Back to Journals » Clinical Interventions in Aging » Volume 10
Joint unloading implant modifies subchondral bone trabecular structure in medial knee osteoarthritis: 2-year outcomes of a pilot study using fractal signature analysis
Authors Miller LE , Sode M, Fuerst T, Block J
Received 4 November 2014
Accepted for publication 9 December 2014
Published 23 January 2015 Volume 2015:10 Pages 351—357
DOI https://doi.org/10.2147/CIA.S76982
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Richard Walker
Larry E Miller,1,2 Miki Sode,3 Thomas Fuerst,3 Jon E Block2
1Miller Scientific Consulting, Inc., Asheville, NC, USA; 2The Jon Block Group, San Francisco, CA, USA; 3Bioclinica, Newark, CA, USA
Background: Knee osteoarthritis (OA) is largely attributable to chronic excessive and aberrant joint loading. The purpose of this pilot study was to quantify radiographic changes in subchondral bone after treatment with a minimally invasive joint unloading implant (KineSpring® Knee Implant System).
Methods: Nine patients with unilateral medial knee OA resistant to nonsurgical therapy were treated with the KineSpring System and followed for 2 years. Main outcomes included Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain, function, and stiffness subscores and independent core laboratory determinations of joint space width and fractal signature of the tibial cortex.
Results: WOMAC scores, on average, improved by 92% for pain, 91% for function, and 79% for stiffness over the 2-year follow-up period. Joint space width in the medial compartment of the treated knee significantly increased from 0.9 mm at baseline to 3.1 mm at 2 years; joint space width in the medial compartment of the untreated knee was unchanged. Fractal signatures of the vertically oriented trabeculae in the medial compartment decreased by 2.8% in the treated knee and increased by 2.1% in the untreated knee over 2 years. No statistically significant fractal signature changes were observed in the horizontally oriented trabeculae in the medial compartment or in the horizontal or vertical trabeculae of the lateral compartment in the treated knee.
Conclusion: Preliminary evidence suggests that the KineSpring System may modify knee OA disease progression by increasing joint space width and improving subchondral bone trabecular integrity, thereby reducing pain and improving joint function.
Keywords: disease modification, KineSpring, joint space, pain, trabecular
Introduction
Osteoarthritis (OA) of the knee is a leading cause of disability in adults that is attributed, in large part, to chronic excessive and aberrant joint loading.1 Despite the recent concerted effort to develop disease modifying OA drugs, none have been shown to alter the natural history of knee OA in human clinical trials. According to the Osteoarthritis Research Society International/Outcome Measures in Rheumatology Initiative,2 pain, functional impairment, and radiographic progression should comprise the key endpoints in trials intended to evaluate potential disease-modifying therapies. To date, there is no known treatment for knee OA that has consistently reported benefit for each of these disease modification characteristics.2
Mounting evidence suggests that minimally invasive joint unloading implants have clinical utility in patients who have unsuccessfully exhausted nonsurgical knee OA therapies, but who are ineligible or unwilling to undergo arthroplasty. Data from several clinical studies have reported that these implants provide durable and clinically meaningful improvements in knee pain and function.3–7 However, none of these studies have reported longitudinal radiographic changes in the knee following implant. While disease progression is frequently assessed by quantifying intraarticular cartilage loss, changes in subchondral bone also play a key role in the pathogenesis of knee OA.8 In fact, subchondral bone trabecular integrity has been shown to be a strong predictor of disease progression.9 Fractal signature analysis (FSA) assesses the complexity of detail in a two-dimensional image (ie, radiograph) to yield a value representative of trabecular number, spacing, and cross-connectivity.10 The purpose of this pilot study was to quantify clinical outcomes and radiographic changes occurring in the knee joint 2 years following treatment with a joint unloading implant, with special emphasis on subchondral bone characteristics quantified by FSA.
Methods
The COAST clinical study prospectively enrolled 40 patients at five European centers who received the KineSpring® Knee Implant System (Moximed, Inc., Hayward, CA, USA) for treatment of unilateral medial compartment knee OA (ISRCTN63048529). The study was approved by local hospital ethics committees and all subjects provided written informed consent before participation. Interim results from the COAST study have been reported elsewhere.3 This report describes a post hoc assessment of subchondral trabecular integrity using fractal signature methods in a randomly selected subgroup of nine patients from the COAST study with available 2-year follow-up data and adequate imaging.
Patients
Inclusion criteria for the COAST study included patients were aged 25 years and older with radiographically confirmed medial knee OA, diagnosed according to the American College of Rheumatology Clinical and Radiographic or Clinical Classification criteria.11 All eligible patients had previously failed at least 3 months of nonoperative care. Exclusion criteria included symptomatic OA in the lateral or patellofemoral compartment, varus or valgus malalignment >10 degrees, inflammatory joint disease, moderate to severe osteoporosis, recent surgery or previous prosthesis at the target knee, ligamentous or meniscal instability, active infection, and clinically significant comorbidity (eg, uncontrolled diabetes mellitus).
Procedures
Baseline assessments included inclusion/exclusion criteria evaluation, a complete clinical and orthopedic examination, and medical history. At baseline and at 2 years follow-up, standing X-rays (anteroposterior, lateral, and sunrise views) were performed on all patients. The Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC, version 3.1) was used to quantify changes in knee pain, function, and stiffness.12
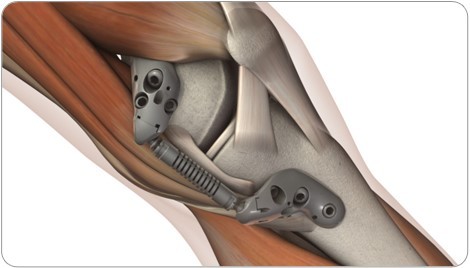
All patients were treated with the KineSpring System (Figure 1), a minimally invasive implant that reduces loading at the diseased medial compartment during the stance phase of gait. The KineSpring System absorbs a maximum load of 13 kg in the medial compartment without transferring additional loads to the lateral knee compartment.13 The magnitude of medial joint unloading provided by the KineSpring System is comparable to the amount of body weight loss that improves joint function and alleviates knee pain in OA patients.14 A detailed description of the surgical procedure for KineSpring System implant has been reported elsewhere.15
  | Figure 1 KineSpring®Knee Implant System. |
Radiographic imaging
An imaging core laboratory (SYNARC, Newark, CA, USA) independently reviewed pretreatment and 2-year radiographs in patients treated with the joint unloading implant. Radiographs of the treated and untreated knee were digitally acquired with a mean resolution of 0.12±0.024 microns/pixel. Image analysis was performed using dedicated software (KneeAnalyzer; Optasia Medical, Cheadle, UK) that incorporates computer-aided detection methods (Figure 2) using statistical modeling to provide quantitative measurements of subchondral trabecular integrity in the medial and lateral compartments of each knee (Figure 3).9 The software also automatically calculated the minimum joint space width (JSW) of the medial and lateral compartments in each knee.
Data analysis
Continuous data were reported as median (interquartile range [IQR]), and categorical data were reported as frequencies. Longitudinal changes in clinical outcomes were assessed with the Wilcoxon signed rank test. Fractal signature data were analyzed using the methods of Kraus et al.9,16 Briefly, morphology of vertically and horizontally aligned trabeculae was assessed by quantifying the fractal dimension across a range of radii, representing trabeculae from 0.4 to 3.0 mm in thickness. Quadratic multiple regression using a noncentered polynomial was applied to model data for each patient, which accounted for differences in pixel width among radiographs. Pretreatment and 2-year radiographs were analyzed using area under the curve methodology.17 Due to the pilot nature of this study, a one-sided P-value <0.1 was considered statistically significant.18 The sample size was selected to provide 80% power to detect a minimum standardized pre–post effect size ≥0.80 (PASS 2013, Kaysville, UT, USA). Data were analyzed using IBM SPSS Statistics (version 22; IBM Corp., Armonk, NY, USA).
Results
Of the 40 patients enrolled in the COAST study, nine patients (five males) with complete clinical and radiographic data at 2 years were randomly selected for inclusion in this pilot study. Median patient age was 53 years (IQR: 48 to 58 years) with body mass index of 30 kg/m2 (IQR: 28 to 33 kg/m2) (Table 1). Clinical and radiographic disease severity of these patients were comparable to patients undergoing total knee arthroplasty.19,20
Statistically significant improvements were observed in all WOMAC scores 2 years following implant with the KineSpring® System. Median WOMAC scores improved 92% for pain, 91% for function, and 79% for stiffness (all P<0.01) over the 2-year follow-up period (Figure 4). All patients exceeded the threshold for clinical success in each WOMAC domain, defined as a ≥20% improvement from baseline.21
Minimum JSW in the medial compartment of the treated knee significantly increased from a median of 0.9 mm at baseline to 3.1 mm at 2 years (P=0.04); JSW in the medial compartment of the untreated knee was unchanged (P=0.32) (Figure 5). No significant differences in JSW were observed in the lateral compartment of either knee.
  | Figure 5 Change in joint space width over 2 years following joint unloading implant. |
Fractal signatures of the vertically oriented trabeculae in the medial compartment decreased by 2.8% in the treated knee and increased by 2.1% in the untreated knee over 2 years postimplant (Figure 6A). The differences in these FSA values between knees were statistically significant (P=0.09) and, from a clinical perspective, was suggestive of OA modification in the treated knee. No statistically significant FSA changes were observed in the horizontally oriented trabeculae in the medial compartment (Figure 6B) or in the horizontal or vertical trabeculae of the lateral compartment in the treated knee.
Discussion
The results of this pilot study suggest that a joint unloading implant may modify knee OA disease progression by increasing JSW and improving subchondral bone trabecular integrity, thereby reducing pain and improving joint function. This is the first report demonstrating radiographic evidence of knee OA disease modification with the KineSpring System.
OA progression is largely mediated through failed attempts at subchondral bone and cartilage repair secondary to excessive mechanical loading at the knee joint. Over time, repetitive impulse loading causes microfractures to develop in the subchondral endplate, resulting in local osteoporosis, subchondral sclerosis, and subsequent cartilage degeneration.22–24 OA progression can be visualized and quantified as thinning and fenestration of vertical trabeculae due to stress shielding and hypomineralization.16
In this study, we observed the opposite phenomenon; that is, medial compartment unloading over 2 years with a minimally invasive implant modified subchondral sclerosis in vertically oriented trabeculae with concomitant increases in JSW in the medial knee compartment. The observed decrease in FSA area under the curve over 2 years is indicative of a less complex radiographic image, with reversal of thinning and fenestration of vertically oriented trabeculae. Fractal signatures of the horizontally oriented trabeculae were unchanged in the current study. This observation was not unexpected; vertical trabecular integrity has a strong relationship with OA progression while the relationship with horizontal trabecular morphometry is inconsistent.9,16,25,26
Interestingly, over 2 years, JSW in the medial compartment of the treated knee increased to approach the baseline JSW of the unaffected knee. These data further support the concept that knee OA progression may be modified when chronic biomechanical overloading forces are reduced.1 Regarding therapies intended to unload the knee joint, Waller et al state:
[…] if the pathological stress pattern across the joint is normalized, it has been hypothesized that extrinsic cells can induce repair by forming fibrocartilage, remodeling of the subchondral plate so a typical trabeculated pattern will ensue, regaining its shock absorption quality and joint space width will be reestablished.1
Studies of surgical joint distraction in patients with knee OA have reported similar findings, with sustained improvements in clinical outcomes, JSW, and cartilage thickness.27,28 The observation of decreased vertical FSA values, increased JSW, and clinically meaningful improvements in patient symptoms with the KineSpring System is supportive of these previous findings.
This pilot study was limited by a small sample size, which limits generalizability of results to the general population with knee OA. Therefore, the results are indeed promising but should not be considered conclusive. A matched control group that did not receive the joint unloading implant would provide more definitive evidence that the observed results were not attributable to confounding factors. Another limitation of this study was that a standardized imaging protocol was not used across study sites, which may introduce additional variability to the radiographic measures. A final limitation of this study was that only patients with available 2-year follow-up were selected for inclusion, which may introduce bias in the study outcomes. A notable strength of the study was use of semiautomated software to determine FSA values, which was used to reduce measurement variability compared to other fractal signature methods.29,30 Additional studies with larger sample sizes are needed to confirm the impact of the KineSpring System on radiographic indexes of knee OA progression.
Conclusion
Preliminary evidence suggests that in patients with knee OA, the KineSpring System may modify knee OA disease progression by increasing JSW and improving subchondral bone trabecular integrity, thereby reducing pain and improving joint function.
Acknowledgment
This research was supported by Moximed, Inc.
Disclosure
The authors report no conflicts of interest in this work.
References
Waller C, Hayes D, Block JE, London NJ. Unload it: the key to the treatment of knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2011;19(11):1823–1829. | ||
Manno RL, Bingham CO, Paternotte S, et al. OARSI-OMERACT initiative: defining thresholds for symptomatic severity and structural changes in disease modifying osteoarthritis drug (DMOAD) clinical trials. Osteoarthritis Cartilage. 2012;20(2):93–101. | ||
London NJ, Smith J, Miller LE, Block JE. Midterm outcomes and predictors of clinical success with the KineSpring Knee Implant System. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:19–28. | ||
Li CS, Seeger T, Auhuber TC, Bhandari M. Cost-effectiveness and economic impact of the KineSpring® Knee Implant System in the treatment for knee osteoarthritis. Knee Surg Sports Traumatol Arthrosc. 2013;21(11):2629–2637. | ||
Farr J, Crawford DC, Diduch DR, Arendt EA, Ma CB, Li CS. Prospective, multi-center, pilot study to evaluate symptom relief in patients with medial knee osteoarthritis (OA) treated with the KineSpring® knee implant for load reduction – the SOAR protocol. J Long Term Eff Med Implants. 2013;23(2–3):161–173. | ||
Li CS, Ayeni OR, Sprague S, Truong V, Bhandari M. Conservative treatments, surgical treatments, and the KineSpring® Knee Implant system for knee osteoarthritis: a systematic review. J Long Term Eff Med Implants. 2013;23(2–3):105–149. | ||
Gabriel SM, Clifford AG, Maloney WJ, O’Connell MK, Tornetta P. Unloading the osteoarthritic knee with a novel implant system. J Appl Biomech. 2013;29(6):647–654. | ||
Kwan Tat S, Lajeunesse D, Pelletier JP, Martel-Pelletier J. Targeting subchondral bone for treating osteoarthritis: what is the evidence? Best Pract Res Clin Rheumatol. 2010;24(1):51–70. | ||
Kraus VB, Feng S, Wang S, et al. Trabecular morphometry by fractal signature analysis is a novel marker of osteoarthritis progression. Arthritis Rheum. 2009;60(12):3711–3722. | ||
Messent EA, Buckland-Wright JC, Blake GM. Fractal analysis of trabecular bone in knee osteoarthritis (OA) is a more sensitive marker of disease status than bone mineral density (BMD). Calcif Tissue Int. 2005;76(6):419–425. | ||
Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–1049. | ||
Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15(12):1833–1840. | ||
Clifford AG, Gabriel SM, O’Connell M, Lowe D, Miller LE, Block JE. The KineSpring(®) Knee Implant System: an implantable joint-unloading prosthesis for treatment of medial knee osteoarthritis. Med Devices (Auckl). 2013;6:69–76. | ||
Zhao D, Banks SA, Mitchell KH, D’Lima DD, Colwell CW Jr, Fregly BJ. Correlation between the knee adduction torque and medial contact force for a variety of gait patterns. J Orthop Res. 2007;25(6):789–797. | ||
Clifford A, O’Connell M, Gabriel S, Miller LE, Block JE. The KineSpring load absorber implant: rationale, design and biomechanical characterization. J Med Eng Technol. 2011;35(1):65–71. | ||
Kraus VB, Feng S, Wang S, et al. Subchondral bone trabecular integrity predicts and changes concurrently with radiographic and magnetic resonance imaging-determined knee osteoarthritis progression. Arthritis Rheum. 2013;65(7):1812–1821. | ||
Matthews JN, Altman DG, Campbell MJ, Royston P. Analysis of serial measurements in medical research. BMJ. 1990;300(6719):230–235. | ||
Lee EC, Whitehead AL, Jacques RM, Julious SA. The statistical interpretation of pilot trials: should significance thresholds be reconsidered? BMC Med Res Methodol. 2014;14:41. | ||
Bachmeier CJ, March LM, Cross MJ, et al; Arthritis Cost and Outcome Project Group. A comparison of outcomes in osteoarthritis patients undergoing total hip and knee replacement surgery. Osteoarthritis Cartilage. 2001;9(2):137–146. | ||
Becker R, Döring C, Denecke A, Brosz M. Expectation, satisfaction and clinical outcome of patients after total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc. 2011;19(9):1433–1441. | ||
Barr S, Bellamy N, Buchanan WW, et al. A comparative study of signal versus aggregate methods of outcome measurement based on the WOMAC Osteoarthritis Index. Western Ontario and McMaster Universities Osteoarthritis Index. J Rheumatol. 1994;21(11):2106–2112. | ||
Burr DB, Radin EL. Microfractures and microcracks in subchondral bone: are they relevant to osteoarthrosis? Rheum Dis Clin North Am. 2003;29(4):675–685. | ||
Lindsey CT, Narasimhan A, Adolfo JM, et al. Magnetic resonance evaluation of the interrelationship between articular cartilage and trabecular bone of the osteoarthritic knee. Osteoarthritis Cartilage. 2004;12(2):86–96. | ||
Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res. 1986;(213):34–40. | ||
Messent EA, Ward RJ, Tonkin CJ, Buckland-Wright C. Tibial cancellous bone changes in patients with knee osteoarthritis. A short-term longitudinal study using Fractal Signature Analysis. Osteoarthritis Cartilage. 2005;13(6):463–470. | ||
Buckland-Wright JC, Messent EA, Bingham CO, Ward RJ, Tonkin C. A 2 yr longitudinal radiographic study examining the effect of a bisphosphonate (risedronate) upon subchondral bone loss in osteoarthritic knee patients. Rheumatology (Oxford). 2007;46(2):257–264. | ||
Wiegant K, van Roermund PM, Intema F, et al. Sustained clinical and structural benefit after joint distraction in the treatment of severe knee osteoarthritis. Osteoarthritis Cartilage. 2013;21(11):1660–1667. | ||
Intema F, Van Roermund PM, Marijnissen AC, et al. Tissue structure modification in knee osteoarthritis by use of joint distraction: an open 1-year pilot study. Ann Rheum Dis. 2011;70(8):1441–1446. | ||
Wolski M, Podsiadlo P, Stachowiak GW, Lohmander LS, Englund M. Differences in trabecular bone texture between knees with and without radiographic osteoarthritis detected by directional fractal signature method. Osteoarthritis Cartilage. 2010;18(5):684–690. | ||
Podsiadlo P, Dahl L, Englund M, Lohmander LS, Stachowiak GW. Differences in trabecular bone texture between knees with and without radiographic osteoarthritis detected by fractal methods. Osteoarthritis Cartilage. 2008;16(3):323–329. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





