Back to Journals » Drug Design, Development and Therapy » Volume 16
Ketamine Attenuates Airway Inflammation via Inducing Inflammatory Cells Apoptosis and Activating Nrf2 Pathway in a Mixed-Granulocytic Murine Asthma Model
Authors Xiao S, Zhou Y, Wang Q , Yang D
Received 23 September 2022
Accepted for publication 15 December 2022
Published 28 December 2022 Volume 2022:16 Pages 4411—4428
DOI https://doi.org/10.2147/DDDT.S391010
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Manfred Ogris
Shilin Xiao, Ying Zhou, Qianyu Wang, Dong Yang
Department of Anesthesiology, Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, People’s Republic of China
Correspondence: Dong Yang, Department of Anesthesiology, Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, 33 Badachu Road, Shijingshan, Beijing, 100144, People’s Republic of China, Tel +86-13661267522, Email [email protected]
Purpose: The use of ketamine, an anesthetic, as a treatment for asthma has been investigated in numerous studies. However, how ketamine affects asthma is unclear. The present study examined the effects of ketamine on a murine model of mixed-granulocytic asthma, and the role of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway.
Methods: The murine model of mixed-granulocytic asthma was established using ovalbumin (OVA) for sensitization and the combination of OVA and lipopolysaccharides (LPS) for challenge. The main characteristics of asthma, oxidative stress biomarkers, and the expression of the Nrf2 pathway were examined. ML385 was administered to verify the role of the Nrf2 pathway.
Results: Mice in the OVA +LPS group developed asthmatic characteristics, including airway hyperresponsiveness, mixed-granulocytic airway inflammation, mucus overproduction, as well as increased levels of oxidative stress and impaired apoptosis of inflammatory cells. Among the three concentrations, ketamine at 75mg/kg effectively attenuated these asthmatic symptoms, activated the Nrf2 pathway, decreased oxidative stress, and induced apoptosis of eosinophils and neutrophils in bronchoalveolar lavage fluid (BALF) with a reducing level of myeloid cell leukemia 1(Mcl-1). ML385 (an Nrf2 inhibitor) eliminated the protective effects of ketamine on the mixed-granulocytic asthma model.
Conclusion: The study concluded that ketamine reduced oxidative stress and attenuated asthmatic symptoms (neutrophilic airway inflammation) by activating the Nrf2-Keap1 pathway, with 75 mg/kg ketamine showing the best results. Ketamine administration also increased neutrophil and eosinophil apoptosis in BALF, which may contribute to the resolution of inflammation. The use of ketamine as a treatment for asthma may therefore be beneficial.
Keywords: ketamine, asthma, inflammation, apoptosis, oxidative stress, NF-E2-related factor 2
Introduction
More than 350 million people worldwide suffer from asthma, and its prevalence has increased over the past 40 years, making it a major public health issue.1 Airway hyperresponsiveness, airway inflammation, and mucus overproduction are the primary features of asthma, and the other two symptoms are closely related to inflammation.2
Eosinophilic, neutrophilic, mixed-granulocytic, and paucigranulocytic asthma are classified according to the percentage of granulocytes in the sputum. Mixed-granulocytic asthma (sputum eosinophils ≥2% and neutrophils ≥40%) has been associated with more severe asthma phenotypes3 and is associated with lower lung function and a greater frequency of wheezing.2 These patients respond poorly to guideline-based therapy, which can account for most of the healthcare costs and economic burden associated with asthma.4 The existence of neutrophilic airway inflammation usually indicates asthma exacerbation5,6 poor response to conventional treatment.7 Aside from the release of inflammatory mediators, impaired or delayed inflammatory cell apoptosis, such as eosinophils8 and neutrophils,9 which is observed in asthma, also contributes to the sustained airway inflammation.
Oxidative stress is a cellular state representing the imbalance of oxidants and antioxidants,10 it not only intensifies inflammation but also regulates cell apoptosis, smooth muscle hypertrophy, mucus overproduction, and fibrosis in the airways.11 The asthmatics have shown increased oxidant substances and impaired antioxidant systems in previous studies,12 reactive oxygen species (ROS) produced by inflammatory responses is one of the major sources of oxidative stress.13
Nrf2 is a transcription factor that plays an important role in maintaining redox balance. Under normal conditions, Nrf2 remains inactive in the cytoplasm bound to its inhibitor Keap1.14 Oxidative stress activates the Nrf2 by dissociating it from Keap1, and its translocation into the nucleus stimulates the expression of some antioxidant genes, such as glutathione peroxidase (GPx), heme oxygenase-1 (HO −1), and so on.15
Ketamine is an uncompetitive N-methyl-D-aspartate receptors (NMDARs) antagonist and has long been used as an anesthetic since 1970.16 Ketamine is the anesthetic drug of choice for patients with heart diseases and active bronchospasm since it not only relaxes the bronchial muscle, but help supports hemodynamic stability as well.17 In addition to anesthesia, ketamine is also widely used for sedation18 and pain management (both acute19,20 and chronic21 pain) with less respiratory adverse effects, more rapid onset of effects, and longer duration in clinical practice. In addition to these common applications, ketamine has received considerable attention for its use in neurological disorders22,23 and depression,24 which indicated some new properties of ketamine, such as the regulation of inflammation, apoptosis, oxidative stress, and the immune system.25 Applications of ketamine in asthma management have been investigated in some clinical trials,26–29 but controversial conclusions have been drawn and the underlying mechanisms need to be examined further.
It has been shown that ketamine can balance the production of oxidants and antioxidants,30,31 which is critical to asthma pathogenesis.32 Using a murine model of mixed-granulocytic asthma, we examined the effects of ketamine on oxidative stress and mixed-granulocytic airway inflammation. The findings indicate that the Nrf2/Keap1 pathway mediates the therapeutic effects of ketamine on the murine model of mixed-granulocytic asthma and may play a role in the regulation of neutrophil apoptosis by ketamine.
Materials and Methods
Animals
Balb/c mice (female; age: 6–7 weeks; weight: 20±2 g, Beijing Vital River Laboratory Animal Technology Co, Ltd.) were housed in a controlled condition (25°C, 12-hour light/dark cycles) in the Animal Experimental Center. Mice were provided with sterilized food and tap water ad libitum. The experiment began after a week of acclimatization. The experiments were approved by the Institutional Animal Care and Use Committee of Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
OVA+LPS-Induced Asthma Model
OVA and LPS were used in the establishment of a mixed-granulocytic asthma model according to the protocol of Yu et al33 with some modifications. There were six groups of mice in the present study (Figure 1). Mice in the Control group and the K100 group were sensitized with 100μL saline on day 0 and 7 intraperitoneally. On day 14–17, the Control group received 30 μL saline intranasally once daily, and the K100 group received an intraperitoneal injection of 100 mg/kg ketamine (100 mg/2 mL Ketamine Hydrochloride Injection (Jiangsu HengRui Medicine Co., Jiangsu, China) diluted to 10 mg/mL with sterile PBS) once a day.
On day 0 and 7, mice in the OVA +LPS, OVA +LPS+K50, OVA +LPS+K75, OVA +LPS+K100, and ML385 groups were i.p. sensitized with 100 μL saline containing 200 μg OVA (Sigma Aldrich; Merck KGaA, Germany) and 2 mg aluminum hydroxide (Sigma Aldrich; Merck KGaA, Germany). From day 14 to 17, mice were administrated intranasally with 30 μL saline containing 25μg OVA + 10μg LPS (Sigma Aldrich; Merck KGaA, Germany) once daily for challenge. Mice in the OVA +LPS+K50, OVA +LPS+K75, and OVA +LPS+K100 group received an i.p. injection of ketamine (50, 75, and 100 mg/kg) one hour before challenge. ML385 (MedChemExpress, New Jersey, USA) was diluted with DMSO and corn oil to a working solution of 2 mg/mL, with a final concentration of DMSO of 1%. Mice in the ML385 group received an intraperitoneal injection of ML385 working solution (30mg/kg) 2h before challenge and an i.p. injection of ketamine (75mg/kg) one hour before challenge. The measurement of AHR and collection of bronchoalveolar lavage fluid (BALF) were performed on day 18.
Assessment of AHR
FlexiVent (SCIREQ) was used to detect airway resistance in response to inhaled methacholine (Mch). Mice were anesthetized with 2% pentobarbital sodium (50 mg/kg). After tracheotomy and catheterization, mice were placed in a chamber for measurement. For each animal, respiratory resistance (Rrs) in response to inhaled Mch of different doses (0, 6, 12, 24, and 48 mg/mL) was measured for 6 min. Data of each concentration was presented as the mean value of 6 min in cmH2O/mL/sec. For each concentration, the Rrs of mice in the Control group is regarded as the baseline (presented as 100%), and Rrs of mice in other groups are described as the percent increase in Rrs over the baseline.
Analysis of BALF
After being euthanized with 2% pentobarbital sodium (150 mg/kg), the left lung was ligated and the right lung was lavaged with 0.8 mL cold DPBS three times through a catheter. After being centrifuged (1500 rpm; 4°C) for 10 minutes, the supernatant of BALF was stored at −80°C for cytokines detection, and the cell deposits were resuspended with 500 μL DPBS for cell counting and BALF smear. Following the manufacturer’s protocol, BALF smears were stained with Wright-Giemsa stain solution, which is purchased from Beijing Solarbio Science & Technology Co., Ltd. (Beijing, China), to differentiate eosinophils and neutrophils. Total cells and differentiated cells were counted using ImageJ software (version 1.8.0; National Institutes of Health).
Histological Analysis
After the collection of BALF, the left lung was collected after the pulmonary circulation perfusion with saline. To fixate the tissue, Lung tissues were placed in 4% paraformaldehyde for 48 hours at room temperature before embedment. For hematoxylin and eosin (H&E) staining, lung tissues were embedded in paraffin and then cut into 5µm-thick slides. For periodic acid Schiff (PAS) staining, lung tissues were embedded in OCT and then cut into 5µm-thick slides. A commercial PAS staining kit from Beijing Solarbio Science & Technology Co., Ltd. was used. Different scoring systems for the evaluation of histological changes in H&E- and PAS-stained sections have been described in our previous work.34 The scoring system for assessment of peribronchial inflammation in H&E-stained sections: 0, normal; 1, a few cells; 2, one cell layer around airways; 3, two-four cell layers around airways; 4, five-seven cell layers around airways; 5, eight-ten cell layers around airways; 6, more than ten cell layers around airways. The percent of PAS-positive cells in the airway epithelium was measured via Image J software (version 1.8.0), and results were graded to assess mucus secretion using a scoring system as follows: 0, less than 2%; 1, less than 20% and more than 2% (including 2%); 2, less than 40% and more than 20% (including 20%); 3, less than 60% and more than 40% (including 40%); 4, less than 80% and more than 60% (including 60%); and 5, more than 80% (including 80%). The slides were observed under an upright BX53 microscope (Olympus Corporation, Tokyo, Japan) and a total of five fields of view were examined by an experienced pathologist blindly.
Enzyme-Linked Immunosorbent Assay (ELISA)
The commercial ELISA kits were purchased from Boster Biological Technology (Wuhan, China). According to the manufacturer’s protocol, the cytokines in BALF, including Th2-cytokine (IL −4, IL −5, and IL −13), IL-6, IL-1β, TNF-α, and IL-8 were detected using ELISA kits.
Assessment of Malondialdehyde (MDA), Superoxide Dismutases (SOD), and GPx
The commercial kits for the detection of MDA, SOD, and GPx were purchased from Beyotime Biotechnology (Shanghai, China) following the instructions of the manufacturer. The sample type used for the measurements was the supernatant of homogenized lung tissue (50mg).
Immunohistochemistry (IHC)
5 μm-thick Paraffin sections were used for the detection of Bax and Mcl-1 proteins by immunohistochemistry. The primary antibodies include anti-Bax (Proteintech. lnc, Rosemont, IL, USA; 1:100) and anti-Mcl-1 (Abmart, Shanghai, China; 1:100). The SPlink Detection Kit and DAB Peroxidase Color substrate kit were purchased from ZSGB-BIO (Beijing, China). The sections were deparaffinized using xylene and dehydrated using alcohol at graded concentrations. For antigen retrieval, sections were incubated in Sodium Citrate solution and heated for 20 minutes using a microwave oven. The following steps followed the manufacturer’s protocol. Incubate the slides with DAB working solution in the dark for 4–5 min, and control the reaction time by checking the color change of slides under the microscope. After achieving the optimal color, stop the reaction by washing the slides with tap water. The nucleus was redyed with hematoxylin, and the slides were observed via an upright BX53 microscope (Olympus Corporation, Tokyo, Japan).
Dihydroethidium Staining
Frozen lung sections were cut into 5 μm-thick slides and stained with Dihydroethidium (Beyotime Biotechnology, Shanghai, China) to detect the content of ROS following the manufacturer’s protocol. The slides were observed via a fluorescence microscope (BX53, Olympus, Tokyo, Japan) at 535 nm.
Western Blot
The specific protein expression in lung tissues were assessed using Western blot. A commercial Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Biotechnology, Shanghai, China) was used for the extraction of nuclear protein following the manufacturer’s protocol. 50mg of lung tissues were homogenized in RIPA lysis buffer (Applygen, Beijing, China) containing protease inhibitors (Beyotime Biotechnology, Shanghai, China) for protein extraction. The commercial BCA protein estimation kit was purchased from Beyotime Biotechnology. Protein was lysed in 5X SDS-PAGE Protein Sample Loading Buffer (Beyotime Biotechnology, Shanghai, China) and then denatured by being heated in a 95 °C water bath for 8 min. A total of 30 μg protein were separated in Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and were transferred to nitrocellulose membranes via wet transfer. After wet transfer, membranes were incubated in 5% BSA and blocked for 2 h at room temperature. The membranes were incubated with primary antibodies overnight at 4 °C and washed with TBST for 10 min 3 times before secondary antibodies incubation. The membranes were incubated with corresponding secondary antibodies for 1 h at room temperature. ECL detection reagent, purchased from Beyotime Biotechnology (Shanghai, China), was used for protein detection following the instructions. The visualization of proteins was performed using Chemi-Doc (Bio-Rad, Hercules, CA, USA) and semi-quantitative analysis was performed via Image J software (version 1.8.0). Primary antibodies used in the experiment included Nrf2 (Proteintech. Lnc, Rosemont, IL, USA, 1:1000), Keap1 (Proteintech. Lnc, Rosemont, IL, USA, 1:1000), HO −1(Santa Cruz, Dallas, TX, USA, 1:1000), GPx4 (Proteintech. Lnc, Rosemont, IL, USA, 1:1000), Bax (Proteintech. Lnc, Rosemont, IL, USA, 1:1000), Bcl-2 (Proteintech. Lnc, Rosemont, IL, USA, 1:1000), Mcl-1 (Abmart, Shanghai, China, 1:1000), Caspase-3 (Proteintech. Lnc, Rosemont, IL, USA, 1:1000), beta-Actin (Proteintech. Lnc, Rosemont, IL, USA, 1:2000), PCNA (Proteintech. Lnc, Rosemont, IL, USA, 1:2000). The secondary antibodies used for the experiment were HRP-conjugated Affinipure Goat Anti-Mouse IgG(H+L). (Proteintech. Lnc, Rosemont, IL, USA, 1:4000), HRP-conjugated Affinipure Goat Anti-Rabbit IgG(H+L) (Proteintech. Lnc, Rosemont, IL, USA, 1:4000) and m-IgGκ BP-HRP (Santa Cruz, Dallas, TX, USA, 1:1000).
Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR)
Total RNA was extracted from homogenized lung tissues (50 mg) in cold TRIzol® (Ambion; Thermo Fisher Scientific, Inc. Waltham, MA, USA) following the manufacturers’ protocol. The A260/A280 ratio (measured via NanoDrop® 2000 ultraviolet spectrophotometer (Thermo Fisher Scientific, Inc. Waltham, MA, USA)) between 1.8 and 2.0 is considered pure. The cDNA synthesis was performed using HiScript 1st Strand cDNA Synthesis Kit-V22.1 (Vazyme, Nanjing, China) according to the manufacturers’ protocol. qPCR was used for the quantification of mRNA using LightCycler® 480 SYBR Green I Master mix on a LightCycler® 96 Instrument (Roche Diagnostics GmbH, Basel, Switzerland). The primers (Invitrogen, Thermo Fisher Scientific, Inc. Waltham, MA, USA) were listed in Table 1. Primer sequences for RT-qPCR. The expression levels of mRNA were normalized to β‑actin. The 2‑ΔΔCq method35 was used to calculate the relative expression levels of genes.
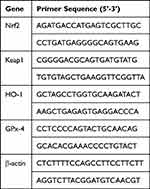 |
Table 1 Primer Sequences for RT-qPCR |
Flow Cytometric Analysis of BALF
After being filtered through a 100μm nylon mesh, BALF was centrifuged at 400 x g at 4°C for 7 minutes and then discarded the supernatant. The cells were incubated with 200 μL ACK lysis at room temperature for 2 min and then 1 mL cold DPBS was to stop the reaction. After being centrifuged at 400 x g at 4°C for 7 minutes, the supernatant was discarded and the cell pellet was resuspended with binding buffer (5% FBS) at 2 x 10^6/mL. Incubate the cells with the fluorescent dye-conjugated mouse antibodies at 4°C for 30 min and avoid direct light. Neutrophils in BALF were marked as LY-6G + (APC-CY7; BD Biosciences, NJ, USA) and CD 11b + (APC; BD Biosciences, NJ, USA); eosinophils in BALF were marked as LY-6G – (APC-CY7; BD Biosciences, NJ, USA) and Siglec-F + (BV421; BD Biosciences, NJ, USA). After being washed with 1mL FBS, the cells were centrifuged at 400 x g at 4°C for 7 minutes before the detection of apoptosis. The FITC Annexin V Apoptosis Detection Kit was purchased from BD Pharmingen (NJ, USA), and the procedures followed the manufacturer’s protocol. Cells were analyzed via a BD Biosciences flow cytometer, and the results were analyzed using FlowJo software (Version 10.8.1).
Statistical Analysis
In the present study, GraphPad Prism 9 (GraphPad Software) was used to analyze the data. For quantitative data, one-way ANOVA followed by Bonferroni’s multiple comparisons test was used for the comparison of groups with different pretreatment, and data were expressed as mean ± standard deviation (SD). The Kruskal–Wallis test was used to analyze histopathological scores, and the comparison of different groups was performed with Dunn’s multiple comparisons test. Histopathological scores were ordinal data and were presented as the median + interquartile range (IQR). The difference was considered statistically significant when P < 0.05.
Results
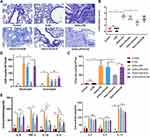
Ketamine Attenuates OVA+LPS-Induced Airway Inflammation
Histological analysis and inflammatory cell counting were used to assess airway inflammation. Significant inflammatory cell infiltration was observed in the lung tissues of asthmatic mice on the basis of histological findings (†P<0.01, vs the control group; Figure 2A and B). In comparison with the controls, both total cell and granulocyte counts (both neutrophils and eosinophils) were significantly higher in BALF (§P<0.0001 for total cell counts, ‡P<0.001 for neutrophils and eosinophils, vs the control group; Figure 2C and D).
There was a discrepancy between ketamine’s therapeutic effects on airway inflammation at three different concentrations. Compared with the OVA+LPS group, ketamine at 75 mg/kg significantly reduced airway inflammation (*P<0.05; Figure 2A and B), and reduced total cell and neutrophil counts in BALF (†P<0.01 for total cell counts and neutrophils counts; Figure 2C and D). There was less improvement in these asthmatic symptoms when ketamine was administered at 50 mg/kg or at the anesthesia concentration (100 mg/kg). However, ketamine treatment did not result in a decrease in the number of eosinophils in BALF.
Several inflammatory cytokines in BALF were examined by ELISA. Significant increases in the release of Th2 cytokines were observed in asthmatic mice (§P<0.0001 for IL-4, IL-5 and IL-13, vs the control group; Figure 2E). In spite of the fact that ketamine-treated mice showed a reduction in the release of Th2 cytokines, no statistical significance was found. The mice in the ketamine (75mg/kg) group showed the greatest reduction in cytokines associated with neutrophilic inflammation among the three concentrations (§P<0.0001 for IL-6, IL-1β and IL-8, †P<0.01 for TNF-α, vs the OVA+LPS group; Figure 2E). The release of inflammatory cytokines and airway inflammation were not affected by ketamine alone.
Ketamine Alleviates AHR and Mucus Overproduction
AHR and mucus hypersecretion are important features of allergic asthma.36 In the present study, airway resistance to inhaled methacholine was assessed and percentages of PAS-positive cells were determined in order to assess AHR and mucus production. Airway hyperresponsiveness to inhaled methacholine (§P<0.0001, vs the control group; Figure 3C) and excessive mucus secretion around airways (†P<0.01, vs the control group; Figure 3A and B) were observed in asthmatic mice in the present study. The difference of PAS score and airway resistance to different concentrations of methacholine were not significantly different between the control group and the K100 group. Compared to the asthmatic group, ketamine at 75 and 100 mg/kg significantly reduced Rrs (§P<0.0001; Figure 3C), with 75 mg/kg showing the best bronchodilating effect (†P<0.01, vs the OVA+LPS+K100 group; §P<0.0001, vs the OVA+LPS+K50 group; Figure 3C). Ketamine at 75 mg/kg greatly improved the mucus overproduction in asthmatic mice (*P<0.05, vs the OVA+LPS group; Figure 3B), whereas the administration of the other two concentrations of ketamine did not.
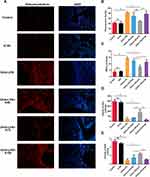
Ketamine Reduces Oxidative Stress in the Mixed-Granulocytic Asthma Model
To evaluate the oxidative stress in lung tissues, dihydroethidium staining was performed in order to determine intracellular ROS content, and MDA levels were assessed in order to determine lipid peroxidation. Asthmatic mice displayed higher ROS levels in lung tissues than the control group (‡P < 0.001; Figure 4A and B), and ketamine at 75 mg/kg significantly reduced ROS levels (†P<0.01, vs the OVA+LPS group; Figure 4A and B). The MDA level was also elevated in asthmatic mice (§P<0.0001, vs the control group; Figure 4C), and ketamine (75 mg/kg) was most effective in reducing this effect (‡P < 0.001, vs the OVA+LPS group; †P<0.01, vs the OVA+LPS+K50 group; *P<0.05, vs the OVA+LPS+K100 group; Figure 4C). Activities of SOD and GPx were measured in lung tissues to determine the antioxidant capacity. Compared with the control group, the activities of GPx (§P<0.0001; Figure 4D) and SOD (§P<0.0001; Figure 4E) were significantly decreased in the OVA + LPS group, whereas 75 mg/kg of ketamine significantly restored these activities (†P<0.01 for SOD and GPx, vs the OVA+LPS group; Figure 4D and E). In comparison with 75 mg/kg of ketamine. Ketamine at 50 and 100 mg/kg showed less improvement in antioxidant capacity and oxidative stress. The redox condition of the lung tissues was not affected by ketamine alone.
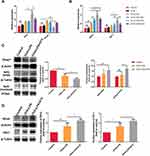
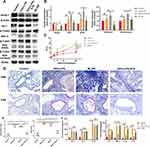
Ketamine Activates the Nrf2/Keap1 Signaling Pathway and Increases the Expression of Nrf2-Targeted Genes
Several antioxidant genes downstream of Nrf2 contribute to the reduction of inflammation. We investigated the expression of the Nrf2 pathway in lung tissues, including Nrf2, Keap1, HO-1, and Gpx4, in order to determine whether Nrf2 contributes to ketamine’s antioxidant properties. qPCR was used to detect the expression of mRNA. When compared with the control group, the expression of Nrf2 and antioxidant genes (GPx4 and HO-1) was increased without statistical significance, and the expression of Keap1 significantly decreased in asthmatic mice (*P < 0.05 for Keap1; Figure 5A and B). A 75mg/kg pretreatment increased the expression of Nrf2 and antioxidant genes (GPx4 and HO-1) significantly, whereas a 100mg/kg or 50mg/kg pretreatment only affected the expression of GPx4 (†P < 0.01 for Nrf2 and HO-1, ‡P < 0.001 for Gpx4, vs the OVA+LPS group; Figure 5A and B). As the Nrf2/Keap1 pathway is activated by the translocation of Nrf2 from the cytoplasm into the nucleus, Western blot was used to determine their expression levels. The administration of OVA and LPS significantly decreased the expression level of Keap1 (†P < 0.01, vs the Control group) and increased the expression of Nrf2 (both total and nucleus) without statistical significance (Figure 5C). It was also found that the expression of GPx4 and HO-1 was also elevated in the asthmatics, but the increase in GPx4 was not statistically significant (*P<0.05 for HO-1, vs the OVA+LPS group; Figure 5D). The results above indicated that ketamine at 75 mg/kg had a better antioxidant effect than the other concentrations, and we detected the protein levels of Keap1, Nrf2, GPx4 and HO-1 in the OVA+LPS+K75 group. As shown in Figure 5C and D, ketamine at 75 mg/kg significantly activated the Nrf2 pathway by significantly elevating the expression of nuclear Nrf2 (*P<0.05 for Nrf2, vs the OVA+LPS group) and reducing the level of Keap1 (*P<0.05 for Keap1, vs the OVA+LPS group). Ketamine pretreatment at 75 mg/kg also significantly increased the expression of Nrf2-related antioxidant proteins (*P<0.05 for GPx4 and HO-1, vs the OVA+LPS group). The results indicated that the Nrf2/Keap1 pathway was involved in the antioxidant properties of ketamine in the present model.
Ketamine Induces Neutrophils and Eosinophils Apoptosis in BALF
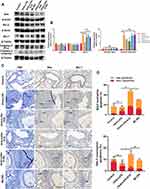
Since excessive oxidative stress caused by asthma tends to induce apoptosis in the lungs,37 we detected some apoptotic and antiapoptotic factors in the lungs using Western blot. There was no significant difference in the expression of Bax or Bcl-2 between these groups. However, administration of OVA+LPS significantly increased the level of cleaved Caspase-3 compared with the Control group (‡P < 0.001 for cleaved Caspase-3, vs the control group; Figure 6A and B). It is noteworthy that the level of Mcl-1 in asthmatic mice was remarkably higher than that in control mice (‡P < 0.001, vs the control group; Figure 6B). The expression of Mcl-1 was significantly reduced by ketamine at 75mg/kg, whereas the expression was not affected by ketamine at 50 or 100 mg/kg (†P < 0.01, vs the OVA+LPS group and OVA+LPS+K50 group; *P<0.05, vs the OVA+LPS+K100 group; Figure 6A and B).
Since Mcl-1 plays a key role in neutrophil and eosinophil survival, we examined whether ketamine could induce granulocyte apoptosis in lung tissues using IHC. The H&E staining revealed inflammatory cells infiltrating the bronchial tube, and the IHC positive cells (Bax and Mcl-1) shared the same locations with the inflammatory cells (Figure 6C). IHC results were consistent with the results of Western blot, showing an increase in expression of Bax and a decrease in expression of Mcl-1 in inflammatory cells of ketamine groups (notably at a concentration of 75) (Figure 6C). Bax and Mcl-1 were observed in inflammatory cells as indicated by the arrows.
In order to further test the hypothesis that ketamine induced the apoptosis of inflammatory cells, the apoptosis of neutrophils and eosinophils in BALF was evaluated by flow cytometric analysis. As shown in the results (Figure 6D), a significant increase in the apoptosis of neutrophils and eosinophils in BALF was observed following ketamine (75mg/kg) treatment.
ML385 was administered to further investigate the possible role of Nrf2 signaling pathway in regulating neutrophils and eosinophils apoptosis by ketamine. Although there was no significant difference in the protein levels of Bcl-1, Bax, and Caspase-3 between the ML385 and the OVA+LPS+K75 groups, ML385 significantly increased the level of Mcl-1 (*P<0.05, vs the OVA+LPS+K75 group; Figure 6A and B). Since Mcl-1 plays an important role in regulating inflammatory cell apoptosis, flow cytometry was used to detect neutrophil and eosinophil apoptosis in BALF. As shown in the results, ML385 significantly abrogated the pro-apoptotic effect of ketamine on neutrophils in BALF (†P < 0.01, vs the OVA+LPS+K75 group; Figure 6D), whereas no statistically significant reduction in apoptotic eosinophils was observed.
Inhibition of Nrf2 Reduced the Therapeutic Effect of Ketamine on the Murine Asthma Model
To investigate the role of the Nrf2/Keap1 pathway in the therapeutic effect of ketamine, ML385 (a Nrf2 inhibitor) was administered. Western blot indicated that ML385 significantly suppressed the activation of Nrf2, as demonstrated by upregulation of Keap1 and downregulation of nuclear Nrf2, as well as decreased the levels of Gpx4 and HO-1 compared to the OVA+LPS+K75 group (Figure 7A and B). In addition, ML385 treatment abrogated ketamine-induced reductions in AHR, airway inflammation, and mucus production (Figure 7C–E). Compared with the OVA+LPS+K75 group, ML385 effectively restored the expression of proinflammatory cytokines, including IL-6, TNF-α, IL-1β, and IL-8, whereas the expression of Th2-cytokines did not differ between the ML385 group and the OVA+LPS+K75 group. The results indicated that the therapeutic effect of ketamine is modulated by the Nrf2 signaling pathway.
Discussion
Ketamine, a non-competitive antagonist of NMDAR, has been used clinically as an intravenous anesthetic since the 1970s.38 Ketamine has a wide range of clinical applications, such as anesthetic doses for rapid sequence induction in patients with hemodynamic instability;39 subanesthetic doses for sedation and analgesia during short medical procedures,18,40 pain management,19,41 bronchodilation for refractory bronchospasm and refractory status asthmaticus,42 rapid-acting antidepressants,43 neuroprotection22 and so on. Severe asthma attacks may lead to dangerous complications such as hypoxia, respiratory arrest, and even cardiac arrest.44 Ketamine has been investigated as a potential treatment for acute asthma attack and refractory status asthmaticus in part due to its sympathomimetic hemodynamic effects, analgesic and sedative effects, and bronchodilating properties. Ketamine has been shown to reduce the likelihood of intubation in children and adults suffering from acute asthma attacks26,27,29,45 as well as alleviate lung injury by decreasing pro-inflammatory cytokines.46–49 In addition to intravenous, inhalational administration of ketamine was shown to improve the peak expiratory flow rates (PEFR) in patients with acute severe asthma that had not responded well to traditional treatments.27 Interestingly, some researchers have reported that children receiving intravenous ketamine for acute asthma exacerbation did not experience significant improvement compared to standard treatment.28 In spite of the growing interest in the use of ketamine for asthma treatment, conflicting conclusions may be due to the limited sample size, insufficient large-scale prospective studies, and incomplete understanding of the mechanisms involved. In order to further investigate the therapeutic effects of ketamine on severe asthma and the underlying mechanisms, we used a murine mixed-granulocytic asthma model to simulate a severe form of asthma, where neutrophils play an important role. The effects of ketamine treatment before every challenge were as follows: 1) it alleviated AHR, mixed-granulocytic inflammation, and mucus overproduction; 2) it helped maintain redox balance by activating the Nrf2/Keap1 signaling pathway; 3) it induced the inflammatory cells apoptosis in BALF. Based on these results, ketamine relieves the mixed-granulocytic asthma in mice by decreasing oxidative stress, enhancing inflammatory cell apoptosis, and modulating the Nrf2/Keap1 pathway.
The immunomodulatory effects of ketamine are demonstrated by its interaction with different aspects of inflammation: recruitment of inflammatory cells, cytokine production, and the regulation of inflammatory mediators. Inflammatory mediators and oxidative stress are released by macrophages in response to aggression. Ketamine has been reported to inhibit the secretion of cytokines (IL-6 and TNF-α)50,51 and oxidative substances31 in macrophages in vitro. Neutrophils are the next cells involved in the inflammatory process. Ketamine can not only suppress neutrophil diapedesis,52 but also inhibit its function. The therapeutic effects of ketamine on allergic airway inflammation have been demonstrated in several studies.53–55 However, there is no conclusive evidence that ketamine can relieve neutrophil-participated airway inflammation. Based on our findings, ketamine significantly reduced the production of some cytokines in BALF, including IL-6, TNF-α, IL-1β, and IL-8, and IL-8 is a crucial chemokine for neutrophils. When ketamine was administrated, the number of neutrophils in BALF decreased, whereas the number of eosinophils did not change. The result was supported by ELISA that ketamine pretreatment did not effectively reduce the levels of Th2 cytokines, which contradicts the previous study.54 The possible cause of this result may be the different model used in our study from other studies, and the lack of statistical significance may be attributed to an insufficient sample size. Our results demonstrated that ketamine reduced inflammatory cytokines and relieved neutrophil-participated airway inflammation.
Oxidative stress occurs when ROS levels in a cell exceed the antioxidant defense mechanisms.10 Excessive intracellular oxidative stress can result from elevated endogenous (products of normal cellular metabolism) or exogenous (air pollutants, cigarette smoke, etc.) ROS, as well as a diminished antioxidant system.56 Neutrophil and eosinophil infiltration are major sources of ROS, and ROS has been indicated to play an important role in pathogenesis of asthma.57,58 A high level of ROS leads to elevated MDA, a biomarker of lipid peroxidation, which impairs the antioxidant system59 and closely related to asthma severity.60 Oxidative stress parameters have been evaluated in many studies on asthma development. The antioxidant capacity of asthma can be assessed by measuring enzymatic antioxidants (SOD, CAT, GPx). Several studies have reported the loss of SOD activity in asthmatic patients, whereas CAT activity does not differ significantly among asthmatic groups.61 Similar deficits in antioxidant capacity were observed in the mixed-granulocytic asthma model used in this study: decreased activities of SOD and GPx, and elevated levels of oxidative stress (MDA and ROS). The administration of ketamine reduced the level of oxidants, thereby conserving some of the antioxidant system’s capacity. On asthmatic mice, ketamine demonstrated a dose-dependent therapeutic effect and alleviated oxidative stress within the sub-anesthesia dosage range. Of the three concentrations of ketamine, 75 mg/kg proved to be the most effective. Ketamine at 50 mg/kg was unable to effectively decrease the oxidative stress due to insufficient dosage. The reason that 100 mg/kg ketamine failed to enhance the protective effects may be that the anesthetic dosage of ketamine is too high to achieve the desired therapeutic effects in asthmatics.
The Nrf2 signaling pathway plays an important role in regulating antioxidant response.30 Oxidative stress induces degradation of Keap1 and translocation of Nrf2 into the nucleus, where it regulates antioxidant genes (NQO1, HO-1, and GPx).62 It has been demonstrated that severe asthma is associated with a lack of Nrf2 activity,63 which suggests that activating the Nrf2 signaling pathway may facilitate asthma treatment. Furthermore, ketamine exhibits antioxidant properties in various cases: activating the Nrf2 pathway and enhancing the expression of antioxidant proteins.30,64,65 We examined the protein expression of Nrf2, Keap1 and its targeted antioxidant enzymes in response to ketamine pretreatment. As shown in the results, the protein expression of Keap1 was decreased in asthmatics, while the Nrf2 expression was elevated without statistical significance. The results of this study are consistent with the traditional pattern of Nrf2 activation by oxidative insult.14,62 Pretreatment with ketamine enhanced expression of downstream antioxidants by further activating the Nrf2/Keap1 pathway. As an inhibitor of the Nrf2 pathway, ML385 was administered to mice receiving ketamine treatment to demonstrate the involvement of the Nrf2 pathway in the therapeutic effects of ketamine in the present model. As the results showed, ML385 inhibited the antioxidant and therapeutic effects of ketamine by inhibiting Nrf2 activation and restraining the expression of antioxidant proteins. As illustrated in several studies, ketamine attenuates traumatic brain injury,30 hepatic injury,64 and reduced LPS-induced HMGB165 via activating Nrf2 pathway; and ketamine exerts its anti-inflammatory properties via activating antioxidant defense system.24,66 In conclusion, the Nrf2/Keap1 pathway mediated the antioxidant and therapeutic effects of ketamine on an OVA+LPS-induced murine model of asthma.
The modulatory effect of oxidative stress on apoptosis has been demonstrated by several studies.10,67,68 The caspases and the Bcl-2 family of proteins are important regulators of apoptosis, and the Bcl-2 family includes both anti-apoptotic (Bcl-2, Bcl-xl, and Mcl-1) and pro-apoptotic (Bad and Bax) proteins.69 The short-lived anti-apoptotic protein Mcl-1 inhibits the apoptosis of neutrophils and eosinophils through a variety of pathways that converge on caspase-3, whose activation is dependent on the degradation of Mcl-1.70,71 We examined the level of several apoptotic proteins and antiapoptotic proteins in lung tissue. A significant reduction in the expression of anti-apoptotic proteins (Mcl-1) was observed following ketamine pretreatment, in contrast to the level of Mcl-1 in asthmatic mice. Since Mcl-1 is essential for neutrophils and eosinophils to survive, we hypothesized that ketamine pretreatment might primarily alter apoptosis in inflammatory cells. To confirm our hypothesis, IHC of Bax and Mcl-1, as well as H&E staining, were performed in continuous slices of the same lung tissue. In the ketamine group, inflammatory cells around the airways expressed more Bax and less Mcl-1, while those in the asthma group expressed almost the same levels of Bax and Mcl-1. In order to investigate the possibility that ketamine might regulate the apoptosis of inflammatory cells in BALF, flow cytometry was used to examine neutrophils and eosinophils apoptosis. Results of the ketamine group supported the observation that apoptosis of inflammatory cells was enhanced, which contributed to the resolution of airway inflammation in asthmatic patients.9 The pro-apoptotic effect of ketamine on inflammatory cells was partially impaired after the administration of ML385, suggesting that the Nrf2 signaling pathway might be involved.
To our knowledge, we are the first to demonstrate the antioxidant and therapeutic benefits of ketamine on a murine model of asthma characterized by mixed-granulated airway inflammation. We also present the evidence that the Nrf2/Keap1 pathway contributes to ketamine attenuating symptoms of severe asthma and the proapoptotic effects of ketamine on eosinophils and neutrophils. According to our results, ketamine is beneficial for the treatment of severe asthma due to its combination of anti-inflammatory, antioxidant, and bronchodilating properties.
The limitations of the present study include i) we have not been able to explain the mechanism of ketamine activating Nrf2 pathway and inducing inflammatory cells apoptosis in BALF; ii) whether ketamine at sub-anesthetic dosages could cause behavioral changes of asthmatic mice has not been investigated in the present study; iii) the administration routes of ketamine other than intravenous were not included in the present study. In order to evaluate the efficacy and safety of ketamine in the treatment of asthma, more large-scale prospective clinical studies are required. Due to its low price and multiple properties, ketamine may also help reduce medical costs when used in asthma treatment.
Conclusion
According to our results, ketamine pretreatment decreased oxidative stress in lung tissues in a murine model of mixed-granulocytic asthma by activating the Nrf2/Keap1 pathway, contributing to the alleviation of asthma symptoms and the apoptosis of inflammatory cells in BALF.
Abbreviations
Nrf2, nuclear factor erythroid 2-related factor 2; OVA, ovalbumin; Alum, aluminum; LPS, lipopolysaccharides; Keap1, Kelch-like ECH-associated protein 1; Bax, BCL2 associated X protein; Mcl-1, myeloid cell leukemia 1; Bcl-2, B-cell lymphoma 2; ROS, reactive oxygen species; GPx, glutathione peroxidase; NQO1, NADP(H)quinone oxidoreductase1; HO-1, heme oxygenase-1; NMDARs, N-methyl-D-aspartate receptors; Mch, methacholine; Rrs, respiratory resistance; BALF, bronchoalveolar lavage fluid; H&E, hematoxylin and eosin; PAS, periodic acid Schiff; ELISA, enzyme-linked immunosorbent assay; MDA, malondialdehyde; SOD, superoxide dismutases; IHC, immunohistochemistry; RT-qPCR, Quantitative Real-Time Polymerase Chain Reaction; SDS-PAGE, sodium dodecyl sulfate-polyacrylamide gel electrophoresis; AHR, airway hyperresponsiveness; PEFR, peak expiratory flow rates; SD, standard deviation; IQR, interquartile range.
Data Sharing Statement
The data used to support the findings of this study are available from the corresponding author upon request.
Ethical Approval
The animal experiments were performed following the Guidelines for Care and Use of Laboratory Animals of the National Institutes of Health. The Institutional Animal Care and Use Committee of Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College has given their approvement to the animal experiment in the present study. Approval no. 2022(211).
Consent for Publication
All authors have approved the manuscript and given their consent for submission and publication.
Acknowledgments
The present study was supported by the Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China) [grant no. YS202006].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The present study was supported by the Plastic Surgery Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College (Beijing, China) [grant no. YS202006].
Disclosure
The authors declare that there is no conflict of interest regarding the publication of this article.
References
1. Menzies-Gow A, Mansur AH, Brightling CE. Clinical utility of fractional exhaled nitric oxide in severe asthma management. Eur Respir J. 2020;55(3):1901633. doi:10.1183/13993003.01633-2019
2. Svenningsen S, Nair P. Asthma endotypes and an overview of targeted therapy for asthma. Front Med. 2017;4:158. doi:10.3389/fmed.2017.00158
3. Hastie AT, Mauger DT, Denlinger LC, et al. Baseline sputum eosinophil + neutrophil subgroups’ clinical characteristics and longitudinal trajectories for NHLBI Severe Asthma Research Program (SARP 3) cohort. J Allergy Clin Immunol. 2020;146(1):222–226. doi:10.1016/j.jaci.2020.01.039
4. Chung KF, Wenzel SE, Brozek JL, et al. International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J. 2014;43(2):343–373. doi:10.1183/09031936.00202013
5. Liu X, Li X, Chen L, et al. Proteomic analysis reveals a novel therapeutic strategy using fludarabine for steroid-resistant asthma exacerbation. Front Immunol. 2022;13:805558. doi:10.3389/fimmu.2022.805558
6. Toussaint M, Jackson DJ, Swieboda D, et al. Host DNA released by NETosis promotes rhinovirus-induced type-2 allergic asthma exacerbation. Nat Med. 2017;23(6):681–691. doi:10.1038/nm.4332
7. Ito K, Herbert C, Siegle JS, et al. Steroid-resistant neutrophilic inflammation in a mouse model of an acute exacerbation of asthma. Am J Respir Cell Mol Biol. 2008;39(5):543–550. doi:10.1165/rcmb.2008-0028OC
8. Felton JM, Lucas CD, Rossi AG, Dransfield I. Eosinophils in the lung - modulating apoptosis and efferocytosis in airway inflammation. Front Immunol. 2014;5:302. doi:10.3389/fimmu.2014.00302
9. Uddin M, Nong G, Ward J, et al. Prosurvival activity for airway neutrophils in severe asthma. Thorax. 2010;65(8):684–689. doi:10.1136/thx.2009.120741
10. Sies H. Oxidative stress: a concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi:10.1016/j.redox.2015.01.002
11. Dut R, Dizdar EA, Birben E, et al. Oxidative stress and its determinants in the airways of children with asthma. Allergy. 2008;63(12):1605–1609. doi:10.1111/j.1398-9995.2008.01766.x
12. Comhair SA, Erzurum SC. Redox control of asthma: molecular mechanisms and therapeutic opportunities. Antioxid Redox Signal. 2010;12(1):93–124. doi:10.1089/ars.2008.2425
13. Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122(6):877–902. doi:10.1161/CIRCRESAHA.117.311401
14. Liu Q, Gao Y, Ci X. Role of Nrf2 and its activators in respiratory diseases. Oxid Med Cell Longev. 2019;2019:7090534. doi:10.1155/2019/7090534
15. Helou DG, Noël B, Gaudin F, et al. Cutting edge: Nrf2 regulates neutrophil recruitment and accumulation in skin during contact hypersensitivity. J Immunol. 2019;202(8):2189–2194. doi:10.4049/jimmunol.1801065
16. Li L, Vlisides PE. Ketamine: 50 years of modulating the mind. Front Hum Neurosci. 2016;10:612. doi:10.3389/fnhum.2016.00612
17. Kurdi MS, Theerth KA, Deva RS. Ketamine: current applications in anesthesia, pain, and critical care. Anesth Essays Res. 2014;8(3):283–290. doi:10.4103/0259-1162.143110
18. Soleimanpour H, Mahmoodpoor A, Eftekhari Milani F, Shahsavari Nia K, Mehdizadeh Esfanjani R, Safari S. Effectiveness of oral ketamine, midazolam, and atropine cocktail versus oral diphenhydramine for pediatric sedation in the emergency department. Iran Red Crescent Med J. 2014;16(9):e21366. doi:10.5812/ircmj.21366
19. Pouraghaei M, Moharamzadeh P, Paknezhad SP, Rajabpour ZV, Soleimanpour H. Intranasal ketamine versus intravenous morphine for pain management in patients with renal colic: a double-blind, randomized, controlled trial. World J Urol. 2021;39(4):1263–1267. doi:10.1007/s00345-020-03319-4
20. Rahmanian M, Leysi M, Hemmati AA, Mirmohammadkhani M. The effect of low-dose intravenous ketamine on postoperative pain following cesarean section with spinal anesthesia: a randomized clinical trial. Oman Med J. 2015;30(1):11–16. doi:10.5001/omj.2015.03
21. Reddi D. Preventing chronic postoperative pain. Anaesthesia. 2016;71(Suppl 1):64–71. doi:10.1111/anae.13306
22. Chang LC, Raty SR, Ortiz J, Bailard NS, Mathew SJ. The emerging use of ketamine for anesthesia and sedation in traumatic brain injuries. CNS Neurosci Ther. 2013;19(6):390–395. doi:10.1111/cns.12077
23. Bhutta AT, Schmitz ML, Swearingen C, et al. Ketamine as a neuroprotective and anti-inflammatory agent in children undergoing surgery on cardiopulmonary bypass: a pilot randomized, double-blind, placebo-controlled trial. Pediatr Crit Care Med. 2012;13(3):328–337. doi:10.1097/PCC.0b013e31822f18f9
24. Weckmann K, Deery MJ, Howard JA, et al. Ketamine’s antidepressant effect is mediated by energy metabolism and antioxidant defense system. Sci Rep. 2017;7(1):15788. doi:10.1038/s41598-017-16183-x
25. Nowacka A, Borczyk M. Ketamine applications beyond anesthesia - A literature review. Eur J Pharmacol. 2019;860:172547. doi:10.1016/j.ejphar.2019.172547
26. Esmailian M, Koushkian Esfahani M, Heydari F. The effect of low-dose ketamine in treating acute asthma attack; a randomized clinical trial. Emerg (Tehran). 2018;6(1):e21.
27. Farshadfar K, Sohooli M, Shekouhi R, Taherinya A, Qorbani M, Rezaei-Kojani M. The effects of nebulized ketamine and intravenous magnesium sulfate on corticosteroid resistant asthma exacerbation; a randomized clinical trial. Asthma Res Pract. 2021;7(1):15. doi:10.1186/s40733-021-00081-1
28. Allen J, Macias CG. The efficacy of ketamine in pediatric emergency department patients who present with acute severe asthma. Ann Emerg Med. 2005;46(1):43–50. doi:10.1016/j.annemergmed.2005.02.024
29. Hendaus MA, Jomha FA, Alhammadi AH. Is ketamine a lifesaving agent in childhood acute severe asthma? Ther Clin Risk Manag. 2016;12:273–279. doi:10.2147/TCRM.S100389
30. Liang J, Wu S, Xie W, He H. Ketamine ameliorates oxidative stress-induced apoptosis in experimental traumatic brain injury via the Nrf2 pathway. Drug Des Devel Ther. 2018;12:845–853. doi:10.2147/DDDT.S160046
31. Zhang X, Feng J, Zhu P, Zhao Z. Ketamine inhibits calcium elevation and hydroxyl radical and nitric oxide production in lipopolysaccharide-stimulated NR8383 alveolar macrophages. Inflammation. 2013;36(5):1094–1100. doi:10.1007/s10753-013-9642-y
32. Chamitava L, Cazzoletti L, Ferrari M, et al. Biomarkers of oxidative stress and inflammation in chronic airway diseases. Int J Mol Sci. 2020;21(12):4339. doi:10.3390/ijms21124339
33. Yu QL, Chen Z. Establishment of different experimental asthma models in mice. Exp Ther Med. 2018;15(3):2492–2498. doi:10.3892/etm.2018.5721
34. Xiao S, Wang Q, Gao H, Zhao X, Zhi J, Yang D. Dexmedetomidine alleviates airway hyperresponsiveness and allergic airway inflammation through the TLR4/NF‑κB signaling pathway in mice. Mol Med Rep. 2022;25(3). doi:10.3892/mmr.2022.12590
35. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi:10.1006/meth.2001.1262
36. Lambrecht BN, Hammad H, Fahy JV. The cytokines of asthma. Immunity. 2019;50(4):975–991. doi:10.1016/j.immuni.2019.03.018
37. Hu J, Wang J, Li C, Shang Y. Fructose-1,6-bisphosphatase aggravates oxidative stress-induced apoptosis in asthma by suppressing the Nrf2 pathway. J Cell Mol Med. 2021;25(11):5001–5014. doi:10.1111/jcmm.16439
38. Choudhury D, Autry AE, Tolias KF, Krishnan V. Ketamine: neuroprotective or neurotoxic? Front Neurosci. 2021;15:672526. doi:10.3389/fnins.2021.672526
39. Morris C, Perris A, Klein J, Mahoney P. Anaesthesia in haemodynamically compromised emergency patients: does ketamine represent the best choice of induction agent? Anaesthesia. 2009;64(5):532–539. doi:10.1111/j.1365-2044.2008.05835.x
40. Ghojazadeh M, Sanaie S, Paknezhad SP, Faghih SS, Soleimanpour H. Using ketamine and propofol for procedural sedation of adults in the emergency department: a systematic review and meta-analysis. Adv Pharm Bull. 2019;9(1):5–11. doi:10.15171/apb.2019.002
41. Launo C, Bassi C, Spagnolo L, et al. Preemptive ketamine during general anesthesia for postoperative analgesia in patients undergoing laparoscopic cholecystectomy. Minerva Anestesiol. 2004;70(10):727–734; 734–728.
42. Goyal S, Agrawal A. Ketamine in status asthmaticus: a review. Indian J Crit Care Med. 2013;17(3):154–161. doi:10.4103/0972-5229.117048
43. Witkin JM, Martin AE, Golani LK, Xu NZ, Smith JL. Rapid-acting antidepressants. Adv Pharmacol. 2019;86:47–96.
44. Garner O, Ramey JS, Hanania NA. Management of life-threatening asthma: severe asthma series. Chest. 2022;162(4):747–756. doi:10.1016/j.chest.2022.02.029
45. Elkoundi A, Bentalha A, El Koraichi A, El Kettani SE. Nebulized ketamine to avoid mechanical ventilation in a pediatric patient with severe asthma exacerbation. Am J Emerg Med. 2018;36(4):734.e733–734.e734. doi:10.1016/j.ajem.2018.01.027
46. Li K, Yang J, Han X. Ketamine attenuates sepsis-induced acute lung injury via regulation of HMGB1-RAGE pathways. Int Immunopharmacol. 2016;34:114–128. doi:10.1016/j.intimp.2016.01.021
47. Xu D, Sun X, Zhang Y, Cao L. Ketamine alleviates HMGB1-induced acute lung injury through TLR4 signaling pathway. Adv Clin Exp Med. 2020;29(7):813–817. doi:10.17219/acem/121936
48. Han WZ, Xu SW, Wang L. Impact of ketamine intervention for acute lung injury on RAGE and TLR9. Eur Rev Med Pharmacol Sci. 2018;22(13):4350–4354. doi:10.26355/eurrev_201807_15432
49. Wang WF, Liu S, Xu B. A study of the protective effect and mechanism of ketamine on acute lung injury induced by mechanical ventilation. Eur Rev Med Pharmacol Sci. 2017;21(6):1362–1367.
50. Wu GJ, Chen TL, Ueng YF, Chen RM. Ketamine inhibits tumor necrosis factor-alpha and interleukin-6 gene expressions in lipopolysaccharide-stimulated macrophages through suppression of toll-like receptor 4-mediated c-Jun N-terminal kinase phosphorylation and activator protein-1 activation. Toxicol Appl Pharmacol. 2008;228(1):105–113. doi:10.1016/j.taap.2007.11.027
51. Chang HC, Lin KH, Tai YT, Chen JT, Chen RM. Lipoteichoic acid-induced TNF-α and IL-6 gene expressions and oxidative stress production in macrophages are suppressed by ketamine through downregulating Toll-like receptor 2-mediated activation oF ERK1/2 and NFκB. Shock. 2010;33(5):485–492. doi:10.1097/SHK.0b013e3181c3cea5
52. Weigand MA, Schmidt H, Zhao Q, Plaschke K, Martin E, Bardenheuer HJ. Ketamine modulates the stimulated adhesion molecule expression on human neutrophils in vitro. Anesth Analg. 2000;90(1):206–212. doi:10.1097/00000539-200001000-00041
53. Zou H, Wang LX, Wang M, et al. MTOR-mediated autophagy is involved in the protective effect of ketamine on allergic airway inflammation. J Immunol Res. 2019;2019:5879714. doi:10.1155/2019/5879714
54. Zhu MM, Zhou QH, Zhu MH, et al. Effects of nebulized ketamine on allergen-induced airway hyperresponsiveness and inflammation in actively sensitized Brown-Norway rats. J Inflamm. 2007;4:10. doi:10.1186/1476-9255-4-10
55. Song L, Sen S, Sun Y, Zhou J, Mo L, He Y. Ketamine inhalation ameliorates ovalbumin-induced murine asthma by suppressing the epithelial-mesenchymal transition. Med Sci Monit. 2016;22:2471–2483. doi:10.12659/MSM.899955
56. Kirkham P, Rahman I. Oxidative stress in asthma and COPD: antioxidants as a therapeutic strategy. Pharmacol Ther. 2006;111(2):476–494. doi:10.1016/j.pharmthera.2005.10.015
57. Liou CJ, Cheng CY, Yeh KW, Wu YH, Huang WC. Protective effects of casticin from vitex trifolia alleviate eosinophilic airway inflammation and oxidative stress in a murine asthma model. Front Pharmacol. 2018;9:635. doi:10.3389/fphar.2018.00635
58. Yoshida M, Minagawa S, Araya J, et al. Involvement of cigarette smoke-induced epithelial cell ferroptosis in COPD pathogenesis. Nat Commun. 2019;10(1):3145. doi:10.1038/s41467-019-10991-7
59. Chen X, Huang Y, Feng J, Jiang XF, Xiao WF, Chen XX. Antioxidant and anti-inflammatory effects of Schisandra and Paeonia extracts in the treatment of asthma. Exp Ther Med. 2014;8(5):1479–1483. doi:10.3892/etm.2014.1948
60. Wood LGM, Fitzgerald DA, Gibson PG, Cooper DM, Garg ML. Lipid peroxidation as determined by plasma isoprostanes is related to disease severity in mild asthma. Lipids. 2000;35(9):967–974. doi:10.1007/s11745-000-0607-x
61. Comhair SA, Ricci KS, Arroliga M, et al. Correlation of systemic superoxide dismutase deficiency to airflow obstruction in asthma. Am J Respir Crit Care Med. 2005;172(3):306–313. doi:10.1164/rccm.200502-180OC
62. Li L, Chen Y, Jiao D, Yang S, Li L, Li P. Protective effect of astaxanthin on ochratoxin A-induced kidney injury to mice by regulating oxidative stress-related NRF2/KEAP1 pathway. Molecules. 2020;25(6):115.
63. Audousset C, McGovern T, Martin JG. Role of Nrf2 in disease: novel molecular mechanisms and therapeutic approaches - pulmonary disease/asthma. Front Physiol. 2021;12:727806. doi:10.3389/fphys.2021.727806
64. Xu WK, Wang P, Wang D, et al. S-ketamine alleviates carbon tetrachloride-induced hepatic injury and oxidative stress by targeting the Nrf2/HO-1 signaling pathway. Can J Physiol Pharmacol. 2021;99(12):1308–1315. doi:10.1139/cjpp-2020-0763
65. YkN T, Wang Q, She Y, Bi X, Zhao B. Ketamine reduces LPS-induced HMGB1 via activation of the Nrf2/HO-1 pathway and NF-κB suppression. J Trauma Acute Care Surg. 2015;78(4):784–792. doi:10.1097/TA.0000000000000588
66. Maciel AL, Abelaira HM, de Moura AB, et al. Acute treatment with ketamine and chronic treatment with minocycline exert antidepressant-like effects and antioxidant properties in rats subjected different stressful events. Brain Res Bull. 2018;137:204–216. doi:10.1016/j.brainresbull.2017.12.005
67. Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153–163. doi:10.1016/S0928-4680(00)00053-5
68. Sahiner UM, Birben E, Erzurum S, Sackesen C, Kalayci Ö. Oxidative stress in asthma: part of the puzzle. Pediatr Allergy Immunol. 2018;29(8):789–800. doi:10.1111/pai.12965
69. Song X, Wang B, Lin S, et al. Astaxanthin inhibits apoptosis in alveolar epithelial cells type II in vivo and in vitro through the ROS-dependent mitochondrial signalling pathway. J Cell Mol Med. 2014;18(11):2198–2212. doi:10.1111/jcmm.12347
70. Wardle DJ, Burgon J, Sabroe I, Bingle CD, Whyte MK, Renshaw SA. Effective caspase inhibition blocks neutrophil apoptosis and reveals Mcl-1 as both a regulator and a target of neutrophil caspase activation. PLoS One. 2011;6(1):e15768. doi:10.1371/journal.pone.0015768
71. Felton JM, Dorward DA, Cartwright JA, et al. Mcl-1 protects eosinophils from apoptosis and exacerbates allergic airway inflammation. Thorax. 2020;75(7):600–605. doi:10.1136/thoraxjnl-2019-213204
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.