Back to Journals » Cancer Management and Research » Volume 12
Knockdown of lncRNA MCM3AP-AS1 Attenuates Chemoresistance of Burkitt Lymphoma to Doxorubicin Treatment via Targeting the miR-15a/EIF4E Axis
Received 7 February 2020
Accepted for publication 28 June 2020
Published 16 July 2020 Volume 2020:12 Pages 5845—5855
DOI https://doi.org/10.2147/CMAR.S248698
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Chao Guo, Ming Gong, Zhenling Li
Department of Hematology, China-Japan Friendship Hospital, Beijing, People’s Republic of China
Correspondence: Zhenling Li Email [email protected]
Purpose: The long-noncoding RNA MCM3AP-AS1 has been shown to participate in the tumorigenesis and growth of several types of cancer, but little is known about the role of MCM3AP-AS1 in the chemoresistance of lymphoma.
Methods: A series of patients with Burkitt lymphoma were enrolled for clinical analysis. Daudi and Namalwa cells were used for further experiments. CCK-8 and apoptosis assays were used to assess the response to doxorubicin. Mitochondrial membrane potential assays and high-resolution respirometry were used to assess mitochondrial function. Western blotting was used to detect the expression of certain molecules. Luciferase assays and microRNA transfection were used to clarify the regulatory mechanisms of MCM3AP-AS1. An in vivo model using BALB/c nude mice was utilized to investigate the effects of MCM3AP-AS1 on cell proliferation and tumor growth.
Results: The expression level of MCM3AP-AS1 was increased in tumors compared with normal lymph nodes, which indicated poor prognosis in patients with Burkitt lymphoma. Moreover, compared with siNC transfection, MCM3AP-AS1 knockdown decreased cell viability and increased apoptosis rates upon doxorubicin treatment compared with siNC. Further studies indicated that upregulation of several antiapoptotic factors, downstream of EIF4E, was partially responsible for MCM3AP-AS1-induced chemoresistance. Moreover, miR-15a functioned as a link between MCM3AP-AS1 and EIF4E, and was sponged by MCM3AP-AS1. Finally, we showed that the MCM3AP-AS1/miR-15a/EIF4E axis regulated the chemoresistance of lymphoma cells in vitro and in vivo.
Conclusion: MCM3AP-AS1/miR-15a/EIF4E axis plays a role in the chemoresistance of Burkitt lymphoma, and it might become a promising target for lymphoma therapeutics.
Keywords: Burkitt lymphoma, MCM3AP-AS1, microRNA-15a, EIF4E, chemosensitivity
Introduction
Lymphoma is a kind of malignant tumor that originates from lymphoid tissue and its incidence and mortality rates have increased greatly in recent years. Lymphoma can be divided into Hodgkin’s lymphoma (HL) and non-Hodgkin’s lymphoma (NHL) depending on the presence of typical Reed-Sternberg cells.1 NHL lymphoma consists of a large group of subtypes that have different morphologic, immunophenotypic, genetic and clinical behaviors. Burkitt lymphoma (BL) is a subtype of B cell NHL (B-NHL) that is highly aggressive, rapidly growing and affecting nearly every organ system. Pathologically, BL is classically characterized by translocations of chromosomes 8 and 14 resulting in upregulation of the c-Myc protein transcription factor with enhanced cell proliferation. BL affects nearly every organ system.2 Currently, the treatment for BL is mainly the chemotherapy, either short or longer duration, employing intensive, multiagent regimens composed of doxorubicin, alkylators, vincristine and etoposide.3 However, many patients experience treatment resistance and develop refractory disease,4 but the mechanism is unclear.
Long noncoding RNAs (lncRNAs), defined as ncRNAs longer than 200 nt, have been found to exert transcriptional regulatory functions via epigenetic regulatory mechanisms.5 Additionally, an increasing number of studies indicate that lncRNAs participate in several pathophysiological processes in cancer, such as tumor invasion, metastasis, proliferation and treatment resistance.6–10 Aberrant expression of lncRNAs has been frequently demonstrated in cancers, especially lymphoma.11–14 One of the most important mechanisms of lncRNAs is their ability to act as ceRNAs (competing endogenous RNAs) to sponge miRNA and regulate the expression levels of miRNA-targeted genes. LncRNA MCM3AP antisense RNA 1 (MCM3AP-AS1) has been reported to regulate certain phenotypes of different types of cancer. Wang et al revealed that lncRNA MCM3AP-AS1 promotes the growth and proliferation of hepatocellular carcinoma by targeting the miR-194-5p/forkhead box A1 (FOXA1) axis.15 Additionally, MCM3AP-AS1 has been shown to promote the proliferation of papillary thyroid cancer via targeting the miR-211-5p/SPARC axis.16 However, little is known about the role of lncRNA MCM3AP-AS1 in B-NHL.
MicroRNAs have been shown to play important roles in both the pathogenesis and treatment of a variety of cancer types.17 Additionally, microRNAs function as connections between lncRNAs and other signaling pathways by functioning as ceRNAs.17 The regulatory networks, such as the lncRNAs/miRNAs/Wnt/β-catenin pathway, the lncRNAs/miRNAs/PI3K/AKT/mTOR pathway, the lncRNAs/miRNAs/Notch pathway and the lncRNAs-miRNAs/MAPK kinase pathway, constitute complex signaling pathways in tumor. These pathways play important roles in the proliferation, migration and chemoresistance of lymphoma, among which the PI3K/AKT/mTOR pathway has been shown to participate in the chemoresistance of lymphoma.18 Eukaryotic translation initiation factor 4E (EIF4E) functions downstream of mTOR and modulates the several cancer phenotypes, but the regulation of EIF4E in lymphoma has not yet been illustrated.19,20 Previous studies indicated that microRNA-202-5p (miR-202-5p), miR-496, miR-15a, miR-34c-3p, miR-455-3p, miR-503 and miR-141 can all bind to the 3′-untranslated region (UTR) of EIF4E. Using bioinformatics analysis of the database lncBase version 2, it was shown that miR-15a can be absorbed by lncRNA MCM3AP-AS1. In our study, we analyzed the expression patterns of MCM3AP-AS1 in B-NHL tissues and showed that MCM3AP-AS1 regulated cellular phenotypes of lymphoma cells via targeting the miR-15a/EIF4E axis, using the B-NHL cell lines Daudi and Namalwa.
Patients and Methods
Patients and Ethics Statement
The protocols for using the lymphoma patients and analyzing patient data were approved by the ethics committee of the China-Japan Friendship Hospital. The enrollment of patients and acquisition of patient tissues were performed in accordance with the Declaration of Helsinki. In general, patients who had tissue biopsies for final diagnosis with Burkitt lymphoma were enrolled in our department and underwent treatment in our hospital. Written informed consent was provided by each patient before inclusion in this study.
Cell Culture and Transfections
Human lymphoma cell lines Daudi and Namalwa were purchased from American Type Culture Collection (ATCC, Manassas, VA, USA). These two cell types were cultured in RPMI-1640 medium supplemented with 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin. The cells were cultured at 37°C and 5% CO2. The cell culture reagents were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Cell transfections were conducted using Sinofection regents (Sino Biological, Beijing, China) according to the protocols of manufacturers. The sequence of MCM3AP-AS1-specific siRNA was: 5′-GCTGCTAATGGCAACACTGA-3′, and siNC: 5′-TTCTCCGAACGTGTCACGTTT-3′. The sequence for EIF4E siRNA was: 5′-AAGCAAACCTGCGGCTGATCT-3′; and siNC: 5′-TAAGGCTATGAAGAGATACTT-3′. The miR-15a mimic, inhibitor and their negative controls were synthesized by and purchased from GenePharma (Shanghai, China).
Cell Viability Assay
Cell viability assays were conducted using the Cell Counting Kit-8 (CCK-8, Dojindo, Kumamoto, Japan) assay as described previously.21
Flow Cytometry
Flow cytometry was conducted as previously described.21 For the detection of apoptosis, lymphoma cells were centrifuged and washed with PBS twice. Then these cells were incubated with annexin-V and PI (propidium iodide) to assess apoptosis and with PI alone to assess the cell cycle. Statistical analysis and diagram generation were performed using FlowJo 7.5.
Western Blotting
Immunoblotting was conducted as previously described.22 The primary antibodies used in this study were as follows: anti-poly (ADP-ribose) polymerase 1 (PARP, Abcam, Cambridge, MA, USA), anti-BCL2 apoptosis regulator (Bcl-2, Santa Cruz, Dallas, TX, USA), anti-MCL1 apoptosis regulator (Mcl-1, Cell Signaling Technology, Danvers, MA, USA), anti-BCL2 like 1 (Bcl-xL, Abcam), anti-BCL2 associated X (BAX, Abcam), anti-BCL2 antagonist/killer 1 (BAK, Abcam), anti-phosphorylated AKT serine/threonine kinase 1 (p-AKT, Cell Signaling Technology), anti-phosphorylated mitogen-activated protein kinase 1 (p-ERK, Cell Signaling Technology), anti-phosphorylated mechanistic target of rapamycin kinase (p-mTOR, Cell Signaling Technology), anti-MYC proto-oncogene (c-Myc, Abcam), anti-EIF4E (Abcam) and anti- glyceraldehyde-3-phosphate dehydrogenase (GAPDH, Beyotime, Shanghai, China).
Luciferase Assay
Dual luciferase reporter assays were performed to verify the direct interactions between MCM3AP-AS1 and miR-15a as well as miR-15a and the 3′-UTR of EIF4E mRNA. PCR was conducted using the PrimeSTAR DNA polymerase (Takara) to amplify the MCM3AP-AS1 complementary DNA (cDNA) containing the predicted miR-15a binding site and the 3′-UTR of EIF4E cDNA containing the predicted miR-15a binding site. The products were purified and incorporated into the pmirGLO vector for further transfections. The pRL-TK plasmid was co-transfected as the internal control. After 48 hours of transfection, a luciferase assay kit (Promega, Madison, WI) and a Promega GloMax 20/20 system were used for final measurement.
In vivo Experiments
Male BALB/c nude mice (4–5 weeks old) were purchased from the Model Animal Research Center of Nanjing University for construction of the tumor-burden model. The protocols for handling the animals and experimental protocols were approved by the Animal Ethics Committee of the China-Japan Friendship Hospital. The conduct of the animal experiments is according to the Animal Welfare Guideline of China-Japan Friendship Hospital and the 3R principle (refinement, reduction and replacement).23 In general, 24 BALB/c mice were randomly divided into four groups (each containing 6 mice, random number generation method) and treated with Daudi-siNC, Daudi-siMCM, Namalwa-siNC and Namalwa-siMCM in the animal house. In detail, Daudi and Namalwa cells (1×107 cells for each mouse) pretreated with siNC or siMCM3AP-AS1 were inoculated subcutaneously in the right flank of the nude mice and then the mice were treated with doxorubicin at a dose of 5 mg/kg/week of via intraperitoneal injection. Then, the tumors were measured every 7 days. After six weeks, the mice were anesthetized by intraperitoneal injection of sodium pentobarbital (50 mg/kg) and euthanized by decapitation; tumor tissues were removed for further analysis by a blinded researcher.
Statistical Analysis
The data are presented as the mean ± SEM. The data analysis was conducted using unpaired Student’s t-test or ANOVA followed by Bonferroni post hoc tests for mean difference analysis. For the survival data analysis, Kaplan-Meier survival curve was drawn, and the Log rank test was used to evaluate the differences between groups. The data analysis and diagram drawing were performed using the Graphpad Prism Software (La Jolla, CA, USA), and with a two-tailed P-value less than 0.05 was considered statistically significant.
Results
MCM3AP-AS1 is Overexpressed in B-NHL Patients with Poor Prognosis
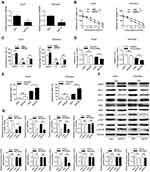
The basic characteristics of the enrolled patients with Burkitt lymphoma were as follows: mean age (56.9±6.5 years), sex (25 male, 16 female), tumor size (3.3±1.4 cm), Ann Arbor stage (I+II 19 cases, III+IV 24 cases), erythrocyte sedimentation rate (40.7±18.9 mm/h), lactic dehydrogenase (301.1±106.9 U/L) and serum albumin (33.7±4.4 g/L). Among the lymphoma patients enrolled, we analyzed the expression patterns of lncRNA MCM3AP-AS1. In Figure 1A, it was demonstrated that the expression levels of MCM3AP-AS1 were significantly increased in tumor tissues compared with normal lymph node tissues (P<0.0001). Additionally, we measured the expression differences between the patients in Ann Arbor stage I or II and stage III or IV. The expression levels of MCM3AP-AS1 in stages III and IV were greater than those in stages I and II (Figure 1B). Furthermore, we divided the enrolled patients into two groups according to the expression levels of MCM3AP-AS1 (low or high) (Figure 1C). The factors of age and sex did not correlate with MCM3AP-AS1 expression levels, while tumor size and tumor stage correlated significantly with MCM3AP-AS1 levels (Table 1). More importantly, patients with low levels of MCM3AP-AS1 expression exhibited better long-term prognosis than those with high levels (Figure 1D, P<0.05).
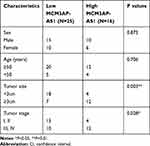 |
Table 1 Correlation Between MCM3AP-AS1 Expression and Clinicopathological Features of Patients with Burkitt’s Lymphoma |
MCM3AP-AS1 Promotes Doxorubicin-Induced Chemoresistance via Inhibiting Apoptosis and Promoting Proliferation
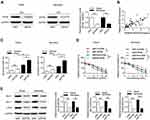
Next, we further investigated the effects and mechanisms of MCM3AP-AS1 on the cellular biological features in two Burkitt lymphoma cell lines, Daudi and Namalwa. Figure 2A shows that treatment with MCM3AP-AS1-specific siRNA significantly decreased its expression levels compared with siNC. Treatment with MCM3AP-AS1-specific siRNA partially increased the sensitivity to doxorubicin in both Daudi and Namalwa cells compared with siNC (Figure 2B). In addition, siMCM3AP-AS1 also suppressed cell cycle progression by increasing the percentage of cells in G0/G1 phases and decreasing the percentage of cells in G2/M phases (Figure 2C). Additionally, the mitochondrial membrane potential of these two cell types treated with siNC or siMCM3AP-AS1 was measured, suggesting that siMCM3AP-AS1 significantly decreased mitochondrial membrane potential upon doxorubicin treatment, compared with siNC, in both Daudi and Namalwa cells (Figure 2D). Furthermore, the apoptosis rates of lymphomatous cells were measured by flow cytometry. It was demonstrated that compared with siNC, siMCM3AP-AS1 significantly enhanced the apoptosis rates upon doxorubicin treatment (Figure 2E). To investigate the mechanism of MCM3AP-AS1-induced cell protections, the key proteins responsible for mitochondrial protection, apoptosis regulation and cell proliferation were detected by Western blotting, as shown in Figure 2F and G. MCM3AP-AS1 knockdown significantly decreased Bcl-2, Mcl-1 and c-Myc expression compared with that in the siNC group upon treatment with doxorubicin, whereas the expression levels of Bcl-xL, BAX, BAK, p-ERK, p-AKT and p-mTOR did not change significantly with MCM3AP-AS1 knockdown. Additionally, siMCM3AP-AS1 enhanced the expression of cleaved PARP. These results indicated that MCM3AP-AS1 affected cell proliferation, apoptosis and mitochondrial function by modulating the expression patterns of several proteins.
Eukaryotic Translation Initiation Factor EIF4E is Regulated by MCM3AP-AS1
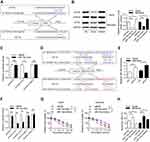
Since a variety of proteins might be modulated by MCM3AP-AS1, we detected the expression levels of EIF4E, which is responsible for the translation of a variety of pro-survival and anti-apoptosis proteins.24 In Figure 3A, we measured the expression patterns of EIF4E, showing that EIF4E protein levels were suppressed by MCM3AP-AS1 knockdown, compared with siNC. In addition, the correlation between mRNA expression levels of EIF4E and MCM3AP-AS1 expression was analyzed. A positive correlation existed among the 41 lymphoma patients enrolled (Figure 3B). Furthermore, we analyzed the effects of EIF4E-specific siRNA on cell apoptosis upon doxorubicin treatment and demonstrated that EIF4E knockdown significantly increased the apoptosis rate in both Daudi and Namalwa cells, compared with siNC (Figure 3C). Additionally, transfection of the EIF4E overexpression plasmid partially attenuated the anti-proliferative effects of siMCM3AP-AS1 in these two cell lines (Figure 3D). Then, we validated the expression patterns of target proteins downstream of EIF4E, showing that EIF4E knockdown also inhibited the expression levels of Bcl-2, Mcl-1 and c-Myc (Figure 3E). Taken together, these results indicated that EIF4E function as the link between MCM3AP-AS1 and downstream proteins.
miR-15a is the Connection Between MCM3AP-AS1 and EIF4E
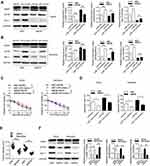
Next, we investigated the regulatory mechanisms between MCM3AP-AS1 and EIF4E. LncRNAs can function as decoys by sponging miRNAs, thus modulating the expression levels of their target genes. It was shown that miR-15a could function as a link between MCM3AP-AS1 and EIF4E. Figure 4A shows that miR-15a can specifically bind to the 3ʹ-UTR of EIF4E mRNA, while mutation of the specific sites in the 3ʹ-UTR of EIF4E might hinder the specific binding between these two elements. Transfection with miR-15a mimic or inhibitor downregulated or upregulated the protein levels of EIF4E in these two cell types, respectively (Figure 4B). Using a luciferase assay, it was shown that specific binding of the miR-15a mimic with wild-type pmirGLO-EIF4E decreased the luciferase activity compared with miR-NC, whereas there was no significant difference in the activity of a mutant form between miR-15a mimic and miR-NC (Figure 4C). Figure 4D illustrates the binding patterns of lncRNA MCM3AP-AS1 with miR-15a, showing that two main binding sites exist in the wild-type form. Figure 4E and F indicate that MCM3AP-AS1 knockdown upregulated the expression levels of miR-15a, while MCM3AP-AS1 mutation inhibited the binding between MCM3AP-AS1 and miR-15a, as revealed by luciferase reporter assay. Moreover, compared with miR-NC transfection, miR-15a mimic transfection decreased the percentage of viable cells and enhanced cell apoptosis, while miR-15a inhibitor transfection increased the percentage of viable cells and suppressed apoptosis in doxorubicin-treated Daudi and Namalwa cells (Figure 4G and H). These results indicated that miR-15a functions as the link between MCM3AP-AS1 and EIF4E.
The MCM3AP-AS1/miR-15a/EIF4E Pathway Regulates the Sensitivity of Doxorubicin in Lymphoma Cells
Then, we investigated the role of the MCM3AP-AS1/miR-15a/EIF4E axis in lymphoma cells or in nude mice with lymphoma tumors. Transfection with miR-15a inhibitor partially attenuated the apoptosis enhancement and EIF4E and Mcl-1 suppression induced by MCM3AP-AS1 knockdown in these two cell lines (Figure 5A and B). In addition, miR-15a inhibitor transfection partially restored cell viability and suppressed apoptosis, induced by MCM3AP-AS1 knockdown in lymphoma cells (Figure 5C and D). Furthermore, in vivo experiments indicated that MCM3AP-AS1 knockdown also suppressed tumor growth, enhanced cleaved PARP expression and decreased Mcl-1 and EIF4E expression in tumor tissues (Figure 5E and F). These results suggest that the MCM3AP-AS1/miR-15a/EIF4E axis functions in doxorubicin response in lymphoma and might be a promising therapeutic target.
Discussion
Improving drug sensitivity for the treatment of lymphoma has been studied recently. In this study, we found that a novel lncRNA, MCM3AP-AS1, had enhanced expression levels in B-NHL tissues compared with normal lymph nodes, which indicated poor prognosis. In detail, MCM3AP-AS1 knockdown promoted drug sensitivity by inhibiting proliferation and facilitating apoptosis. The expression patterns of several oncogenes downstream of EIF4E were decreased upon MCM3AP-AS1 knockdown. Further studies indicated that miR-15a functioned as the link between MCM3AP-AS1 and EIF4E and that the MCM3AP-AS1/miR-15a/EIF4E axis functioned in doxorubicin response in lymphoma both in vitro and in vivo. Our study revealed that lncRNA MCM3AP-AS1 might be a promising target for the understanding the pathogenesis of B-NHL and for investigating effective therapeutics.
The role of lncRNAs in lymphoma has been studied widely in recent years. Since the development of genomics, lncRNAs have become a region of increased interest within cancer research and recent reports implicated that lncRNAs are dysregulated in human malignancies.25 Currently, reverse transcription-quantitative polymerase chain reaction (RT-qPCR) is the most useful and commonly used method for measuring lncRNA levels. Tayari et al analyzed lncRNA expression profiles of normal B cell subsets and HL and found some differentially expressed lncRNAs that may be useful as tumor biomarkers.26 In later studies, several key lncRNAs were identified to be aberrantly expressed in lymphoma and had important roles in regulating cellular phenotypes. For instance, lncRNA HOTAIR is upregulated in DLBCL (diffuse large B cell lymphoma) tumor tissues and cell lines, while functions by modulating cell proliferation, cell cycle progression and PI3K/AKT/NF-κB pathways.27 Moreover, FAS-AS1 showed low expression in primary B-cell lymphomas and lymphoma-derived cell lines. FAS-AS1 is a novel modulator of the soluble Fas receptor expression and finally impairs the Fas-mediated apoptosis pathway in human lymphomas.28 However, currently, little is known about the role of lncRNAs in Burkitt lymphoma. Doose et al reported that lncRNA MINCR has a strong correlation with MYC expression in MYC-positive lymphomas, which is related to cellular proliferation and cell cycle progression.29,30 In this study, we demonstrated that lncRNA MCM3AP-AS1 was overexpressed in Burkitt lymphoma tissues compared with normal lymph node tissues, which regulated cell proliferation and chemoresistance by sponging miR-15a. However, several issues regarding lncRNA studies still exist in this field. First, no uniform cutoff values are available for lncRNA detection, and there is no standard detection method. Moreover, dysregulated lncRNAs are detected in more than one cancer, and the role of lncRNAs in lymphomas needs more investigation. Additionally, lncRNAs being used as biological therapies in lymphoma need to be rigorously evaluated.
Translational control plays a critical role in the regulation of gene expression in eukaryotes and affects many essential cellular processes, including cell cycle progression, proliferation, apoptosis and differentiation.24 Since EIF4E is activated or overexpressed in a large number of tumors, there has been considerable effort to target EIF4E for cancer treatment. EIF4E could be regulated by several pathways, such as the PI3K/AKT/mTOR and MAPK pathways.19 Therefore, previous studies indicated that inhibition of these pathways might inhibit the activities of EIF4E, therefore affecting the expression of target genes. However, these inhibitors might influence a variety of signals, and accurately targeting of EIF4E phosphorylation or EIF4E-EIF4G interactions is still quite difficult.31,32 In addition, studies that reported the epigenetic regulation of EIF4E are still quite few. Hu et al reported that lncRNA GAS5 cooperates with EIF4E to regulate c-Myc translation.33 In our study, we showed that lncRNA MCM3AP-AS1 promoted the expression of EIF4E by sponging miR-15a in lymphoma cells. Therefore, targeting MCM3AP-AS1 might become a promising strategy for precisely modulating the expression of EIF4E and its downstream proteins.
MiR-15a has been shown to be downregulated and might function as a tumor suppressor in several kinds of cancer. It was firstly discovered in chronic lymphocytic leukemia (CLL), while restoring its expression enhances apoptosis and arrests the cell cycle of cancer cells. Therefore, researchers aim to enhance the chemosensitivity of tumors by upregulating miR-15a. Del13 (q14) is the most common cytogenetic abnormality in CLL and is usually associated with good prognosis and is located exactly in the 13 (q14) region. It was shown that miR-15a downregulates Bcl-2 at the posttranscriptional level.34 Furthermore, another study showed that p53 directly or indirectly upregulates miR-15a/16-1, whereas the increased viability of leukemia cells may be due to the loss of p53 and the downregulation of miR-15a.35 In this study, we demonstrated that miR-15a might regulate the expression profile of these anti-apoptotic proteins by directly regulating upstream EIF4E. MiR-15a mimic transfection also increased drug sensitivity and enhanced apoptosis compared to that in the miR-NC group.
Conclusion
This study identified a novel lncRNA, MCM3AP-AS1, that was upregulated in Burkitt lymphoma tissues and indicated poor prognosis. Knockdown of MCM3AP-AS1 increased drug sensitivity, enhanced cell cycle progression and facilitated apoptosis by regulating EIF4E and its downstream anti-apoptotic proteins in vitro and in vivo. MiR-15a functioned as the link between MCM3AP-AS1 and EIF4E. Therefore, targeting the MCM3AP-AS1/miR-15a/EIF4E axis might become a promising target in the treatment of B-NHL.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Jakic-Razumovic J, Aurer I. The World Health Organization classification of lymphomas. Croat Med J. 2002;43(5):527–534.
2. Kalisz K, Alessandrino F, Beck R, et al. An update on Burkitt lymphoma: a review of pathogenesis and multimodality imaging assessment of disease presentation, treatment response, and recurrence. Insights Imaging. 2019;10(1):56. doi:10.1186/s13244-019-0733-7
3. Jacobson C, LaCasce A. How I treat Burkitt lymphoma in adults. Blood. 2014;124(19):2913–2920. doi:10.1182/blood-2014-06-538504
4. Kim H, Park ES, Lee SH, et al. Clinical outcome of relapsed or refractory Burkitt lymphoma and mature B-cell lymphoblastic leukemia in children and adolescents. Cancer Res Treat. 2014;46(4):358–365. doi:10.4143/crt.2013.047
5. Ponting CP, Oliver PL, Reik W. Evolution and functions of long noncoding RNAs. Cell. 2009;136(4):629–641. doi:10.1016/j.cell.2009.02.006
6. Wei L, Wang X, Lv L, Zheng Y, Zhang N, Yang M. The emerging role of noncoding RNAs in colorectal cancer chemoresistance. Cell Oncol (Dordr). 2019;42(6):757–768. doi:10.1007/s13402-019-00466-8
7. Puvvula PK. LncRNAs regulatory networks in cellular senescence. Int J Mol Sci. 2019;20(11):
8. Mirhosseini SA, Sarfi M, Samavarchi Tehrani S, Mirazakhani M, Maniati M, Amani J. Modulation of cancer cell signaling by long noncoding RNAs. J Cell Biochem. 2019;120(8):12224–12246. doi:10.1002/jcb.28847
9. Wang J, Zhang X, Chen W, Hu X, Li J, Liu C. Regulatory roles of long noncoding RNAs implicated in cancer hallmarks. Int J Cancer. 2020;146(4):906–916. doi:10.1002/ijc.32277
10. Jiang W, Xia J, Xie S, et al. Long non-coding RNAs as a determinant of cancer drug resistance: towards the overcoming of chemoresistance via modulation of lncRNAs. Drug Resist Updat. 2020;50:100683. doi:10.1016/j.drup.2020.100683
11. Wang QM, Lian GY, Song Y, Huang YF, Gong Y. LncRNA MALAT1 promotes tumorigenesis and immune escape of diffuse large B cell lymphoma by sponging miR-195. Life Sci. 2019;231:116335. doi:10.1016/j.lfs.2019.03.040
12. Ngoc PCT, Tan SH, Tan TK, et al. Identification of novel lncRNAs regulated by the TAL1 complex in T-cell acute lymphoblastic leukemia. Leukemia. 2018;32(10):2138–2151. doi:10.1038/s41375-018-0110-4
13. Huang PS, Chung IH, Lin YH, Lin TK, Chen WJ, Lin KH. The long non-coding RNA MIR503HG enhances proliferation of human ALK-negative anaplastic large-cell lymphoma. Int J Mol Sci. 2018;19(5):
14. Zhou M, Zhao H, Xu W, Bao S, Cheng L, Sun J. Discovery and validation of immune-associated long non-coding RNA biomarkers associated with clinically molecular subtype and prognosis in diffuse large B cell lymphoma. Mol Cancer. 2017;16(1):16. doi:10.1186/s12943-017-0580-4
15. Wang Y, Yang L, Chen T, et al. A novel lncRNA MCM3AP-AS1 promotes the growth of hepatocellular carcinoma by targeting miR-194-5p/FOXA1 axis. Mol Cancer. 2019;18(1):28. doi:10.1186/s12943-019-0957-7
16. Liang M, Jia J, Chen L, et al. LncRNA MCM3AP-AS1 promotes proliferation and invasion through regulating miR-211-5p/SPARC axis in papillary thyroid cancer. Endocrine. 2019;65(2):318–326. doi:10.1007/s12020-019-01939-4
17. Lou W, Ding B, Fu P. Pseudogene-derived lncRNAs and their miRNA sponging mechanism in human cancer. Front Cell Dev Biol. 2020;8:85. doi:10.3389/fcell.2020.00085
18. Tarantelli C, Lupia A, Stathis A, Bertoni F. Is there a role for dual PI3K/mTOR inhibitors for patients affected with lymphoma? Int J Mol Sci. 2020;21(3):
19. Siddiqui N, Sonenberg N. Signalling to eIF4E in cancer. Biochem Soc Trans. 2015;43(5):763–772. doi:10.1042/BST20150126
20. Hua H, Kong Q, Zhang H, Wang J, Luo T, Jiang Y. Targeting mTOR for cancer therapy. J Hematol Oncol. 2019;12(1):71. doi:10.1186/s13045-019-0754-1
21. Lee YH, Martin-Orozco N, Zheng P, et al. Inhibition of the B7-H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27(8):1034–1045. doi:10.1038/cr.2017.90
22. Li C, Peng W, Song X, Wang Q, Wang W. Anticancer effect of icaritin inhibits cell growth of colon cancer through reactive oxygen species, Bcl-2 and cyclin D1/E signaling. Oncol Lett. 2016;12(5):3537–3542. doi:10.3892/ol.2016.5089
23. Vitale A, Manciocco A, Alleva E. The 3R principle and the use of non-human primates in the study of neurodegenerative diseases: the case of Parkinson’s disease. Neurosci Biobehav Rev. 2009;33(1):33–47. doi:10.1016/j.neubiorev.2008.08.006
24. Bitterman PB, Polunovsky VA. eIF4E-mediated translational control of cancer incidence. Biochim Biophys Acta. 2015;1849(7):774–780. doi:10.1016/j.bbagrm.2014.09.007
25. Jiang C, Li X, Zhao H, Liu H. Long non-coding RNAs: potential new biomarkers for predicting tumor invasion and metastasis. Mol Cancer. 2016;15(1):62. doi:10.1186/s12943-016-0545-z
26. Tayari MM, Winkle M, Kortman G, et al. Long noncoding RNA expression profiling in normal B-cell subsets and Hodgkin lymphoma reveals Hodgkin and reed-sternberg cell-specific long noncoding RNAs. Am J Pathol. 2016;186(9):2462–2472. doi:10.1016/j.ajpath.2016.05.011
27. Yan Y, Han J, Li Z, Yang H, Sui Y, Wang M. Elevated RNA expression of long noncoding HOTAIR promotes cell proliferation and predicts a poor prognosis in patients with diffuse large B cell lymphoma. Mol Med Rep. 2016;13(6):5125–5131. doi:10.3892/mmr.2016.5190
28. Sehgal L, Mathur R, Braun FK, et al. FAS-antisense 1 lncRNA and production of soluble versus membrane Fas in B-cell lymphoma. Leukemia. 2014;28(12):2376–2387. doi:10.1038/leu.2014.126
29. Doose G, Haake A, Bernhart SH, et al. MINCR is a MYC-induced lncRNA able to modulate MYC’s transcriptional network in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 2015;112(38):E5261–5270. doi:10.1073/pnas.1505753112
30. Liu D, Zhang XX, Xi BX, et al. Sine oculis homeobox homolog 1 promotes DNA replication and cell proliferation in cervical cancer. Int J Oncol. 2014;45(3):1232–1240. doi:10.3892/ijo.2014.2510
31. Konicek BW, Dumstorf CA, Graff JR. Targeting the eIF4F translation initiation complex for cancer therapy. Cell Cycle. 2008;7(16):2466–2471. doi:10.4161/cc.7.16.6464
32. Papadopoulos E, Jenni S, Kabha E, et al. Structure of the eukaryotic translation initiation factor eIF4E in complex with 4EGI-1 reveals an allosteric mechanism for dissociating eIF4G. Proc Natl Acad Sci U S A. 2014;111(31):E3187–3195. doi:10.1073/pnas.1410250111
33. Hu G, Lou Z, Gupta M, Kim YK. The long non-coding RNA GAS5 cooperates with the eukaryotic translation initiation factor 4E to regulate c-Myc translation. PLoS One. 2014;9(9):e107016. doi:10.1371/journal.pone.0107016
34. Cimmino A, Calin GA, Fabbri M, et al. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102(39):13944–13949. doi:10.1073/pnas.0506654102
35. Fabbri M, Bottoni A, Shimizu M, et al. Association of a microRNA/TP53 feedback circuitry with pathogenesis and outcome of B-cell chronic lymphocytic leukemia. JAMA. 2011;305(1):59–67. doi:10.1001/jama.2010.1919
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.