Back to Journals » OncoTargets and Therapy » Volume 13
KIF5B-EGFR Fusion: A Novel EGFR Mutation in Lung Adenocarcinoma
Received 25 May 2020
Accepted for publication 3 August 2020
Published 20 August 2020 Volume 2020:13 Pages 8317—8321
DOI https://doi.org/10.2147/OTT.S263994
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Federico Perche
Hui Xu,1 Chuan Shao1– 3
1Department of Pulmonary and Critical Care Medicine, Ningbo Medical Center Lihuili Hospital, Ningbo, Zhejiang, People’s Republic of China; 2Department of Special Procurement Ward, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China; 3Department of General Practice, The First Affiliated Hospital of Soochow University, Suzhou, Jiangsu, People’s Republic of China
Correspondence: Chuan Shao Department of Pulmonary and Critical Care Medicine
Ningbo Medical Center Lihuili Hospital, No. 1111 Jiangnan Road, Ningbo, Zhejiang 315040, People’s Republic of China
Tel +86 18758353202
Email [email protected]
Abstract: With the rapid development of detection methods, next-generation sequencing (NGS) technology as a new technology, some novel epidermal growth factor receptor (EGFR) gene fusions comprising the EGFR linked to various fusion partners, most commonly RAD51, are detected in the non-small cell lung cancer (NSCLC). We described a case of an unreported kinesin family member 5B (KIF5B)-EGFR in NSCLC and the efficacy of EGFR tyrosine kinase inhibitors (TKIs) to this specific fusion. Considering this rare EGFR fusion and remarkable response to EGFR-TKI, we should realize the importance of these rare EGFR fusions, the advances in diagnostic techniques and personalized care for lung cancer patients.
Keywords: non-small cell lung cancer, adenocarcinoma, KIF5B-EGFR, EGFR-TKIs, next-generation sequencing
Introduction
Oncogenic mutations in the epidermal growth factor receptor (EGFR) gene serve as important predictive biomarkers in the non-small cell lung cancer (NSCLC). Most of these mutations commonly occur as in-frame deletions, insertions, or point mutations within exons 18–21 consisted of the kinase domain in EGFR and confer constitutive activity and sensitivity to EGFR tyrosine kinase inhibitors (TKIs).1 For EGFR wild-type patients, EGFR-TKIs still had a low ORR (1–9%).2 Uncommon EGFR mutation may be existing in these wild-type patients. Currently, next-generation sequencing (NGS) emerged as a new diagnostic approach for detection, more and more rare or atypical EGFR mutations have been identified. EGFR fusions are detected which are rare events in NSCLC.3 Several EGFR fusion partner genes have been reported, including RAD51 recombinase gene (RAD51), purine-rich element binding protein B gene (PURB), tensin 3 gene (TNS3), zinc finger CCHC-type containing 6 gene (ZCCHC6) and septin 14 gene (SEPTIN14) in NSCLC, most commonly EGFR-RAD51.3–6 Herein, we reported for the first time, the presence of EGFR fusion, kinesin family member 5B (KIF5B)-EGFR in NSCLC. Moreover, we have demonstrated that the patient experienced a remarkable response to EGFR-TKIs. Institutional approval was required to publish the case details. The informed consent was obtained from the patient prior to study commencement and the patient provided informed consent to have their case details, and accompanying images, published. The case report was approved by the ethics committee of Ningbo Medical Center Lihuili Hospital.
Case Report
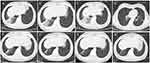
A 45-year-old Chinese woman presented with half a month history of cough and expectoration to our hospital in May 2019. Physical examinations were unremarkable. No obvious abnormalities were found in laboratory tests, including blood routine, serum biochemistry and tumor biomarkers. A computed tomography (CT) scan of the chest on May 25 revealed a high-density shadow in inferior lobe of right lung (Figure 1A). The enhanced CT was performed after 2 weeks’ treatment with oral moxifloxacin on June 7, which revealed a little progress of the lesion (Figure 1B). The patient underwent bronchoscopy and aspergillus fumigatus was detected in bronchoalveolar lavage fluid. Therefore, the patient was treated with oral voriconazole (200mg q12h) for more than 1 months. However, the patient’s symptoms worsened. The re-checked CT on July 27 demonstrated marked progression of the lesion in inferior lobe of right lung (Figure 1C) and a new lesion of ground grass opacity (GGO) was also found in the lower left lobe (Figure 1D). Then, voriconazole treatment was discontinued and the patient underwent CT-guided percutaneous lung biopsy and pathological results showed lung adenocarcinoma (Figure 2). The tumor tissue was subjected to next-generation sequencing (NGS), using a commercial gene panel targeting 168 cancer-related genes (Burning Rock Biotech Ltd, Guangzhou, China). The NGS reports revealed the KIF5B-EGFR, a novel rearrangement of the EGFR gene that has not been reported previously (Figure 3). The patient began treatment with afatinib (40mg qd po) and bevacizumab (400mg q3w ivgtt) since August 14. She experienced sustained clinical improvement after anti-tumor therapy and a CT scan on October 13 revealed marked decrease of the infiltrations on the lower right lung (Figure 1E) and the absence of GGO on the left lung, assessed as partial remission. The patient had mild rash and diarrhea during treatment, which was considered an adverse reaction associated with EGFR-TKI. Encouragingly, the lesion on the right lower lung continued to shrink during the treatment (Figure 1F–H). Thus far, the patient feels well and the disease has remained stable. She has been continuing treatment with afatinib and bevacizumab and will be followed up regularly.
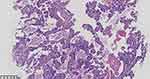 |
Figure 2 Pathological results of tissue obtained through CT-guided percutaneous lung biopsy showing adenocarcinoma (hematoxylin and eosin; magnification ×200). |
 |
Figure 3 Integrative Genomics Viewer snapshot of EGFR-KIF5B fusion. |
Discussion
In clinical practice, EGFR fusions are rare in NSCLC. Previously, EGFR fusions were observed in large cohorts such as Memorial Sloan Kettering–Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT), Foundation Medicine (FM), which are found at a frequency of 0.05% to 0.13% in the MSK-IMPACT and FM cohorts.6 EGFR-RAD51 is the most common type of EGFR fusions. It is oncogenic, able to mediate downstream signaling through the MAPK and PI3K/AKT pathways and can be therapeutically targeted with available EGFR-TKIs.4 Other EGFR fusion partner genes have also been reported, including PURB, TNS3, ZCCHC6 and SEPTIN14 in NSCLC.3–6 In this case, we provided the first report of a patient with the fusion of KIF5B exon15 to EGFR exon 18 in a primary lung adenocarcinoma through NGS. She responded well to treatment with afatinib and bevacizumab.
We identified the novel KIF5B-EGFR rearrangement through NGS. Traditional methods for detection of EGFR fusion have limitations. Immunohistochemistry and fluorescence in situ hybridization depend on diagnostic expertise, and reverse-transcriptase polymerase chain reaction is unable to detect novel chromosomal rearrangements. Compared with traditional methods, NGS allows for multiplex testing and enables the detection of known as well as uncommon genomic events. More and more rare or atypical EGFR mutations have been identified through NGS.
KIF5B is located on the short arm of human chromosome 10 and encodes member 5B of the kinesin family of proteins. Its product is a component of a motor protein complex that is associated with microtubules and mediates the transport of organelles within eukaryotic cells.7 There is currently no report on KIF5B-EGFR. In this case, we provided the first report of a patient with the fusion of KIF5B exon15 to EGFR exon 18 that contains the EGFR tyrosine kinase domain in a primary lung adenocarcinoma. Konduri et al described five patients with metastatic lung cancer whose tumors harbored EGFR fusions and verified that Ba/F3 cells expressing EGFR-RAD51 were sensitive to different first-third generation EGFR-TKI drugs in vitro.4 Afatinib is an irreversible inhibitor of the ErbB family that is expected to inhibit tumors with activating EGFR mutations more strongly than are reversible EGFR-TKIs. Several clinical trials have shown favorable PFS with acceptable toxicity profiles for combination therapy consisting of bevacizumab and EGFR-TKIs in untreated EGFR-mutant tumors.8–10 In consideration of the synergistic effects of afatinib and bevacizumab, we experimentally treated the KIF5B-EGFR patient with afatinib and bevacizumab. The patient exhibited a significant partial response after treatment with afatinib and bevacizumab for 2 months.
It is worth noting that in this patient, although the lesion progressed after treatments of moxifloxacin and voriconazole, co-infection cannot be ruled out absolutely. Besides, the possibility of delayed absorption of lung infiltrations caused by fungal infection should not be neglected. In addition, the favorable response of the patient to anti-tumor therapy should be attributed to the combined effects of afatinib and bevacizumab. We did not explore the mechanism of EGFR-TKI on this new target, which can be a direction for future efforts.
Conclusion
In the present case, the patient of lung adenocarcinoma with KIF5B-EGFR fusion achieved a remarkable remission from treatment with EGFR-TKIs and the monoclonal antibody to vascular endothelial growth factor A. We provided case experiences supporting the use of afatinib and bevacizumab for treatment of the patients with NSCLC who are harboring KIF5B-EGFR fusion.
Acknowledgments
We thank Dr. Yuanyuan Xu and Yibing He for their great efforts in the care for this patient, and we also thank Dr. Xianfa Xu for his great help in the preparation of pathological images.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sharma SV, Bell DW, Settleman J, Haber DA. Epidermal growth factor receptor mutations in lung cancer. Nat Rev Cancer. 2007;7(3):169–181. doi:10.1038/nrc2088
2. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a Phase III, randomized, open-label, first-line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non-small-cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29(21):2866–2874. doi:10.1200/JCO.2010.33.4235
3. Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703–713. doi:10.1038/nm.4333
4. Konduri K, Gallant JN, Chae YK, et al. EGFR fusions as novel therapeutic targets in lung cancer. Cancer Discov. 2016;6(6):601–611. doi:10.1158/2159-8290.CD-16-0075
5. Zhu YC, Wang WX, Li XL, et al. Identification of a novel icotinib-sensitive EGFR-SEPTIN14 fusion variant in lung adenocarcinoma by next-generation sequencing. J Thorac Oncol. 2019;14(8):e181–e183. doi:10.1016/j.jtho.2019.03.031
6. Raez LE, Pinto JA, Schrock AB, Ali SM. EGFR - RAD51 fusion: a targetable partnership originated from the tumor evolution? J Thorac Oncol. 2018;13(3):e33–e34. doi:10.1016/j.jtho.2017.10.005
7. Sablin EP. Kinesins and microtubules: their structures and motor mechanisms. Curr Opin Cell Biol. 2000;12(1):35–41. doi:10.1016/S0955-0674(99)00054-X
8. Stinchcombe TE, Jänne PA, Wang X, et al. Effect of erlotinib plus bevacizumab vs erlotinib alone on progression-free survival in patients with advanced EGFR-mutant non-small cell lung cancer: a phase 2 randomized clinical trial. JAMA Oncol. 2019. doi:10.1001/jamaoncol.2019.1847
9. Ichihara E, Hotta K, Nogami N, et al. Phase II trial of gefitinib in combination with bevacizumab as first-line therapy for advanced non-small cell lung cancer with activating EGFR gene mutations: the Okayama lung cancer study group trial 1001. J Thorac Oncol. 2015;10(3):486–491. doi:10.1097/JTO.0000000000000434
10. Ninomiya T, Nogami N, Kozuki T, et al. A Phase I trial of afatinib and bevacizumab in chemo-naïve patients with advanced non-small-cell lung cancer harboring EGFR mutations: Okayama lung cancer study group trial 1404. Lung Cancer. 2018;115:103–108. doi:10.1016/j.lungcan.2017.11.025
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.