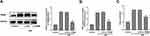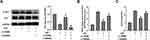Back to Journals » Drug Design, Development and Therapy » Volume 15
Knockdown of TRIM8 Protects HK-2 Cells Against Hypoxia/Reoxygenation-Induced Injury by Inhibiting Oxidative Stress-Mediated Apoptosis and Pyroptosis via PI3K/Akt Signal Pathway
Authors Zhang BH , Liu H , Yuan Y, Weng XD, Du Y , Chen H, Chen ZY, Wang L, Liu XH
Received 9 August 2021
Accepted for publication 30 November 2021
Published 10 December 2021 Volume 2021:15 Pages 4973—4983
DOI https://doi.org/10.2147/DDDT.S333372
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Manfred Ogris
Bang-Hua Zhang,1,2 Hao Liu,1,2 Yan Yuan,1,2 Xiao-Dong Weng,1 Yang Du,1 Hui Chen,1 Zhi-Yuan Chen,1 Lei Wang,1 Xiu-Heng Liu1
1Department of Urology, Renmin Hospital of Wuhan University, Wuhan, Hubei, People’s Republic of China; 2Hubei Key Laboratory of Digestive System Disease, Wuhan, Hubei, People’s Republic of China
Correspondence: Xiu-Heng Liu; Lei Wang
Department of Urology, Renmin Hospital of Wuhan University, No. 238 Jiefang Road, Wuhan, 430060, Hubei, People’s Republic of China
Tel/Fax +86 27 8804 1911
Email [email protected]; [email protected]
Background: Acute kidney injury (AKI) emerges as an acute and critical disease. Tripartite motif 8 (TRIM8), one number of the TRIM protein family, is proved to participate in ischemia/reperfusion (I/R) injury. However, whether TRIM8 is involved in renal I/R injury and the associated mechanisms are currently unclear.
Purpose: This study aimed to investigate the precise role of TRIM8 and relevant mechanisms in renal I/R injury.
Materials and Methods: In this study, human renal proximal tubular epithelial cells (HK-2 cells) underwent 12 hours of hypoxia and 2 h, 3 h or 4 h of reoxygenation to establish an in vitro hypoxia/reoxygenation (H/R) model. The siRNAs specific to TRIM8 (si-TRIM8) were transfected into HK-2 cells to knockdown TRIM8. The cell H/R model included various groups including Control, H/R, H/R+DMSO, H/R+NAC, si-NC+H/R, si-TRIM8+H/R and si-TRIM8+LY294002+H/R. The cell viability and levels of reactive oxygen species (ROS), hydrogen peroxide (H2O2), mRNA, apoptotic proteins, pyroptosis-related proteins and PI3K/AKT pathway-associated proteins were assessed.
Results: In vitro, realtime-quantitative PCR and western-blot analysis showed that the mRNA and protein expression of TRIM8 were obviously upregulated after H/R treatment in HK-2 cells. Compared with the H/R model group, knockdown of TRIM8 significantly increased cell viability and reduced the levels of ROS, H2O2, apoptotic proteins (Cleaved caspasebase-3 and BAX) and pyroptosis-related proteins (NLRP3, ASC, Caspase-1, Caspase-11, IL-1β and GSDMD-N). Western-blot analysis also authenticated that PI3K/AKT pathway was activated after TRIM8 inhibition. The application of 5 mM N-acetyl-cysteine, one highly efficient ROS inhibitor, significantly suppressed the expression of apoptotic proteins and pyroptosis-related proteins. Moreover, the combined treatment of TRIM8 knockdown and LY294002 reversed the effects of inhibiting oxidative stress.
Conclusion: Knockdown of TRIM8 can alleviate H/R-induced oxidative stress by triggering the PI3K/AKT pathway, thus attenuating pyropyosis and apoptosis in vitro.
Keywords: TRIM8, renal I/R injury, oxidative stress, apoptosis, pyroptosis
Introduction
Acute kidney injury (AKI) has high incidence and mortality, which emerges as an acute and critical disease. Moreover, AKI is often the result from kidney transplantation, sepsis, nephrectomy, nephrotoxicity, hypovolemic conditions.1,2 Among these, renal ischemia/reperfusion (I/R) injury is one of the most common cause of AKI.3 The occurrence and progress of renal I/R injury are connected with a variety of molecular events, such as ion accumulation, mitochondrial dysfunction, activation of oxidative stress, inflammation, and apoptosis.2,4 Although numerous cellular activities are associated with renal I/R injury, reactive oxygen species (ROS) are proved to be crucial mediators, as the enhancement of ROS scavenging can relieve renal I/R injury.5 Previous work has illustrated that alleviation of oxidative stress is able to mitigate renal I/R injury.6,7 Therefore, it is increasingly meaningful to find strategies to reduce renal I/R injury-generated oxidative stress.
Tripartite motif 8 (TRIM8) interacts with and ubiquitinates a great deal of substrates,8 and it is involved in modulating extensive biological processes including immune response, cell survival and differentiation.9,10 Besides, TRIM8 is mainly expressed in the kidney, gut and central nervous system.11 Studies testified that severity of insulin resistance and hepatic steatosis was increased along with the overexpression of TRIM8.12 Up-regulation of TRIM8 exaggerated the progression of cardiac hypertrophy and enhanced inflammatory response in Pseudomonas aeruginosa-induced keratitis.13,14 Interestingly, some evidence suggested that TRIM8 inhibition mediated beneficial effects in I/R-related diseases and had a critical function in regulating oxidative stress. TRIM8 deficiency relieved hepatic I/R-induced damage and restrained I/R-mediated cerebral injury.15,16 Downregulation of TRIM8 contributed to the elimination of intracellular ROS generation and apoptosis in oxygen-glucose deprivation/re-oxygenation-induced HT22 neurons.17 Moreover, the decrease of TRIM8 expression resulted in the activated PI3K/Akt signaling pathway to protect H9c2 cells against H/R-induced oxidative stress and apoptosis.18 Despite the intensive research of TRIM8 on various pathological processes, the role of TRIM8 in renal I/R injury remains unclarified. According to these findings, we speculated that TRIM8 could regulate renal I/R injury. Herein, this study was aimed to investigate the underlying mechanism of TRIM8 in regulating H/R-induced injury in HK-2 cells, which were often used to build in vitro renal I/R models.19,20
Materials and Methods
Antibodies and Reagents
N-acetyl-cysteine (NAC) was purchased from Sigma–Aldrich (St. Louis, MO, USA). LY294002 was supplied by Cell Signaling Technology (Danvers, MA, USA). The rabbit anti-TRIM8 and anti-GAPDH were obtained from Santa Cruz Biotechnology (Santa Cruz, CA, USA). These antibodies including Cleaved caspase-3, BAX, AKT, p-AKT, PI3K and p-PI3K were purchased from Cell Signaling Technology (Danvers, MA, USA), while antibodies against Bcl-2 and IL-1β were obtained from Abcam (Cambridge, UK). Primary antibodies against NLRP3, Caspase-11 and Caspase-1 and ASC and GSDMD-N were supplied by Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell Culture
Human renal proximal tubular epithelial cell line (HK-2 cell line) was provided by China Center for Type Culture Collection (CCTCC, China). The HK-2 cells were incubated in plates and cultured in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, USA) containing 5% fetal bovine serum, nonessential amino acids, 50 ng/mL human recombinant epidermal growth factor, 50ng/mL bovine pituitary extract, 100μg/mL streptomycin, 100 U/mL penicillin. The parameters of the incubator were set to 5% CO2, 95% air and 37°C.
Knockdown of TRIM8
The siRNAs specific to TRIM8 (si-TRIM8) were provided by GenePharma in Shanghai. According to the manufacturer’s instructions, Lipofectamine™ 3000 kit (Invitrogen) was used to transfect 100μM si-TRIM8 into HK-2 cells for experiments. For the accuracy of the transfection, negative control siRNA was transfected into HK-2 cells in si-NC group for 36 h. After completing the above operation, we incubated the cells in the DMEM/F12 medium containing 5% FBS for 36h. Western-blot analysis detected the level of target protein in the si-TRIM8 group to verify the effect of siRNA transfection. The sequence of siRNAs specific to TRIM8 and Negative control (listed below): TRIM8: 5′-GCAAGCACUCAACGGCCUUTT-3′(sense), 5′-AAGGCCGUUGAGUGCUUGCTT-3′(antisense); Negative control: UUCUCCGAACGUGUCACGUTT-ACGUGACACGUUCGGAGAATT.
H/R Model
The HK-2 cells were treated with 12 h hypoxic incubation in hypoxic incubator (94% N2, 1% O2 and 5% CO2) without nutrients (glucose-free, serum-free) to construct the H/R model, which is an in vitro renal I/R model.21 Then, treated cells were transferred into refreshed medium to accomplish 2 h, 3 h and 4 h of reoxygenation under normoxic conditions (95% air and 5% CO2). Adequate nutrition and normoxic environment were always maintained in the control group as mentioned above.
Real Time-Quantitative PCR (RT-qPCR)
RNA extraction kit (Applied Biosystems, Foster, CA, USA) was used to extract total RNA in HK-2 cells. Reverse transcription of the extracted RNA was performed by a PrimeScript™ RT Reagent Kit. GAPDH served as internal control in the process of real-time PCR reactions. The following evaluation was completed through the operation of ABIViiA7DX System (Foster City, CA, USA). The specific primer of target genes (listed below):
GAPDH: 5′-GTCTCCTCTGACTTCAACAGCG- 3′(F),
5′-ACCACCCTGTTGCTGTAGCCAA-3′(R)
TRIM8: 5′-AAGACGGAGGATGTCAGCTTC- 3′(F)
5′-GTGGCCGATCTTAGTGGGG- 3′(R)
Western Blot
Proteins in HK-2 cells were extracted according to standard operating procedures. Subsequently, protein concentrations could be assessed by the BCA Protein Assay kit (Abcam, Cambridge, UK). Same amounts of proteins (40μg) subjected to separation through the procedure of 10% SDS-PAGE and then were transferred electrophoretically to PVDF membranes. The next experimental step was to block membranes with Protein Free Rapid Blocking Buffer (5×) (Servicebio Technology, Wuhan, China) for 2 h at 37°C according to instructions. For protein detection, the membranes demanded overnight incubation at 4°C with primary antibodies against TRIM8 (1:500), Cleaved caspase-3 (1:1000), BAX (1:1000), Bcl-2 (1:1000), NLRP3 (1:600), Caspase-11 (1:500), Caspase-1 (1:500), IL-1β (1:1000), ASC (1:200), GSDMD-N (1:600), AKT (1:1000), p-AKT (1:200), PI3K (1:1000), p-PI3K (1:1000) and GAPDH (1:500). After incubation with secondary antibody for 2 h at 37°C, TBST buffer was configured to flush membranes so as to reduce non-specific binding. Then, the Western blots were visualized through the Chemiluminescent HRP Substrate. The data analysis was performed using Image Software (NIH, USA) to quantify the protein levels.
Cell Viability Assay
Cell viability was detected by the CCK-8 Assay Kit (Jiancheng, Nanjing, China). The HK-2 cells in 96-well plates were at a density of 5×103 cells/well, followed by supplement of 10μL of CCK-8 reagent per well. After three hours of incubation in the incubator at 37°C, these samples were taken to measure absorbance at 450 nm by a PerkinElmer Microplate reader. Optical density measurements of the different groups were used to measure cell viability. The results were determined as follows: (Treatment group/Control group) × 100%.
Measurement of Intracellular ROS
DCFH-DA, a cell-permeable indicator for ROS, was applied to measure ROS production. HK-2 cells were seeded at a density of 104 cells/well in 96-well plates. 10 mM of DCFH-DA was added into HK-2 cells in plates under 30 minutes exposure at 37°C. Next, the fluorescent microscope with excitation/emission set at 502/523 nm was used for visualizing the fluorescence of formed DCF.
Assay for the Production of Hydrogen Peroxide (H2O2)
Production of H2O2 was detected by the Amplex Red assay as previously described.22 Briefly, HK-2 cells were pretreated and cultured in DMEM//F12 with 0.2% FBS. All data were corrected by cell number at the same condition. After 24 hours treatment, the level of H2O2 production in HK-2 cells could be measured through the application of Amplex Red reagent, which functioned as a substrate that reacted with H2O2. Generated fluorescent resorufin (ex/em maxima = 570/585nm) was normalized and assessed at last.
Rescue Assay
LY294002, an established inhibitor of PI3K/Akt, was added into siRNA-transfected HK-2 cells for 48 h, followed by H/R exposure as previously described. The rescue assay was to further validate that the cytoprotective effect of TRIM8 inhibition relied on PI3K/Akt signaling pathway.
Statistical Analysis
GraphPad Prism version 8.0 (GraphPad Software, Inc., San Diego, CA, USA) was applied to analyze all quantitative results at three separate experiments. The results were expressed as mean + standard deviation. Quantitative results were compared by Student’s t-tests or one-way analysis of variance with Tukey’s post hoc tests. Statistical significance was confirmed by P < 0.05.
Results
TRIM8 Expression Was Up-Regulated in HK-2 Cells After H/R Stimulation
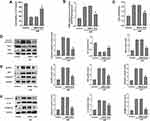
Firstly, we established the H/R model to investigate whether TRIM8 participated in renal I/R injury. We chose different reoxygenation time to observe the changes of TRIM8 expression. HK-2 cells were cultured for 12 h under hypoxic condition and then moved to complete medium for 2 h, 3 h, and 4 h under normal culture environment. Interestingly, TRIM8 expression including mRNA and protein levels were gradually upregulated with increased reoxygenation time after hypoxia (Figure 1A and B). So, we elected the reoxygenation time at 4 h in our study.
H/R-Induced Apoptosis and Pyroptosis Depended on Oxidative Stress in vitro
Next, we investigated whether oxidative stress could regulate H/R-induced apoptosis and pyroptosis in vitro. One highly efficient ROS inhibitor, N-acetyl-cysteine (NAC), was reasonably employed to suppress oxidative stress. As shown in Figure 2A, cell viability in the H/R group declined obviously, while NAC pretreatment led to a recovery of cell viability. The experiment of ROS detection indicated that H/R caused accumulation of intracellular ROS. Meanwhile, the level of H2O2 was also significantly elevated in the H/R group. Nevertheless, the up-regulation of ROS and H2O2 was efficiently inhibited by the use of NAC (Figure 2B and C). Simultaneously, the Western-blot analysis suggested that H/R injury caused notable enhancement of apoptotic markers (BAX and Cleaved caspase-3) and downregulation of Bcl-2 expression. As a result, the ablation of ROS with NAC contributed to evident decline of Cleaved caspase-3 and BAX expression and increased expression of Bcl-2 in the NAC group (Figure 2D). Proteins associated pyroptosis included NLRP3, ASC, Caspase-1, Caspase-11, IL-1β and GSDMD-N. Our analysis validated that H/R stimulation obviously activated pyroptosis in HK-2 cells. However, the levels of pyroptosis-related proteins were diminished in NAC group (Figure 2E and F). Thus, oxidative stress displayed a key regulatory role in H/R-induced apoptosis and pyroptosis in vitro.
TRIM8 Inhibition Attenuated Oxidative Stress Induced by H/R in HK-2 Cells
Subsequently, siRNA was transfected into vibrant cells to knockdown TRIM8, which helped us to better illuminate how TRIM8 inhibition exerted its effect. The transfection efficacy was confirmed by protein detection using Western blot analysis. Results revealed that small interfering RNAs effectively inhibited the expression of the target molecule (Figure 3A). Comparing the H/R group with the control group, we clearly analyzed that there existed significant increase in the production of ROS and H2O2, while TRIM8 inhibition induced meaningful reduction of ROS and H2O2 (Figure 3B and C).
TRIM8 Inhibition Alleviated Apoptosis and Pyroptosis in HK-2 Cells After H/R Exposure
Then, we observed that the loss of cell viability of HK-2 cells in H/R group was reversed greatly by siTRIM8 transfection (Figure 4A). As for apoptotic proteins, TRIM8 inhibition abrogated the enhancement in the expression of Cleaved caspase-3 and BAX. Bcl-2 expression was elevated again in si-TRIM8 group (Figure 4B). To testify that TRIM8 inhibition was able to alleviate pyroptosis as well, the above mentioned pyroptosis-related proteins were detected. Respectively, transfection with siTRIM8 exerted a similar influence to that observed in apoptotic proteins. The levels of pyroptosis-related protein expression were markedly depressed, which manifested that TRIM8 inhibition was momentous in regulating H/R-induced pyroptosis in HK-2 cells (Figure 4C and D).
PI3K/Akt Signaling Pathway Was Involved in TRIM8 Inhibition-Regulated H/R Injury in HK-2 Cells
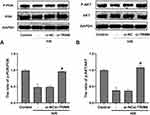
To further verify potential mechanisms related to TRIM8 inhibition against H/R injury, we studied the possible pathway involved. It was reported that knockdown of TRIM8 suppressed H/R-generated oxidative stress and apoptosis in cardiac H9C2 cells, which was achieved through PI3K/Akt signaling pathway.18 Notably, blockage of TRIM8 enhanced phosphorylation of PI3K/Akt in si-TRIM8 group versus si-NC group, which represented that PI3K/Akt signaling pathway was activated by TRIM8 inhibition (Figure 5A and B).
Inhibition of PI3K/AKT Reversed the Effects of TRIM8 Knockdown on Attenuating Oxidative Stress in HK-2 Cells
LY294002, an inhibitor of PI3K/Akt pathway, was used to further illustrate the relationship between PI3K/Akt signaling pathway and TRIM8 inhibition. As shown in Figure 6A, LY294002 was capable of abolishing the phosphorylation of Akt. Suppression of PI3K/Akt signaling pathway was suggested to abrogate the antioxidative effects of TRIM8 inhibition in HK-2 cells, as evidenced by increased ROS production and H2O2 level in the LY294002 group versus the si-TRIM8 group (Figure 6B and C).
Discussion
Acute kidney injury (AKI) is one challenging clinical problem with increasing incidence, unsatisfactory consequences and a great economic burden to society.23 Renal ischemia/reperfusion (I/R) injury is the most important risk factor for AKI.24 Despite decades of pioneering basic work invested in the study of molecular mechanisms associated with renal I/R injury, its occurrence and progression remain incompletely elucidated, as well as how its damage could be effectively mitigated. Herein, we innovatively explored the molecular role and potential mechanism of TRIM8 in renal I/R injury based on the establishment of classical H/R injury model in HK-2 cells. In this research, we firstly proved that H/R exposure increased the expression of TRIM8 in HK-2 cells. Next, knocking down TRIM8 protected against H/R damage, including reduced cellular oxidative stress responses and the inhibition of apoptosis and pyroptosis. Furthermore, the application of NAC demonstrated that H/R-induced apoptosis and pyroptosis could be regulated by oxidative stress in vitro. Simultaneously, the PI3K/AKT phosphorylation was enhanced in the si-TRIM8 group, which indicated PI3K/Akt signaling pathway contributed to the TRIM8 inhibition-mediated cytoprotective effect during H/R injury. The treatment of LY294002 again confirmed this involved pathway. Overall, our study testified that TRIM8 inhibition could alleviate oxidative stress-mediated apoptosis and pyroptosis during H/R injury in HK-2 cells by promoting activation of PI3K/Akt signal pathway.
Oxidative stress is confirmed by more evidence to occupy an increasingly momentous part in biological process of renal I/R injury.6,7,25,26 When reperfusion is restored during I/R injury, a large amount of reactive oxygen species is thus generated by NADPH oxidases, and/or by mitochondrial respiration chain.27–29 Excessive ROS and electrophiles will function as oxidative stress and impair cellular homeostasis, which intensifies the inflammatory response and apoptosis damage.30,31 Apoptosis has widespread participation in a mass of pathological activities, including renal I/R injury, though extrinsic or intrinsic pathways.32 Oxidative stress exacerbating apoptosis in ischemia reperfusion has been demonstrated by several experiments.33,34 Pyroptosis acts as one quite intractable biological event in I/R injury, which is closely associated with increased inflammation after pathological stimulation.35–37 The occurrence and progression of kidney diseases are more or less related to pyroptosis, and the inflammatory body NLRP3 is the most well-studied.38,39 Pyroptosis-related cell death is similar to apoptosis in that both are characterized by DNA fragmentation. However, pyroptosis is regulated by inflammation-associated proteins rather than classical apoptotic-related proteins.40 The large amount of ROS generated in ischemia/reperfusion injury exacerbates inflammatory activity, contributing to the formation of inflammatory signaling factors, including NLRP3 inflammasome.41 The NLRP3 inflammasome is composed of NLRP3, procaspase-1 and ASC. It catalyzes the conversion of procaspase-1 to activated caspase-1, which enhances the maturation and secretion of IL-1β and induces pyroptosis.42 The classical pyroptotic markers that have been confirmed also include Caspase-11 and Gasdermin D.43 Related studies have shown that intracellular apoptosis and pyroptosis can be regulated simultaneously.44–47 Consistent with previous studies, our experiments proved that H/R-induced apoptosis and pyroptosis can be modulated by oxidative stress in HK-2 cells.48,49 In our investigation, phenotypes including apoptosis and pyroptosis are obviously activated during H/R injury, as evidenced by up-regulated expression of apoptotic proteins Cleaved caspase-3, BAX, and pyroptosis-related proteins including NLRP3 and others. However, these effects were obviously reversed by NAC, one highly efficient ROS inhibitor that can eliminate products of oxidative stress. All these results indicated that the severity of H/R-induced apoptosis and pyroptosis was connected with the degree of oxidative stress.
TRIM8, a member of the TRIM family, is involved in the regulation of cell survival, differentiation and apoptosis.50–52 whether TRIM8 is able to regulate renal I/R injury has not been explored. TRIM8 was proved to be a tumor suppressor inhibiting the proliferation of tumor cells.8 TRIM8 overexpression would stabilize P53 and reduce cell proliferation.9 In chemo-resistant renal cell carcinoma, up-regulation of TRIM8 could enhance chemo-sensitivity by reinforcing the apoptosis of tumor cells.10 These findings showed that overexpression of TRIM8 contributed to cell cycle arrest and apoptosis initiation. Further studies confirmed that TRIM8 inhibition could confer a protective effect against pathological stimuli. It was revealed that TRIM8 inhibition attenuated oxidative stress and inflammation to reduce cardiac hypertrophy or acute lung injury.14,53 In addition, TRIM8 was testified to participate in ischemia-related diseases. TRIM8 deficiency could regulate TAK1-JNK1/2/p38 signaling to alleviate inflammation and apoptosis triggered by hepatic I/R injury.15 Inflammation and apoptosis in I/R-mediated cerebral injury can be reduced by TRIM8 knockdown.16 Downregulation of TRIM8 was confirmed to mitigate damage by relieving oxidative stress and apoptosis in oxygen–glucose deprivation/re-oxygenation-induced injury in neurons,17 as well as in H/R-induced injury in cardiac H9C2 cells.18 The results suggested that the protective effect of TRIM8 inhibition in neurons was associated with reinforcement of the AMPK/Nrf2/ARE pathway, which was an important antioxidant pathway. In cardiac H9C2 cells, its protective effect was connected with the activation of PI3K/Akt pathway. Thus, we could hypothesize that TRIM8 might also be of great importance in renal I/R injury. In the experiment, the results innovatively demonstrated that TRIM8 was a key positive regulator in renal HK-2 cells. The mRNA and protein expression of TRIM8 was upregulated after H/R stimulation. The results authenticated that knockdown of TRIM8 attenuated oxidative stress including reducing the production of ROS and H2O2. Moreover, TRIM8 inhibition relieved apoptosis by down regulating Cleaved caspase-3 and BAX and upregulating Bcl-2. The function of TRIM8 inhibition in suppressing oxidative stress and apoptosis was consistent with the studies in I/R-related diseases mentioned above. Interestingly, the expression of pyroptosis-related proteins was also downregulated in si-TRIM8 group versus si-NC group, which indicated that inhibition of TRIM8 alleviated H/R-induced pyroptosis in renal HK-2 cells. As mentioned above, we also verified that the attenuation of apoptosis and pyroptosis was also partly due to the suppressing of oxidative stress.
The phosphoinositide 3-kinase (PI3K)/Akt pathway was demonstrated to regulate cell growth and survival in physiological or pathological conditions.54–56 Recent studies identified that upregulation of PI3K/Akt phosphorylation could eliminate oxidative stress, proinflammatory and apoptotic events in renal I/R injury.19,57,58 Besides, PI3K/Akt signaling pathway was proved to participate in the regulation of pyroptosis in response to I/R stimuli as well.59–62 Therefore, we explored the underlying mechanism that was responsible for the cytoprotective effect of TRIM8 inhibition after H/R stimulation in HK-2 cells. A previous study reported that downregulation of TRIM8 protected cardiac H9C2 cells against H/R-induced oxidative stress and apoptosis via PI3K/Akt signaling pathway.18 Consistent with this study, we observed that the expression of the p-PI3K and p-AKT proteins was markedly increased in siRNA-pretreated renal HK-2 cells exposed to H/R. In addition, the cytoprotective effects of TRIM8 knockdown in attenuating H/R-induced oxidative stress were abrogated by LY294002, an established PI3K/AKT pathway inhibitor. These results validated that inhibition of TRIM8 suppressed oxidative stress induced by hypoxia/reoxygenation via activation of PI3K/Akt signaling pathway in HK-2 cells.
Conclusion
In conclusion, the present study demonstrated the protective effects of TRIM8 inhibition in H/R-stimulated renal HK-2 cells. Moreover, we proved that knockdown of TRIM8 protected HK-2 cells against apoptosis and pyroptosis through suppressing oxidative stress via PI3K/Akt signaling pathway. Therefore, TRIM8 may be a promising therapeutic target for renal I/R injury. The development of specific inhibitor of TRIM8 may be of great interest. However, further investigation is needed to validate the precise function of TRIM8 in regulating renal ischemia/reperfusion injury in vivo.
Acknowledgments
Our work was supported by the National Natural Science Foundation of China (No.82000639).
Disclosure
The authors have declared that there are no conflicts of interest in this work.
References
1. Han SJ, Lee HT. Mechanisms and therapeutic targets of ischemic acute kidney injury. Kidney Res Clin Pract. 2019;38:427–440. doi:10.23876/j.krcp.19.062
2. Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17:1503–1520. doi:10.1681/ASN.2006010017
3. Wang L, Chen H, Liu XH, et al. Ozone oxidative preconditioning inhibits renal fibrosis induced by ischemia and reperfusion injury in rats. Exp Ther Med. 2014;8:1764–1768. doi:10.3892/etm.2014.2004
4. Tammaro A, Kers J, Scantlebery A, Florquin S. Metabolic flexibility and innate immunity in renal ischemia reperfusion injury: the fine balance between adaptive repair and tissue degeneration. Front Immunol. 2020;11:1346. doi:10.3389/fimmu.2020.01346
5. Rovcanin B, Medic B, Kocic G, Cebovic T, Ristic M, Prostran M. Molecular dissection of renal ischemia-reperfusion: oxidative stress and cellular events. Curr Med Chem. 2016;23:1965–1980. doi:10.2174/0929867323666160112122858
6. Liu H, Wang L, Weng X, et al. Inhibition of Brd4 alleviates renal ischemia/reperfusion injury-induced apoptosis and endoplasmic reticulum stress by blocking FoxO4-mediated oxidative stress. Redox Biol. 2019;24:101195. doi:10.1016/j.redox.2019.101195
7. Diao C, Chen Z, Qiu T, et al. Inhibition of PRMT5 attenuates oxidative stress-induced pyroptosis via activation of the Nrf2/HO-1 signal pathway in a mouse model of renal ischemia-reperfusion injury. Oxid Med Cell Longev. 2019;2019:2345658. doi:10.1155/2019/2345658
8. Caratozzolo MF, Marzano F, Mastropasqua F, Sbisa E, Tullo A. TRIM8: making the right decision between the oncogene and tumour suppressor role. Genes. 2017;8:354.
9. Caratozzolo MF, Micale L, Turturo MG, et al. TRIM8 modulates p53 activity to dictate cell cycle arrest. Cell Cycle. 2012;11:511–523. doi:10.4161/cc.11.3.19008
10. Caratozzolo MF, Valletti A, Gigante M, et al. TRIM8 anti-proliferative action against chemo-resistant renal cell carcinoma. Oncotarget. 2014;5:7446–7457. doi:10.18632/oncotarget.2081
11. Reymond A, Meroni G, Fantozzi A, et al. The tripartite motif family identifies cell compartments. Embo J. 2001;20:2140–2151. doi:10.1093/emboj/20.9.2140
12. Yan FJ, Zhang XJ, Wang WX, et al. The E3 ligase tripartite motif 8 targets TAK1 to promote insulin resistance and steatohepatitis. Hepatology. 2017;65:1492–1511. doi:10.1002/hep.28971
13. Guo L, Dong W, Fu X, et al. Tripartite Motif 8 (TRIM8) positively regulates pro-inflammatory responses in pseudomonas aeruginosa-induced keratitis through promoting K63-linked polyubiquitination of TAK1 protein. Inflammation. 2017;40:454–463. doi:10.1007/s10753-016-0491-3
14. Chen L, Huang J, Ji YX, et al. Tripartite motif 8 contributes to pathological cardiac hypertrophy through enhancing transforming growth factor beta-activated kinase 1-dependent signaling pathways. Hypertension. 2017;69:249–258. doi:10.1161/HYPERTENSIONAHA.116.07741
15. Tao Q, Tianyu W, Jiangqiao Z, et al. Tripartite motif 8 deficiency relieves hepatic ischaemia/reperfusion injury via TAK1-dependent signalling pathways. Int J Biol Sci. 2019;15:1618–1629. doi:10.7150/ijbs.33323
16. Bai X, Zhang YL, Liu LN. Inhibition of TRIM8 restrains ischaemia-reperfusion-mediated cerebral injury by regulation of NF-kappaB activation associated inflammation and apoptosis. Exp Cell Res. 2020;388:111818. doi:10.1016/j.yexcr.2020.111818
17. Zhao W, Zhang X, Chen Y, Shao Y, Feng Y. Downregulation of TRIM8 protects neurons from oxygen-glucose deprivation/re-oxygenation-induced injury through reinforcement of the AMPK/Nrf2/ARE antioxidant signaling pathway. Brain Res. 2020;1728:146590. doi:10.1016/j.brainres.2019.146590
18. Dang X, Qin Y, Gu C, Sun J, Zhang R, Peng Z. Knockdown of tripartite motif 8 protects H9C2 cells against hypoxia/reoxygenation-induced injury through the activation of PI3K/Akt signaling pathway. Cell Transplant. 2020;29:2138941951. doi:10.1177/0963689720949247
19. Liu C, Chen K, Wang H, et al. Gastrin attenuates renal ischemia/reperfusion injury by a PI3K/Akt/Bad-mediated anti-apoptosis signaling. Front Pharmacol. 2020;11:540479.
20. Shen B, Mei M, Pu Y, et al. Necrostatin-1 attenuates renal ischemia and reperfusion injury via meditation of HIF-1alpha/mir-26a/TRPC6/PARP1 signaling. Mol Ther Nucleic Acids. 2019;17:701–713. doi:10.1016/j.omtn.2019.06.025
21. Tang TT, Lv LL, Pan MM, et al. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. 2018;9:351. doi:10.1038/s41419-018-0378-3
22. Tong X, Khandelwal AR, Qin Z, et al. Role of smooth muscle Nox4-based NADPH oxidase in neointimal hyperplasia. J Mol Cell Cardiol. 2015;89:185–194. doi:10.1016/j.yjmcc.2015.11.013
23. Ronco C, Bellomo R, Kellum JA. Acute kidney injury. Lancet. 2019;394:1949–1964. doi:10.1016/S0140-6736(19)32563-2
24. Kasuno K, Shirakawa K, Yoshida H, et al. Renal redox dysregulation in AKI: application for oxidative stress marker of AKI. Am J Physiol Renal Physiol. 2014;307:F1342–51. doi:10.1152/ajprenal.00381.2013
25. Nezu M, Suzuki N. Roles of Nrf2 in protecting the kidney from oxidative damage. Int J Mol Sci. 2020;21:2951.
26. Aboutaleb N, Jamali H, Abolhasani M, Pazoki TH. Lavender oil (Lavandula angustifolia) attenuates renal ischemia/reperfusion injury in rats through suppression of inflammation, oxidative stress and apoptosis. Biomed Pharmacother. 2019;110:9–19. doi:10.1016/j.biopha.2018.11.045
27. Ohsaki Y, O’Connor P, Mori T, et al. Increase of sodium delivery stimulates the mitochondrial respiratory chain H2O2 production in rat renal medullary thick ascending limb. Am J Physiol Renal Physiol. 2012;302:F95–102. doi:10.1152/ajprenal.00469.2011
28. Quijano C, Castro L, Peluffo G, Valez V, Radi R. Enhanced mitochondrial superoxide in hyperglycemic endothelial cells: direct measurements and formation of hydrogen peroxide and peroxynitrite. Am J Physiol Heart Circ Physiol. 2007;293:H3404–14. doi:10.1152/ajpheart.00761.2007
29. Layton AT. Recent advances in renal hypoxia: insights from bench experiments and computer simulations. Am J Physiol Renal Physiol. 2016;311:F162–5. doi:10.1152/ajprenal.00228.2016
30. Ratliff BB, Abdulmahdi W, Pawar R, Wolin MS. Oxidant mechanisms in renal injury and disease. Antioxid Redox Signal. 2016;25:119–146. doi:10.1089/ars.2016.6665
31. Yamamoto M, Kensler TW, Motohashi H. The KEAP1-NRF2 system: a thiol-based sensor-effector apparatus for maintaining redox homeostasis. Physiol Rev. 2018;98:1169–1203. doi:10.1152/physrev.00023.2017
32. Fukazawa K, Lee HT. Volatile anesthetics and AKI: risks, mechanisms, and a potential therapeutic window. J Am Soc Nephrol. 2014;25:884–892. doi:10.1681/ASN.2013111215
33. Kishimoto M, Suenaga J, Takase H, et al. Oxidative stress-responsive apoptosis inducing protein (ORAIP) plays a critical role in cerebral ischemia/reperfusion injury. Sci Rep. 2019;9:13512. doi:10.1038/s41598-019-50073-8
34. Kar F, Hacioglu C, Senturk H, Donmez DB, Kanbak G. The role of oxidative stress, renal inflammation, and apoptosis in post ischemic reperfusion injury of kidney tissue: the protective effect of dose-dependent boric acid administration. Biol Trace Elem Res. 2020;195:150–158. doi:10.1007/s12011-019-01824-1
35. Yang JR, Yao FH, Zhang JG, et al. Ischemia-reperfusion induces renal tubule pyroptosis via the CHOP-caspase-11 pathway. Am J Physiol Renal Physiol. 2014;306:F75–84. doi:10.1152/ajprenal.00117.2013
36. Toldo S, Mauro AG, Cutter Z, Abbate A. Inflammasome, pyroptosis, and cytokines in myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2018;315:H1553–68. doi:10.1152/ajpheart.00158.2018
37. Qiu Z, Lei S, Zhao B, et al. NLRP3 inflammasome activation-mediated pyroptosis aggravates myocardial ischemia/reperfusion injury in diabetic rats. Oxid Med Cell Longev. 2017;2017:9743280. doi:10.1155/2017/9743280
38. Kim YG, Kim SM, Kim KP, Lee SH, Moon JY. The role of inflammasome-dependent and inflammasome-independent NLRP3 in the kidney. Cells-Basel. 2019;8:1389.
39. Zhang KJ, Wu Q, Jiang SM, et al. Pyroptosis: a new frontier in kidney diseases. Oxid Med Cell Longev. 2021;2021:6686617. doi:10.1155/2021/6686617
40. Miao EA, Rajan JV, Aderem A. Caspase-1-induced pyroptotic cell death. Immunol Rev. 2011;243:206–214. doi:10.1111/j.1600-065X.2011.01044.x
41. Devi TS, Lee I, Huttemann M, Kumar A, Nantwi KD, Singh LP. TXNIP links innate host defense mechanisms to oxidative stress and inflammation in retinal Muller glia under chronic hyperglycemia: implications for diabetic retinopathy. Exp Diabetes Res. 2012;2012:438238. doi:10.1155/2012/438238
42. Horng T. Calcium signaling and mitochondrial destabilization in the triggering of the NLRP3 inflammasome. Trends Immunol. 2014;35:253–261. doi:10.1016/j.it.2014.02.007
43. Tavakoli DZ, Singla DK. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am J Physiol Heart Circ Physiol. 2019;317:H460–71. doi:10.1152/ajpheart.00056.2019
44. Huang Y, Tan F, Zhuo Y, et al. Hypoxia-preconditioned olfactory mucosa mesenchymal stem cells abolish cerebral ischemia/reperfusion-induced pyroptosis and apoptotic death of microglial cells by activating HIF-1alpha. Aging. 2020;12:10931–10950. doi:10.18632/aging.103307
45. Kofahi HM, Taylor NG, Hirasawa K, Grant MD, Russell RS. Hepatitis C virus infection of cultured human hepatoma cells causes apoptosis and pyroptosis in both infected and bystander cells. Sci Rep. 2016;6:37433. doi:10.1038/srep37433
46. Jang Y, Lee AY, Jeong SH, et al. Chlorpyrifos induces NLRP3 inflammasome and pyroptosis/apoptosis via mitochondrial oxidative stress in human keratinocyte HaCaT cells. Toxicology. 2015;338:37–46. doi:10.1016/j.tox.2015.09.006
47. Li DX, Wang CN, Wang Y, et al. NLRP3 inflammasome-dependent pyroptosis and apoptosis in hippocampus neurons mediates depressive-like behavior in diabetic mice. Behav Brain Res. 2020;391:112684. doi:10.1016/j.bbr.2020.112684
48. Liu H, Chen Z, Weng X, et al. Enhancer of zeste homolog 2 modulates oxidative stress-mediated pyroptosis in vitro and in a mouse kidney ischemia-reperfusion injury model. FASEB J. 2020;34:835–852. doi:10.1096/fj.201901816R
49. Choi EK, Jung H, Kwak KH, et al. Inhibition of oxidative stress in renal ischemia-reperfusion injury. Anesth Analg. 2017;124:204–213. doi:10.1213/ANE.0000000000001565
50. Micale L, Fusco C, Fontana A, et al. TRIM8 downregulation in glioma affects cell proliferation and it is associated with patients survival. BMC Cancer. 2015;15:470. doi:10.1186/s12885-015-1449-9
51. Ye W, Hu MM, Lei CQ, et al. TRIM8 negatively regulates TLR3/4-mediated innate immune response by blocking TRIF-TBK1 interaction. J Immunol. 2017;199:1856–1864. doi:10.4049/jimmunol.1601647
52. Mastropasqua F, Marzano F, Valletti A, et al. TRIM8 restores p53 tumour suppressor function by blunting N-MYC activity in chemo-resistant tumours. Mol Cancer. 2017;16:67. doi:10.1186/s12943-017-0634-7
53. Xiaoli L, Wujun Z, Jing L. Blocking of tripartite motif 8 protects against lipopolysaccharide (LPS)-induced acute lung injury by regulating AMPKalpha activity. Biochem Biophys Res Commun. 2019;508:701–708. doi:10.1016/j.bbrc.2018.11.072
54. Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi:10.1006/excr.1999.4690
55. Jafari M, Ghadami E, Dadkhah T, Akhavan-Niaki H. PI3k/AKT signaling pathway: erythropoiesis and beyond. J Cell Physiol. 2019;234:2373–2385. doi:10.1002/jcp.27262
56. Hoxhaj G, Manning BD. The PI3K-AKT network at the interface of oncogenic signalling and cancer metabolism. Nat Rev Cancer. 2020;20:74–88. doi:10.1038/s41568-019-0216-7
57. Xu Y, Wang W, Jin K, et al. Perillyl alcohol protects human renal tubular epithelial cells from hypoxia/reoxygenation injury via inhibition of ROS, endoplasmic reticulum stress and activation of PI3K/Akt/eNOS pathway. Biomed Pharmacother. 2017;95:662–669. doi:10.1016/j.biopha.2017.08.129
58. Liu HB, Meng QH, Huang C, Wang JB, Liu XW. Nephroprotective effects of polydatin against ischemia/reperfusion injury: a role for the PI3K/Akt signal pathway. Oxid Med Cell Longev. 2015;2015:362158. doi:10.1155/2015/362158
59. Li Z, Zhao F, Cao Y, et al. DHA attenuates hepatic ischemia reperfusion injury by inhibiting pyroptosis and activating PI3K/Akt pathway. Eur J Pharmacol. 2018;835:1–10. doi:10.1016/j.ejphar.2018.07.054
60. Guo X, Hu S, Liu JJ, et al. Piperine protects against pyroptosis in myocardial ischaemia/reperfusion injury by regulating the miR-383/RP105/AKT signalling pathway. J Cell Mol Med. 2021;25:244–258. doi:10.1111/jcmm.15953
61. Zhang Y, Wang H, Li H, et al. Gualou guizhi granule protects against OGD/R-induced injury by inhibiting cell pyroptosis via the PI3K/Akt signaling pathway. Evid Based Complement Alternat Med. 2021;2021:6613572. doi:10.1155/2021/6613572
62. Diao MY, Zhu Y, Yang J, et al. Hypothermia protects neurons against ischemia/reperfusion-induced pyroptosis via m6A-mediated activation of PTEN and the PI3K/Akt/GSK-3beta signaling pathway. Brain Res Bull. 2020;159:25–31. doi:10.1016/j.brainresbull.2020.03.011
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.