolescent agreement with receiving a text message reminder corresponded with an increased willingness to be vaccinated (OR=3.21, 95% CI=1.07– 9.57, p-value=0.0368). Sexually active adolescents were older (mean age, 17 years) than those who reported no sexual activity (mean age, 15 years) (p< 0.0001).
Conclusion: Increased knowledge about HPV influences vaccine acceptance. Parents and adolescents may disagree in accepting HPV vaccination. A majority of adolescents and their parents were agreeable to receiving HPV vaccination in the ED and subsequent text message reminders. The ED should be explored further as a non-traditional healthcare setting for HPV vaccination of adolescents.
Keywords: adolescent, vaccination, vaccine, prevention, public health
Introduction
Genital human papilloma virus (HPV) infection is the most common sexually transmitted disease (STD) in the USA infecting approximately 79 million, mostly adolescent and young adult, Americans.1 HPV infection underlies the pathogenesis of cervical, oropharyngeal and anogenital cancers and genital warts.2 A prevalence rate of 33% has been reported in female teenagers in the US aged between 14 and 19 years.3 Data on the prevalence of HPV infection in adolescent boys in the USA are sparse though one study reports a prevalence rate of 12.7% in young men aged 18–20 years.4 HPV vaccination has been shown to reduce the incidence of high-grade cervical abnormalities in girls under 18 years old and the incidence of genital warts in both young men and women under 21 years old.5 Despite recommendations by the Centers for Disease Control and Prevention (CDC) and the American Academy of Pediatrics to immunize all 11–12-year-old children, HPV vaccination coverage in the US remains poor.6
The 2012 National Immunization Survey–Teen showed that of girls and boys aged 13–17 years, 33.4% and 6.8% respectively had completed the three-dose HPV vaccine series.6 Since October 2016 the CDC has recommended a two-dose HPV vaccination schedule for adolescents who start the vaccine series on or before their fifteenth birthday.7 For anyone older or who is immunocompromised three doses are still recommended. HPV vaccine coverage data in the US for 2017 varies from state to state. The highest coverage is the District of Columbia with a rate of 91.9%, the lowest is Wyoming with a rate of 46.9%.8
HPV vaccination has population-level effects with a 64% decrease in quadrivalent HPV vaccine type prevalence among females aged 14–19 years within 6 years of vaccine introduction in the US.9 Novel strategies are urgently needed to increase HPV vaccination coverage in children in the US. A comprehensive review of barriers to HPV vaccination among US adolescents suggested that providing opportunities for HPV vaccination in less traditional healthcare settings and using reminder and recall systems may facilitate vaccine series completion.10 We explore the non-traditional setting of the Emergency Department (ED) in New York City (NYC) as a setting for HPV vaccination of adolescents.
There are few published data available on routine childhood immunization in the ED and the data that do exist are out of date. A cohort study in 1994 showed minimal effect on compliance with primary care after immunization in the ED and noted that unreliable information from parents at the ED visit lead to unnecessary immunizations.11 This is less of an issue now in NYC with the existence of the Citywide Immunization Registry (CIR). Since 1997, all providers have been mandated to enter vaccines administered to all children <8 years of age into CIR. The age requirement was increased to 18 years old in 2005. A second study in 1996 found that an urban ED immunization program only temporarily improved immunization rates of the ED population.12
Text messaging is growing in potential as a vehicle for health promotion given the advantages of low cost, ease of use and high accessibility to large numbers of healthcare consumers.13 Adolescents are learning to navigate their own health care and other studies have investigated the acceptability and effectiveness of text messages sent to adolescents in the context of sexual health promotion.14,15
We describe demographics, knowledge about HPV and HPV vaccine coverage among adolescents aged 13–18 years and their parents/guardians presenting to two urban Emergency Departments and describe the willingness of these adolescents to receive a first dose of HPV vaccine followed by text messaging reminders to return to a primary care clinic for a subsequent dose. Note that hereafter parents/guardians will be referred to as parents while recognizing that an adult caregiver who is not a parent could have accompanied the adolescent to the ED.
Patients and Methods
This study utilized a cross-sectional design and was carried out at two pediatric emergency departments in New York City: NYC Health+ Hospitals/Bellevue Pediatric Emergency Service and the KiDS Emergency Department within the New York University (NYU) Ronald O. Perelman Center for Emergency Services. Inclusion criteria were all adolescents aged 13–18 years presenting to the study sites. Exclusion criteria were: parent refusal to participate when approached, refusal of adolescent to participate regardless of parental consent, adolescents critically ill at presentation (ie triage category I or II), and adolescents with a mental health presenting complaint. Participants were able to withdraw from the study at any time.
Informed consent was obtained from parents and an assent form was signed by adolescents. Recruited adolescents and their parent took part in a researcher-administered questionnaire based on the validated Carolina HPV Attitudes and Beliefs Scale (CHIAS).16,17 Additionally, demographic information was collected for each participating adolescent.
This was a convenience sample with participants approached and recruited from the ED between 0800 and 2000 in 4-hour blocks from 0800 to 1200, 1200 to 1600, 1600 to 2000. Consecutive participant counts occurred from the departmental patient white board census as it stood at the beginning of the recruitment time block. A maximum of 8 patients were recruited per 4-hour block in order to not affect ED patient flow with study activities. Parents of all adolescents approached and older adolescents aged 17–18 years were given a copy of the CDC HPV Vaccine information statement. A telephone interpreter was available for non-English language translation.
The patient survey questions consisted of demographic questions including age, sex, insurance type, place of birth, and the primary language of the patient and parent. There were also five knowledge-based questions centering around who qualifies for the HPV vaccine and short-term side effects of the vaccine. Additional questions assessed whether the adolescent had previous discussions about HPV vaccination, engaged in sexual activity, and knew of the HPV vaccination status of friends. For the parental questionnaire, there were nine HPV vaccine knowledge questions in addition to questions related to the adolescent’s vaccination status, whether the parent had heard of the HPV vaccine, vaccine cost and access, and vaccination of other children in the community. Final questions in both the adolescent and parental survey pertained to cell phone use and the willingness to be texted by a physician for vaccination reminders. The full survey questionnaire is provided as supplementary material.
There were two outcomes of interest. Willingness to receive vaccination in the ED and willingness to receive vaccination reminders via text message. Whether the adolescent and/or their parent were willing to have the adolescent receive the HPV vaccine in the ED, was assessed separately. Adolescents were asked, “if you were offered an HPV vaccine shot today and your parent agreed would you have it done?” while parents were asked, “if your child was due a vaccine shot and it was offered in the ED today would you get it done?” Survey response categories were yes, no, and not sure. To simplify the responses, no and not sure categories were combined to give a dichotomous outcome variable. To assess willingness to receive text reminders about vaccinations, both adolescents and their parent were asked if they had a cell phone and how they felt about text message reminders for vaccinations.
Descriptive statistics detailed adolescent characteristics. Overall mean knowledge scores were calculated for the survey questions. Adolescent and parental agreement with willingness to have the adolescent receive the HPV vaccine in the ED and with willingness to receive text reminders were assessed using the kappa statistic. Moreover, a T-test was used to assess association of mean knowledge scores with willingness to have the HPV vaccine and separately with willingness to receive text reminders. A chi-square test was used to assess if there were differences in correct responses to knowledge assessment questions between adolescents and parents. Predictors of willingness to have the adolescent receive the HPV vaccine in the ED and to receive a text message were identified by logistic regression. Cell phone use was assessed descriptively for adolescents. Finally, a missing data analysis was conducted to identify the characteristics of patients whose parents did not complete the survey, using two-sample T-tests and a chi-square test.
There are different methods to determine the sample size for a pilot study and no consensus on which method should be used. We chose the approach of a pilot study sample size of 10% of the estimated final study size.18 A final study size was estimated as follows: NYC Health+ Hospitals/Bellevue Emergency Department sees approximately 25,000 children and young adults per year. KiDS Emergency Department within the NYU Ronald O. Perelman Center for Emergency Services opened in 2014 with no patient numbers available at the time of study planning; therefore, an estimate of 18,000 children seen there per year was made. This gave a total of 43,000 children from 0 to 18 years old presenting to the study sites per year. Based on data from the United States Census Bureau for New York City,19 approximately 5% of these children will be aged 13–18 years giving a population size of 2150 rounded up to 2200. Using a confidence level of 95% and a confidence interval (margin of error) of 0.075 and a conservative estimate of variance of 50% (0.5) the calculated minimum sample size was 159. At 10% of the final study size, a minimum pilot study sample size of 16 was required. Study data were collected on paper and entered into Research Electronic Data Capture (REDCap), a secure, web-based database software application.20 All analyses were conducted using Statistical Analysis Software (SAS) version 9.4 (Cary, NC: SAS Institute Inc.). Institutional Review Board (IRB) approvals were obtained from NYU Langone Health IRB and NYC Health+ Hospitals/Bellevue IRB and informed consent was obtained as required.
Results
Ninety-six adolescents completed the survey between 12 December 2015 and 29 September 2016. Table 1 shows adolescent demographics. The mean age (SD) of participating adolescents was 15.9 (1.8) years with most responders being female (59%), having Medicaid (59%), being born in the US (84%), speaking English (76%) and having Spanish-speaking parents (38%).
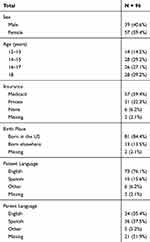 |
Table 1 Characteristics of Participating Adolescents |
The knowledge score ranges were from 0% to 100% for both adolescents and parents. The mean adolescent knowledge score was 63% (SD=29.7) and the mean knowledge score for the parents was 60% (SD=22.1). Figure 1 shows that agreement to receive HPV vaccine had a higher mean knowledge score than disagreement to HPV vaccination for both adolescents (p=0.01) and parents (p=0.02). Two knowledge questions were associated with agreeing to receive the vaccine among adolescents: whether one is too young to receive the vaccine (p=0.01) and whether the HPV vaccine is only for younger people (p=0.01) (Table 2). The following correctly answered parental responses were associated with agreeing with the HPV vaccine for their adolescent children: HPV vaccine might cause future health problems (p=0.03), HPV vaccine is effective in preventing genital warts (p=0.01) and HPV vaccine is effective in preventing cervical cancer (p=0.02) (Table 3). Sixty-seven percent of adolescents and 68% of parents reported that they were agreeable to the adolescent receiving vaccination in the ED (kappa = 0.24). There were 10 cases where the parents disagreed to the HPV vaccine and the adolescents agreed. The mean knowledge score among parents disagreeing was 47 compared to 62 among the remaining parents (p=0.04). The mean of knowledge score of those 10 adolescents who agreed to receiving HPV, but their parents disagreed was not significantly different from the knowledge score of the rest of adolescents who participated in knowledge assessment.
 |
Table 2 Association of Correct HPV Question Answers with Adolescent Acceptance of HPV Vaccine |
 |
Table 3 Association of Correct HPV Question Answers with Parental Acceptance of HPV Vaccine |
 |
Figure 1 Mean knowledge score by HPV acceptance. |
Adolescent predictors of willingness to receive the HPV vaccine in the ED included their knowledge score, with an increase in score corresponding with an increase in willingness to be vaccinated (OR=1.02, 95% CI=1.01, 2.16, p=0.0102) and agreement with receiving a text message from their physician corresponding with an increase in willingness to be vaccinated (OR=3.21, 95% CI=1.07–9.57, p-value=0.0368). Predictors of parent willingness to have their child vaccinated included whether they had enough information about HPV, with parents who reported not having enough knowledge less likely to agree to have their child vaccinated (OR=0.18, 95% CI=0.04–0.78, p-value=0.0216). Additionally, parents who were agreeable to receiving text messages were also agreeable to their child receiving the vaccine (OR=11.5, 95% CI=2.25–58.8, p-value=0.0034).
Seventy-five percent of adolescents and 71% of parents reported that they were agreeable to receiving text message reminders regarding vaccination (kappa = 0.20). There were no statistically significant differences among knowledge scores and the subset of parents/adolescents who disagreed to receiving text messages. Overall, there was no statistically significant difference among adolescents agreeing to receive text messages and their mean knowledge scores; the same was true for parents. However, parents who correctly answered questions about the HPV vaccine causing future health problems and that HPV vaccine is not as necessary as a Pap smear were associated with agreeing to receiving text reminders (p=0.02 and 0.05, respectively - Table 4). Predictors of whether an adolescent was agreeable to receiving text reminders included age whereby as age increased, willingness to receive a text reminder decreased (OR=0.66, 95% CI=0.44–0.99, p-value=0.0475) and, whether an interpreter was used, with patients who used an interpreter less keen on being contacted with a text message reminder (OR=0.10, 95% CI=0.02–0.065, p-value=0.0156). Also, adolescents who had not had anyone talk to them about the HPV vaccine were more likely to agree to text messages reminders than those who had conversations about the HPV vaccine (OR=7.02, 95% CI=1.88–26.23, p-value=0.0038). Ninety percent of adolescents had a cell phone and reported between 118 and 130 text messages a day being sent or received.
 |
Table 4 Assocation of Correct HPV Question Answers with Parental Acceptance of Text Message Reminders |
Parental data were consistently missing for adolescents that reported sexual activity and for older adolescents. Specifically, patients who reported sexual activity were older (mean age 17 years) than patients who reported no sexual activity (mean age 15 years), p<0.0001. This also corresponded with a missing parental survey with all older adolescents with a mean age of 17 years not having a parent survey completed and younger adolescents with a mean age of 15 having one completed (p<0.0001).
Discussion
Uptake of HPV vaccination remains poor overall across the USA. As part of their HPV Cancer Free campaign, The American Cancer Society has a goal that by 2026 80% of adolescents will be up to date with HPV vaccination before their thirteenth birthday.21 The majority of states have not achieved this goal. A recent study estimated that at least 14.39 million additional adolescents aged 11–12 years would need to receive two doses of the HPV vaccine to reach this goal.22 Continued efforts are needed to find innovative ways to improve HPV vaccination rates for adolescents in the United States. The ED is often, for a variety of reasons used by parents for non-urgent care of their children23,24 and an ED presentation may represent an opportunity for HPV vaccination.
We found that the higher the HPV knowledge score among both adolescents and parents, the more likely they were to accept HPV vaccination in ED. The correct age for vaccination and misconceptions about sexual activity were not shown as gaps in knowledge. However, just over half the parents thought that HPV vaccination could cause health problems in the future and 70% of them thought that it could cause side effects. Vaccine safety was therefore a concern with parents and this may be a barrier to vaccine uptake.
While the majority of adolescents and parents were agreeable to HPV vaccination in the ED, the predictors of willingness to receive HPV vaccination in this setting were different. The willingness of adolescents to receive the HPV vaccine in the ED was predicted by their willingness to receiving text reminders. Additionally, as their HPV knowledge score increased so did their odds of accepting the HPV vaccine in the ED.
Parental agreement to their children receiving HPV vaccination in the ED was predicted by having HPV information and also willingness to receiving text reminders. Predicators of both adolescents and their parents being agreeable to receiving a text message reminder for HPV vaccination were younger age and not having someone who had previously talked to them about the vaccine. Mobile phone ownership is widespread and text messaging is popular among teenagers. One survey showed that 95% of young adults aged 18–24 years in the United States own mobile phones and send/receive an average of 50 messages per day, compared to adults sending and receiving an average of 10 messages per day.25 Our findings were similar with 9/10 of adolescents having a cell phone. Text messaging volume was, however, much higher with between 118 and 130 text messages a day being sent or received. This heavy use of text messaging can be leveraged for text message reminders about vaccinations, particularly with older adolescents who are starting to take more ownership of their healthcare needs. Our finding that parental data was consistently missing (parents elected not to fill the survey information) with both older adolescents and sexually active adolescents could indicate that parents in these circumstances are also starting to “let go” with respect to health care for their children. Text messaging has been shown by two studies to be an effective intervention to increase HPV vaccine uptake adolescent and young adults presenting to a clinic setting. In one study parents received up to three weekly reminders that a vaccine dose was due.26 In another study, although adolescents were the main target for intervention, more parents than adolescents elected to receive the text reminders.27 There are no data on text message reminders and its effect specifically on HPV vaccine uptake following first dose vaccination in the ED.
Our pilot study shows that in the Emergency Department, certainly a less traditional healthcare setting for childhood immunization, an initial HPV vaccine followed by text message reminders for subsequent doses would be acceptable for adolescents and their parents. The content of the health intervention text message can influence its effectiveness. A study using focus groups of seventh graders with an average age of 13 years examined text message preferences about HPV vaccination.28 It was found that the best composite score for likeability, trustworthiness and motivation to seek more information was for a message emphasizing reduction in HPV infection if vaccinated. Messages emphasizing threats of disease if not vaccinated scored lower. A recent randomized control trial found that embedding health literacy information improved the effectiveness of text message reminders for a second dose of influenza vaccine for children in a low-income, minority community in New York City.29 If routine HPV vaccination is to be initiated in an ED with follow-up text messaging reminders, then care will have to be taken with the content of the text message reminders to ensure maximal effectiveness.
It was found that there was a discordance between parents and their adolescent children with respect to HPV vaccine acceptance and text message reminder acceptance. It is not clear what the reason for this discordance is. It is possible that a contributing factor may be the generational gap that often exists between parents and their children or differences that come with adolescents being born and raised in the US in contrast to their parents. This is not unlikely in a multicultural location like New York city and though place of birth data were not collected, 38% of parents were Spanish speaking.
The role of the clinician in improving vaccine uptake cannot be underestimated and a clinician recommendation is one of the most important determinants of HPV vaccine uptake.10 It was interestingly noted in a recent study that the lack of school entry requirements for HPV vaccination may contribute to decreased vaccine uptake.30 Conflicting messages were given by the clinicians interviewed with strong recommendations given by emphasizing the importance of the vaccine for cancer prevention degraded by the lack of urgency suggested by stating that the vaccine is not needed to attend school. Only three states have school entry HPV vaccine requirements and for two out of the three the requirements are only for female adolescents. As such, not focusing on school entry requirements may be beneficial in discussions about HPV vaccination. Increasing HPV vaccination in adolescents across the USA will require a multipronged approach targeting not just barriers for the adolescents and their parents but also barriers for clinicians and barriers at the health and education system levels.
This study is limited by a potential lack of generalizability. Though data were collected at two emergency departments, they are located in one city in a single state. Furthermore, findings may not be generalizable to emergency departments in more rural and remote locations in the US. A second limitation is the dependence of self-reported data on the participant understanding of the question being asked and the completeness and integrity of participant responses.31 This was mitigated by the more time-consuming and labor-intensive process of questionnaire administration by researchers in an interview format. However, even with this approach, there are still missing data. A final limitation is inherent in the cross-sectional study design meaning that there may be alternate explanations for the study findings.32 This limitation is usually addressed by comparing data to that collected in similar studies in other institutions. However, there is a paucity of published data on HPV vaccination in the ED setting and this comparison was not possible.
Conclusion
While the ED setting has been explored for other vaccine-preventable disease such as influenza, it has not been explored as an option for HPV vaccination. We show in this pilot study that receipt of HPV vaccination is acceptable to both adolescents and their parents in the ED. This approach, combined with text message reminders for subsequent vaccine doses by primary care providers which were also found to be acceptable, warrants further investigation. The ED can be a setting for public health interventions and is a potential non-traditional setting to be considered in efforts to increase HPV vaccination rates in adolescents in the US.
Acknowledgments
Thanks are extended to the volunteer research assistants who participate in Project Healthcare, a student volunteer program of the NYU Ronald O. Perelman Department of Emergency Medicine for assistance in recruitment of patients and study questionnaire administration.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Centers for Disease Control and Prevention (CDC). Genital HPV infection - CDC fact sheet. Available from: https://www.cdc.gov/std/hpv/HPV-FS-July-2017.pdf.
2. IARC Working. Group on the evaluation of carcinogenic risks to humans. Human papillomaviruses. IARC Monogr Eval Carcinog Risks Hum. 2007;90:1–636.
3. Hariri S, Unger ER, Sternberg M, et al. Prevalence of genital human papillomavirus among females in the United States, the National Health And Nutrition Examination Survey, 2003–2006. J Infect Dis. 2011;204(4):566–573. doi:10.1093/infdis/jir341
4. Giuliano AR, Lu B, Nielson CM, et al. Age-specific prevalence, incidence, and duration of human papillomavirus infections in a cohort of 290 US men. J Infect Dis. 2008;198(6):827–835. doi:10.1086/591095
5. Ali H, Donovan B, Wand H, et al. Genital warts in young Australians five years into national human papillomavirus vaccination programme: national surveillance data. BMJ. 2013;346:f2032.
6. Centers for Disease Control and Prevention (CDC). National and state vaccination coverage among adolescents aged 13–17 years–United States, 2012. Morb Mortal Wkly Rep. 2013;62(34):685–693.
7. Centers for Disease Control and Prevention (CDC). HPV vaccine schedule and dosing. Available from: https://www.cdc.gov/hpv/hcp/schedules-recommendations.html.
8. Centers for Disease Control and Prevention (CDC). Results for adolescent HPV vaccination coverage. Available from: https://www.cdc.gov/vaccines/imz-managers/coverage/teenvaxview/data-reports/hpv/index.html.
9. Markowitz LE, Liu G, Hariri S, Steinau M, Dunne EF, Unger ER. Prevalence of HPV after introduction of the vaccination program in the United States. Pediatrics. 2016;137(3):e20151968. doi:10.1542/peds.2015-1968
10. Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among US adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76–82. doi:10.1001/jamapediatrics.2013.2752
11. Joffe MD, Luberti A. Effect of emergency department immunization on compliance with primary care. Pediatr Emerg Care. 1994;10(6):317–319. doi:10.1097/00006565-199412000-00002
12. Szilagyi PG, Rodewald LE, Humiston SG, et al. Effect of 2 urban emergency department immunization programs on childhood immunization rates. Arch Pediatr Adolesc Med. 1997;151(10):999–1006. doi:10.1001/archpedi.1997.02170470033007
13. Head KJ, Noar SM, Iannarino NT, Grant Harrington N. Efficacy of text messaging-based interventions for health promotion: a meta-analysis. Soc Sci Med. 2013;97:41–48. doi:10.1016/j.socscimed.2013.08.003
14. Gold J, MS L, Hocking JS, Keogh LA, Spelman T, Hellard ME. Determining the impact of text messaging for sexual health promotion to young people. Sex Transm Dis. 2011;38(4):247–252. doi:10.1097/OLQ.0b013e3181f68d7b
15. Perry RC, Kayekjian KC, Braun RA, Cantu M, Sheoran B, Chung PJ. Adolescents’ perspectives on the use of a text messaging service for preventive sexual health promotion. J Adolesc Health. 2012;51(3):220–225. doi:10.1016/j.jadohealth.2011.11.012
16. Dempsey AF, Fuhrel-Forbis A, Konrath S. Use of the Carolina HPV Immunization Attitudes and Beliefs Scale (CHIAS) in young adult women. PLoS One. 2014;9(6):e100193. doi:10.1371/journal.pone.0100193
17. Gowda C, Carlos RC, Butchart AT, et al. CHIAS: a standardized measure of parental HPV immunization attitudes and beliefs and its associations with vaccine uptake. Sex Transm Dis. 2012;39(6):475–481. doi:10.1097/OLQ.0b013e318248a6d5
18. Hertzog MA. Considerations in determining sample size for pilot studies. Res Nurs Health. 2008;31(2):180–191. doi:10.1002/nur.20247
19. United States Census Bureau. Annual estimates of the resident population for selected age groups by sex for the United States, States, Counties, and Puerto Rico Commonwealth and Municipios: april 1, 2010 to July 1, 2013. Available from: http://factfinder.census.gov/faces/tableservices/jsf/pages/productview.xhtml?pid=PEP_2013_PEPAGESEX&prodType=table.
20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. doi:10.1016/j.jbi.2008.08.010
21. American Cancer Society. Press release - American Cancer Society Launches Campaign to Eliminate Cervical Cancer. Available from: http://pressroom.cancer.org/HPVcancerfreelaunch.
22. Fedewa SA, Preiss AJ, Fisher-Borne M, Goding Sauer A, Jemal A, Saslow D. Reaching 80% human papillomavirus vaccination prevalence by 2026: how many adolescents need to be vaccinated and what are their characteristics? Cancer. 2018;124(24):4720–4730. doi:10.1002/cncr.31763
23. Costet Wong A, Claudet I, Sorum P, Mullet E. Why do parents bring their children to the emergency department? A systematic inventory of motives. Int J Family Med. 2015;2015:978412. doi:10.1155/2015/978412
24. Smith V, Mustafa M, Grafstein E, Doan Q. Factors influencing the decision to attend a pediatric emergency department for nonemergent complaints. Pediatr Emerg Care. 2015;31(9):640–644. doi:10.1097/PEC.0000000000000392
25. Pew Internet and American Life Project. Americans and text messaging. Available from: https://www.pewinternet.org/2011/09/19/americans-and-text-messaging/.
26. Kharbanda EO, Stockwell MS, Fox HW, Andres R, Lara M, Rickert VI. Text message reminders to promote human papillomavirus vaccination. Vaccine. 2011;29(14):2537–2541. doi:10.1016/j.vaccine.2011.01.065
27. Matheson EC, Derouin A, Gagliano M, Thompson JA, Blood-Siegfried J. Increasing HPV vaccination series completion rates via text message reminders. J Pediatr Health Care. 2014;28(4):e35–e39. doi:10.1016/j.pedhc.2013.09.001
28. Cates JR, Ortiz RR, North S, Martin A, Smith R, Coyne-Beasley T. Partnering with middle school students to design text messages about HPV vaccination. Health Promot Pract. 2014.
29. Stockwell MS, Hofstetter AM, DuRivage N, et al. Text message reminders for second dose of influenza vaccine: a randomized controlled trial. Pediatrics. 2015;135(1):e83–e91. doi:10.1542/peds.2014-2475
30. Niccolai LM, North AL, Footman A, Hansen CE. Lack of school requirements and clinician recommendations for human papillomavirus vaccination. J Public Health Res. 2018;7(1):1324. doi:10.4081/jphr.2018.1324
31. Passmore C, Dobbie AE, Parchman M, Tysinger J. Guidelines for constructing a survey. Fam Med. 2002;34(4):281–286.
32. Carlson MD, Morrison RS. Study design, precision, and validity in observational studies. J Palliat Med. 2009;12(1):77–82. doi:10.1089/jpm.2008.9690
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
