Back to Journals » OncoTargets and Therapy » Volume 13
KCNH3 Predicts Poor Prognosis and Promotes Progression in Ovarian Cancer
Authors Li Z, Huang L, Wei L, Zhang B, Zhong S, Ou Y, Wen C, Huang S
Received 28 June 2020
Accepted for publication 21 September 2020
Published 13 October 2020 Volume 2020:13 Pages 10323—10333
DOI https://doi.org/10.2147/OTT.S268055
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Leo Jen-Liang Su
Zhongjun Li,1,2,* Lishan Huang,1,* Li Wei,1 Bin Zhang,1 Shulin Zhong,1 Yijing Ou,1 Chuangyu Wen,1 Suran Huang1
1Department of Obstetrics and Gynecology, Affiliated Dongguan People’s Hospital, Southern Medical University, Dongguan, Guangdong, 523059, China; 2Department of Obstetrics and Gynecology, Nanfang Hospital, Southern Medical University, Guangzhou, Guangdong, 510515, China
*These authors contributed equally to this work
Correspondence: Chuangyu Wen; Suran Huang
Department of Obstetrics and Gynecology, Affiliated Dongguan People’s Hospital, Southern Medical University, 3 Wangdao Road, Dongguan, Guangdong 523059, China
Email [email protected]; [email protected]
Background: Ovarian cancer (OC) is one of the most common causes of cancer-related death among women; accordingly, new biomarkers of OC are urgently needed. Potassium voltage-gated channel sub-family H member 3 (KCNH3) is a voltage-gated potassium channel member involved in cognitive function and diabetes. Here, we aimed to elucidate the role and potential molecular mechanisms of KCNH3 in OC.
Materials and Methods: KCNH3 expression levels in OC tissues were analyzed using TCGA data and confirmed by RT-qPCR and immunohistochemistry in OC tissues. The cell counting kit-8 was used to assess cell proliferation in OC cells in which KCNH3 was knocked-down with small interference RNA (siRNA). Wound-healing and transwell invasion assays were used to assess migratory and invasive abilities, respectively. Cell cycle distribution and apoptosis were determined using a flow cytometer. Gene set enrichment analysis and Western blot were used to investigate the potential pathways of KCNH3 in OC development.
Results: TCGA data and RT-qPCR results from patients with OC revealed high KCNH3 expression in OC tissues compared to normal ovarian tissues. Survival analysis in patients with OC suggested that high KCNH3 expression might be an independent predictor for poor overall survival and disease-free survival. In vitro studies showed that KCNH3 silencing in OC cells could inhibit cell proliferation and migration ability, and induce apoptosis and G2/M phase arrest. Furthermore, Western blot results showed that KCNH3 silencing might induce downregulation of RPA1 and RPA2 expression level in both SKOV3 and COC1 cells.
Conclusion: KCNH3 plays an important role in cancer progression in patients with OC. Further investigation might reveal KCNH3 as a potential biomarker for prognosis or diagnosis in OC.
Keywords: KCNH3, ovarian cancer, prognosis, replication protein A
Introduction
Ovarian cancer (OC) is one of the most common causes of cancer-related death in women. It is estimated that 295,414 new OC cases and 184,799 OC-related deaths occurred worldwide in 2018.1 Despite innovations in OC treatment over the last decade, five-year survival rates of OC have remained at around 47%, which is much lower than those of breast cancer (85%).2–4 Additionally, most patients with OC are diagnosed with stage III/IV due to the lack of specific clinical manifestations in early-stage. More than 75% of patients with OC die from their disease, but OC is highly curable if diagnosed in early-stages.3,5 To overcome OC, it’s necessary to find some predictors with high sensitivity and specificity for OC in clinical treatment, prognosis and diagnosis.6,7
Over the last few decades, cancer genome sequencing has been used to identify and develop biomarkers for personalized cancer treatment and preventive and predictive medicine.7 Core high throughput sequencing technology data has been saved in public databases for researchers to integrate and re-analyze. The Cancer Genome Atlas (TCGA) is a commonly used public source containing sequence information from thousands of tumor samples. The TCGA provides vast amounts of information, including about gene expression, gene methylation, copy number variation, and somatic mutation.8,9 Using TCGA data, we found a gene that is highly associated with OC, called potassium voltage-gated channel sub-family H member 3 (KCNH3). Voltage-gated potassium channels are the largest group of ion channels and have been reported to play important roles in tumor progression.10 For example, KCNH5 controls mitotic entry and tumor growth in medulloblastoma.11 Additionally, silencing of KCNA1 inhibits cervical cancer progression by mitochondria damage.12 KCNH3 is a member of the voltage-gated potassium channel family that is involved in cognitive function and diabetes.13–15 Here, we present the first biological data for KCNH3 in OC.
Using TCGA datasets, we found that KCNH3 mRNA levels were significantly higher in OC tissues than in normal tissues, and that high KCNH3 expression was related to poor prognosis. KCNH3 downregulation not only suppressed tumor proliferation and invasion, but also induced cell cycle arrest and apoptosis in vitro. Gene set enrichment analysis (GSEA) and Western blot were used to investigate KCNH3 biological pathways in OC. Our results show that KCNH3 might induce cancer progression by activating the DNA repair signaling pathway through RPA1 and RPA2. We hypothesize that KCNH3 might play an important role in prognosis, diagnosis and treatment of OC.
Materials and Methods
Patients and Tissue Samples
This study was approved by the Regional Institutional Review Board of Affiliated Dongguan People’s Hospital, Southern Medical University and performed according to the principles of the Declaration of Helsinki. Written informed consent was obtained from all patients. Specimens were handled and made anonymous following ethical and legal standards.
The tissue microarray (TMA), including 82 OC tissues and 82 normal ovarian tissues and the relevant clinical records for all patients, was obtained from Affiliated Dongguan People’s Hospital, Southern Medical University. The clinical characteristics of patients are shown in Table 1. Overall survival (OS) is defined as the period between surgery and death or the last contact. Disease-free survival (DFS) is defined as the period between surgery and any form of tumor recurrence.
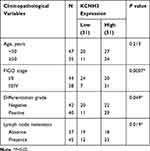 |
Table 1 Correlation Between KCNH3 Expressions with Clinic-Pathological Characteristics of Ovarian Cancer |
High-Throughput Data Processing
OC expression data were obtained from TCGA database. All data were converted to Log2 and analyzed using R and GraphPad Prism software (San Diego, CA, USA). The expression of KCNH3 in OC tumor tissues and normal tissues were obtained from GEPIA, a web server for cancer and normal gene expression profiling and interactive analyses. For GSEA, the expression level of KCNH3 from TCGA datasets (374 tumor tissues) was considered as a phenotype label. The normalized enrichment score (NES) and nominal P value were applied to sort the pathways enriched in the phenotypes.
Cell Culture and Transfection
Human OC SKOV3 and COC1 cell lines were purchased from BeNa Culture Collection (Beijing, China). SKOV3 and COC1 cell lines were cultured in DMEM (Life Technologies, Carlsbad, CA, USA) with 10% fetal bovine serum (Life Technologies), 100 U/mL penicillin, and 100 μg/mL streptomycin (Life Technologies) at 37°C in an atmosphere containing 5% CO2. We transfected three small interfering RNAs (siRNAs) (RiboBio Co., Ltd., Guangzhou, Guangdong, China) targeting KCNH3. The siRNAs sequences were: siRNA1-5ʹ-UCUUUAUGGGUAUCACAUCCA-3ʹ, siRNA2-5ʹ-UUUAAUAUAAAAAUUUGGGGA-3ʹ, siRNA3-5ʹ-UUUUAAUAUAAAAAUUUGGGG-3ʹ. Scrambled siRNAs were constructed as the control treatment.
Quantitative Real-Time Polymerase Chain Reaction (RT-qPCR) Assay
Total RNA was extracted from OC cell lines or frozen tissues using RNAiso Plus (Takara Biotechnology Co., Ltd., Kusatsu, Shiga, Japan) and following the manufacturer’s instructions. KCNH3 mRNA expression was assessed by qPCR using a SYBR Green PCR kit (Takara Biotechnology Co., Ltd.). The reaction conditions were: preincubation at 95°C for 10 min, followed by 40 cycles of 95°C for 10 s, 60°C for 10 s, and 72°C for 20 s. The primers used were:
 |
|
KCNH3 mRNA expression was calculated using the 2−ΔΔCT method and normalized to β-actin mRNA expression levels.
Western Blot Analysis
OC cells were collected and the protein concentrations were measured using the BCA Protein Quantitation Assay (Nanjing KeyGen Biotech Co., Ltd., Nanjing, Jiangsu, China). SDS-PAGE was used to separate the total protein and proteins were then transferred to PVDF membranes. PVDF membranes were blocked in 5% non-fat dry milk and probed with primary antibodies overnight at 4°C. PVDF membranes were incubated with alkaline phosphatase-conjugated secondary antibody and finally detected by chemiluminescence. Primary antibodies against KCNH3, RPA1, RPA2, and β-actin were obtained from Abcam (Cambridge, MA, USA). The alkaline phosphatase-conjugated secondary antibody was purchased from Cell Signaling Technology (Beverly, MA, USA).
Cell Viability
The viability of OC cells was evaluated using the cell counting kit-8 (CCK-8, Nanjing KeyGen Biotech Co., Ltd.). Cells were seeded (5×103 cells/well) in 96-well plates and cultured in an incubator for 0, 24, 48, and 72 h. Then, 20 μL of CCK-8 solution was added. After culturing for 2 h, a spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA) was used to measure absorbance at 450 nm.
Apoptosis Assay
Apoptotic cells were determined using an Annexin V-FITC/propidium iodide (PI) Apoptosis Detection Kit (Thermo Fisher Scientific) and following the manufacturer’s instructions. In brief, cells were harvested and washed with ice-cold phosphate buffered saline (PBS). After centrifugation, cells were resuspended in binding buffer (100 μL) and incubated with PI and Annexin V-FITC for 15 min in the dark. Finally, the samples were resuspended in binding buffer (400 μL) and detected using a flow cytometer (FACSCalibur, BD Biosciences, Franklin Lakes, NJ, USA).
Cell Cycle Distribution
The effect of KCNH3 on cell cycle arrest in OC cells was assessed using a flow cytometer. Briefly, cells were harvested, washed with PBS, and fixed with 70% ice-cold ethanol at 4°C overnight. Then, the cells were collected and stained with PI/RNase staining solution (BD Biosciences) for 30 min at room temperature in the dark, followed by flow cytometric analysis.
Transwell Invasion Assay
OC cells were seeded in the upper side of a Transwell chamber (5×104 cells/well) and 10% FBS medium was added into the lower chamber. After culture, 1% paraformaldehyde was used to fix OC cells in the lower chamber, and the cells were stained with hematoxylin. Cells were counted and cells numbers expressed as the average number of cells per field of view.
Wound-Healing Assay
Cell migration ability was assessed using a scratch wound-healing motility assay. First, 1×106 cells/well were seeded and cultivated in 6-well plates. Then, a pipette tip (10 μL) was used to make a scratch in culture plate. Serum-free medium with 4 μg/mL mitomycin (Sigma-Aldrich, St. Louis, MO, USA) was added to the cells to inhibit cell division. Cells that had migrated from the wound edge were imaged and counted after 24 h.
Immunohistochemistry (IHC) Staining and Evaluation
TMA specimens were stained using Dako EnVision Detection Systems (Glostrup, Denmark) following the manufacturer’s instructions. In brief, samples underwent a proteolytic digestion and peroxidase blocking, they were incubated with a KCNH3 primary antibody (Boster Biological Technology Co., Ltd., Wuhan, Hubei, China) at 4°C overnight. Then, the slide was washed with PBS. Finally, substrate-chromogen and peroxidase labeled polymer were added to visualize the stained protein.
Aperio ImageScope software (Leica Biosystems Inc. Buffalo Grove, IL, USA) was used to analyze TMA specimens. Two experienced pathologists, blinded to patient information, independently scored the intensity of immunostaining in a semi-quantitative manner. The scores recorded by these two pathologists were compared and any inconsistencies were reevaluated for a consensus score. Staining intensity was defined using the following criteria: absent, 0; weak, 1; moderate staining, 2; and strong staining, 3. The percentage of staining was scored as follows: 0 (<5%); 1 (5–25%); 2 (26–50%); and 3 (51–100%). The immunoreactivity scores (IRS) of each specimen were the sum of the immunostaining intensity and percentage scores. Samples were divided into low (IRS≤1) and high (IRS>1) groups based on KCNH3 expression.
Statistical Analysis
Statistics were analyzed using SPSS 21.0 software (IBM, New York, USA). The association of KCNH3 expression with clinicopathological characteristics were calculated using Fisher’s exact and Pearson’s Chi-squared tests. Overall survival and disease-free survival were analyzed by Kaplan-Meier method, and the Log rank test was applied to evaluate the differences. Univariate and multivariate Cox regression analyses were applied to assess survival data. Statistical significance between groups was calculated by Student’s t-test. P<0.05 was considered as statistically significant.
Results
Increased KCNH3 Expression is Associated with OC Progression and Poor Prognosis
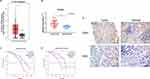
Using the TCGA database, we found that the KCNH3 expression level was significantly higher in OC tissues (n=426) than that in normal tissues (n=88) (Figure 1A). Similarly, increased KCNH3 expression was also observed in 29 fresh cancer tissues from patients with OC when compared to that in 29 normal ovarian tissues (Figure 1B). To confirm KCNH3 expression level in OC tissues, IHC analysis was used to measure the KCNH3 protein expression in a TMA containing 82 OC tissues and 82 normal ovarian tissues. Higher KCNH3 expression levels were observed in OC tissues (Figure 1E). Moreover, KCNH3 expression was significantly associated with FIGO stage (P=0.0007), differentiation grade (P=0.049), and lymph node metastasis (P=0.019) (Table 1). No significant association was observed between KCNH3 expression level and age.
To explore the relationship between KCNH3 expression level and overall survival or disease-free survival in patients with OC, we used the Kaplan-Meier method and log rank tests to assess the TMA data. Overall survival (P=0.009) and disease-free survival (P=0.005) were shorter in the KCNH3 TMA high expression group than in the KCNH3 TMA low expression group (Figure 1C and D). Univariate and multivariate analyses using the Cox proportional hazards regression model were used to determine whether KCNH3 expression was a potential independent predictor of overall survival or disease-free survival in patients with OC. Increased KCNH3 expression was an independent predictor of shorter overall survival (HR: 2.098, 95% CI: 1.004–4.383, P=0.048) (Table 2). Similarly, higher KCNH3 expression (HR: 2.254, 95% CI: 1.029–4.937, P=0.042) was also an independent predictor of shorter disease-free survival time (Table 3).
 |
Table 2 Univariate and Multivariate Cox Regression Analyses of Risk Factors Associated with Overall Survival |
 |
Table 3 Univariate and Multivariate Cox Regression Analyses of Risk Factors Associated with Disease Free Survival |
KCNH3 Silencing Inhibits OC Cell Proliferation and Migration
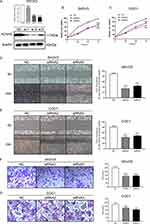
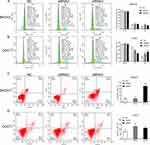
As the KCNH3 expression in SKOV3 and COC1 cells were higher than the other ovarian cancer cell lines (Supplement Figure 1), we chose SKOV3 and COC1 cells to knock-down KCNH3 and evaluate the biological functions of KCNH3 in OC, including cell proliferation, cell cycle arrest, apoptosis, invasion, and migration abilities. Three siRNAs were used for KCNH3 silencing. We chose siRNA2 and siRNA3 for the following experiments because of their better silencing efficiency (Figure 2A). Obvious inhibitory effects were observed on cell proliferation in the KCNH3-silencing groups in both in SKOV3 and COC1 cells compared to the control groups (NC) (Figure 2B and C). A significant G2/M phase block was also observed in KCNH3 silencing groups in both SKOV3 and COC1 cancer cells (Figure 3A and B). The silencing of KCNH3 in both SKOV3 and COC1 cancer cells also resulted in a higher percentage of apoptotic cells than in the control groups (Figure 3C and D). These results suggest that KCNH3 might enhance the cell growth of OC.
The effect of KCNH3 on the migratory and invasive abilities of OC cells were determined using transwell and wound-healing assays, respectively. Healing in SKOV3 and COC1 cells were suppressed in the KCNH3 silencing group compared to the control groups (Figure 2D and E). Transwell invasion assay results indicated that KCNH3 silencing could suppress the invasive abilities of both SKOV3 and COC1 cells (Figure 2F and G). These results indicate that KCNH3 plays an important role in cell migration and invasion in OC.
KCNH3 is Associated with the DNA Repair Signaling Pathway in OC Cells Through RPA
To elucidate the biological pathways in which KCNH3 functions in the development of OC, gene set enrichment analysis (GSEA) was performed between low and high KCNH3 expression dataset (TCGA). Ten important pathways were found to enrich in phenotype high (high KCNH3 expression) in OC progression (Figure 4A). Figure 4B has shown some enrichment plot of the corresponding signaling pathways, such as mismatch repair, DNA replication, spliceosome and ribosome. The normalized enrichment score (NES) and nominal P value were listed in Table 4. Five signaling pathways had significance with P<0.05, including spliceosome, RNA degradation, propanoate metabolism, mismatch repair and beta alanine metabolism. Mismatch repair with P=0.008 showed better association with KCNH3 expression, which was further investigated in this study. Replication protein A (RPA) is a conserved single-stranded DNA-binding protein complex with three subunits: RPA1, RPA2, and RPA3, which is essential for DNA replication and repair.16,17 Therefore, we performed Western blot to investigate the relationship between RPA1, RPA2, and KCNH3 in OC. Silencing of KCNH3 could downregulate RPA1 and RPA2 expression in both SKOV3 and COC1 cells (Figure 4C and D). Different downregulated rates of KCNH3, RPA1 and RPA2 were shown in the results, which might due to the heterogeneity of these two cell lines.
 |
Table 4 Gene Sets Enriched in Phenotype High |
Discussion
Ovarian cancer (OC) is the second most common cause of gynecologic cancer-related death worldwide. For patients with OC, outcomes are complicated because of late diagnosis and treatment. Over the past decades, only marginal improvement in overall survival has been observed in patients with OC. Therefore, it is crucial to identify a series of precise prognostic factors that can be used to improve patients’ outcomes.3,18
In current study, using TCGA database analysis and our samples, we found that KCNH3 expression levels were significantly higher in OC tissues than in normal ovarian tissues. OC patients with higher KCNH3 expression levels had shorter overall survival and disease-free survival times. Moreover, KCNH3 silencing suppressed cell proliferation and cell invasion, and induced apoptosis and cell cycle arrest. Taken together, these results demonstrate that KCNH3 plays an important role in promoting OC progression. KCNH3 is one of the members of potassium voltage-gated channel subfamily H, which has been studied in a limited research field, such as cognitive function and diabetes.14,15,19 Voltage-gated potassium channels are the most diverse group of ion channels and are proposed as therapeutic targets for tumor regression. Voltage-gated potassium channels can regulate a check point in the initial stages of the cell cycle to influence cell volume control, membrane potential, and other ion channel regulation. The blockade of voltage-gated potassium channels could impair cancer cell proliferation. Once voltage-gated potassium channels are remodeled in cancer cells, biological processes in cancers such as proliferation, transformation, adhesion, apoptosis, migration, and cell volume can be altered.20–23 However, in OC cells, the effect of KCNH3 and underlying mechanism of action remain unclear.
To better understand the molecular mechanisms of KCNH3 in OC, we performed GSEA analysis. We found that mismatch repair with P=0.008 showed better association with KCNH3 expression. To investigate the relationship between the mismatch repair pathway and KCNH3, RPA was used for Western blot in this study. RPA, a heterotrimeric complex of RPA1, RPA2 and RPA3, binds to single-stranded DNA and is important for DNA recombination, replication and repair. RPA is hyperphosphorylated in response to DNA damage.24 In addition, RPA is also essential in DNA resynthesis and lesion recognition during NER and it is reported that replication fork protection due to RPA might be a potential mechanism of cisplatin resistance.25,26 Our results show that KCNH3 silencing leads to downregulation of RPA1 and RPA2 expression in both SKOV3 and COC1 cells. This suggests that KCNH3 may induce cancer progression by activating the DNA repair signaling pathway through RPA1 and RPA2. However, precisely how KCNH3 regulates RPA expression in OC should be determined in future studies.
In addition, OC is increasingly considered as a disease of DNA repair deficiency and DNA repair targeted therapies have been developed.27–29 There are six main DNA repair pathways that strengthen genomic integrity throughout the cell cycle and DNA replication, including homologous recombination repair, base excision repair, mismatch repair, nucleotide excision repair (NER), Fanconi Anemia, and non-homologous end joining.30,31 Interestingly, homozygous deletions, non-synonymous mutations, and splice mutations of NER genes have been reported in around 8% of patients with OC, and these mutations are related to improved overall survival and progression-free survival.32 This indicates that inactivation of the NER pathway in OC may suppress tumor progression.
Conclusion
We aimed to investigate the role of KCNH3 in OC. Our findings suggest that high KCNH3 expression is associated with poor overall survival and disease-free survival in patients with OC. KCNH3 silencing could suppress cell proliferation and metastasis in OC cells, and induce apoptosis, and G2/M phase block. Furthermore, the results of Western blot showed that the silencing of KCNH3 might induce downregulation of RPA1 and RPA2 expression level in both SKOV3 and COC1 cells. Based on these results, KCNH3 may play an important role for OC prognosis or diagnosis.
Acknowledgment
This work was supported by grants from the National Natural Science Foundation of China (grant no. 81702399), the Social Science and Technology Development Key Project of Dongguan City (grant no. 2018507150011651), the Guangdong Province Medical Scientific Research Foundation (grant nos. C2017034, C2018053, C2018054 and C2019097) and the National Medical Science and Technology Foundation (grant no. W2016CWGD05)
Disclosure
The authors report no conflicts of interest in this work.
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
2. Torre LA, Trabert B, DeSantis CE, et al. Ovarian cancer statistics, 2018. CA Cancer J Clin. 2018;68(4):284–296. doi:10.3322/caac.21456
3. Lheureux S, Braunstein M, Oza AM. Epithelial ovarian cancer: evolution of management in the era of precision medicine. CA Cancer J Clin. 2019;69(4):280–304.
4. Lee JM, Minasian L, Kohn EC. New strategies in ovarian cancer treatment. Cancer-Am Cancer Soc. 2019;125(Suppl 24):4623–4629.
5. Chen SN, Chang R, Lin LT, et al. MicroRNA in ovarian cancer: biology, pathogenesis, and therapeutic opportunities. Int J Environ Res Public Health. 2019;16:9.
6. Altman DG, Riley RD. Primer: an evidence-based approach to prognostic markers. Nat Clin Pract Oncol. 2005;2(9):466–472. doi:10.1038/ncponc0287
7. Au KK, Josahkian JA, Francis JA, Squire JA, Koti M. Current state of biomarkers in ovarian cancer prognosis. Future Oncol. 2015;11(23):3187–3195. doi:10.2217/fon.15.251
8. Deng M, Bragelmann J, Schultze JL, Perner S. Web-TCGA: an online platform for integrated analysis of molecular cancer data sets. BMC Bioinform. 2016;17:72. doi:10.1186/s12859-016-0917-9
9. Wang Z, Jensen MA, Zenklusen JC. A practical guide to The Cancer Genome Atlas (TCGA). Methods Mol Biol. 2016;1418:111–141.
10. Serrano-Novillo C, Capera J, Colomer-Molera M, Condom E, Ferreres JC, Felipe A. Implication of voltage-gated potassium channels in neoplastic cell proliferation. Cancers (Basel). 2019;11(3):3. doi:10.3390/cancers11030287
11. Huang X, Dubuc AM, Hashizume R, et al. Voltage-gated potassium channel EAG2 controls mitotic entry and tumor growth in medulloblastoma via regulating cell volume dynamics. Genes Dev. 2012;26(16):1780–1796. doi:10.1101/gad.193789.112
12. Liu L, Chen Y, Zhang Q, Li C. Silencing of KCNA1 suppresses the cervical cancer development via mitochondria damage. Channels (Austin). 2019;13(1):321–330. doi:10.1080/19336950.2019.1648627
13. Takahashi S, Ohmiya M, Honda S, Ni K. The KCNH3 inhibitor ASP2905 shows potential in the treatment of attention deficit/hyperactivity disorder. PLoS One. 2018;13(11):e207750. doi:10.1371/journal.pone.0207750
14. Miyake A, Takahashi S, Nakamura Y, et al. Disruption of the ether-a-go-go K+ channel gene BEC1/KCNH3 enhances cognitive function. J Neurosci. 2009;29(46):14637–14645. doi:10.1523/JNEUROSCI.0901-09.2009
15. Pan J, Liu S, Farkas M, et al. Serum molecular signature for proliferative diabetic retinopathy in Saudi patients with type 2 diabetes. Mol Vis. 2016;22:636–645.
16. Ni Z, Yao C, Zhu X, et al. Ailanthone inhibits non-small cell lung cancer cell growth through repressing DNA replication via downregulating RPA1. Br J Cancer. 2017;117(11):1621–1630.
17. Byrne BM, Oakley GG. Replication protein A, the laxative that keeps DNA regular: the importance of RPA phosphorylation in maintaining genome stability. Semin Cell Dev Biol. 2019;86:112–120. doi:10.1016/j.semcdb.2018.04.005
18. Jammal MP, Martins-Filho A, Silveira TP, Murta EF, Nomelini RS. Cytokines and prognostic factors in epithelial ovarian cancer. Clin Med Insights Oncol. 2016;10:71–76. doi:10.4137/CMO.S38333
19. Takahashi S, Inamura K, Yarimizu J, Yamazaki M, Murai N, Ni K. Neurochemical and neuropharmacological characterization of ASP2905, a novel potent selective inhibitor of the potassium channel KCNH3. Eur J Pharmacol. 2017;810:26–35. doi:10.1016/j.ejphar.2017.05.045
20. Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448(3):274–286. doi:10.1007/s00424-004-1258-5
21. Pardo LA, Stuhmer W. The roles of K(+) channels in cancer. Nat Rev Cancer. 2014;14(1):39–48. doi:10.1038/nrc3635
22. Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda). 2004;19:285–292.
23. Muratori L, Petroni G, Antonuzzo L, et al. hERG1 positivity and Glut-1 negativity identifies high-risk TNM stage I and II colorectal cancer patients, regardless of adjuvant chemotherapy. Onco Targets Ther. 2016;9:6325–6332. doi:10.2147/OTT.S114090
24. Hac A, Brokowska J, Rintz E, Bartkowski M, Wegrzyn G, Herman-Antosiewicz A. Mechanism of selective anticancer activity of isothiocyanates relies on differences in DNA damage repair between cancer and healthy cells. Eur J Nutr. 2019.
25. Fortier E, Drobetsky E, Wurtele H. Know your limits: RPA availability and chemoresistance in ovarian cancer. Oncotarget. 2019;10(8):800–802. doi:10.18632/oncotarget.26607
26. Belanger F, Fortier E, Dube M, et al. Replication Protein A availability during DNA replication stress is a major determinant of cisplatin resistance in ovarian cancer cells. Cancer Res. 2018;78(19):5561–5573. doi:10.1158/0008-5472.CAN-18-0618
27. Konstantinopoulos PA, Matulonis UA. Targeting DNA damage response and repair as a therapeutic strategy for ovarian cancer. Hematol Oncol Clin North Am. 2018;32(6):997–1010. doi:10.1016/j.hoc.2018.07.006
28. Li G, Han L, Ren F, Zhang R, Qin G. Prognostic value of the tumor-specific ceRNA network in epithelial ovarian cancer. J Cell Physiol. 2019;234(12):22071–22081. doi:10.1002/jcp.28770
29. Gong C, Yang Z, Zhang L, Wang Y, Gong W, Liu Y. Quercetin suppresses DNA double-strand break repair and enhances the radiosensitivity of human ovarian cancer cells via p53-dependent endoplasmic reticulum stress pathway. Onco Targets Ther. 2018;11:17–27.
30. Damia G, Broggini M. Platinum resistance in ovarian cancer: role of DNA repair. Cancers (Basel). 2019;11:1. doi:10.3390/cancers11010119
31. Chartron E, Theillet C, Guiu S, Jacot W. Targeting homologous repair deficiency in breast and ovarian cancers: biological pathways, preclinical and clinical data. Crit Rev Oncol Hematol. 2019;133:58–73. doi:10.1016/j.critrevonc.2018.10.012
32. Ceccaldi R, O’Connor KW, Mouw KW, et al. A unique subset of epithelial ovarian cancers with platinum sensitivity and PARP inhibitor resistance. Cancer Res. 2015;75(4):628–634. doi:10.1158/0008-5472.CAN-14-2593
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.