Back to Journals » HIV/AIDS - Research and Palliative Care » Volume 13
Joint Modeling of Incidence of Tuberculosis and Change in Viral Load Over Time Among Adult HIV/AIDS Patients on Anti-Retroviral Therapy at Zewditu Memorial Hospital in Addis Ababa, Ethiopia
Authors Ayana GM , Akalu TY , Ayele TA
Received 12 November 2020
Accepted for publication 29 January 2021
Published 1 March 2021 Volume 2021:13 Pages 239—249
DOI https://doi.org/10.2147/HIV.S291872
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bassel Sawaya
Galana Mamo Ayana,1 Temesgen Yihunie Akalu,2 Tadesse Awoke Ayele2
1Department of Epidemiology and Biostatistics, School of Public Health, Haramaya University, Harar, Ethiopia; 2Department of Epidemiology and Biostatistics, Institute of Public Health, University of Gondar, Gondar, Ethiopia
Correspondence: Temesgen Yihunie Akalu Email [email protected]
Background: Globally, approximately 37.9 million people were living with HIV and one-third of these people are co-infected with tuberculosis (TB). However, little is known about predictors of tuberculosis incidence and its association with viral load. Thus, this study was aimed at assessing the incidence of tuberculosis and its predictors and its association with the longitudinal change in viral load over time among adult HIV/AIDS patients at Zewditu memorial hospital, Addis Ababa Ethiopia.
Methods: A retrospective follow-up study was conducted among 471 HIV patients. The proportional hazard assumption was checked for the survival sub-model and the longitudinal sub-model. &Ngr;ormality assumption was checked. Then the joint model with time-dependent lagged parameterizations was fitted. The goodness of fit was checked using the Cox-Snell residual test and Akaike Information Criteria (AIC) was used for model selection. Finally, the hazard ratio with a 95% confidence interval (CI) with a corresponding P-value < 0.05 was used.
Results: A total of 471 patients were followed for a minimum of 12 and a maximum of 48 months with a median follow-up time of 44 months [IQR (33, 46)]. The incidence rate was 3.08/1000 person-years (PY) with a 95% CI of [0.0023788, 0.003998). Age above 65 years adjusted hazard ratio (AHR) = 2.07, 95% CI: 1.06, 4.06), underweight at baseline (AHR = 2.29, 95% CI: 1.20, 4.35), past opportunistic infection (AHR = 2.98, CI: 1.23, 7.17) and 6th month lagged value of the viral load were significant predictors for being co-infected with TB.
Conclusion: The incidence of TB among HIV/AIDS patients in Zewditu memorial hospital was low. Older age, underweight at baseline, and past opportunistic infection were significant predictors of time to TB co-infection. Thus, addressing significant predictors and strengthening continuous follow-up are highly recommended in the study setting.
Keywords: TB incidence, viral load change, joint modeling, adults, HIV/AIDS
Background
Tuberculosis (TB) is the most common co-infection and cause of death among people living with human immunodeficiency virus (HIV).1 At least 1 out of 4 HIV-related deaths were caused as a result of TB and many of these deaths occur in developing countries. People living with HIV are around 20 times more likely to develop TB than HIV free individuals.2,3
According to the World health organization (WHO) 2019 report, TB is one of the top 10 causes of death with an incidence of about 10 million cases annually. Of these, about 1.5 million were died and one-sixth (251,000) were people living with HIV and Africa accounts for 84% of the total deaths.1,4 Ethiopia is one of the 30 high TB and HIV burden countries in the world with an incidence of 164 and 17 cases per 100,000 population, respectively.5 In Ethiopia, the incidence of TB among HIV patients varies from 3.3 in Addis Ababa to 8.6 in Afar region per 100 Person years (PY) observation.1,6
The effectiveness of anti-retro-viral therapy (ART) is assessed by clinical observations and longitudinal biomarkers including CD4 cell counts and plasma viral load.7 The incidence of TB was affected by several factors including CD4 cell count and the number of viral loads,8,9 household family size, cigarette smoking, use of IPT, CPT, baseline CD4 cell counts, WHO clinical stage, and having a history of diabetics.10–13 However, little is known about the association between longitudinal viral load and the incidence of TB.
The Ethiopian ministry of health planned to reduce TB-related deaths and incidence by 90% and 80%, respectively by the year 2030.5 To evaluate this target, current, and up-to-date information on the incidence of TB is important. Besides, the finding of the current study was important to identify potential determinants that will be valuable for program managers to produce appropriate intervention and will serve as a baseline for future researchers. Therefore, the current study aim to identify the predictors of incidence of TB with the association with viral load change over time among HIV-Infected adult clients at Zewditu Memorial Hospital, Addis Ababa, Ethiopia.
Methods
Study Design and Setting
A retrospective follow-up study was conducted among HIV/AIDS patients on ART between February 2016 and December 2019. Based on the 2007 census Addis Ababa, the capital city of Ethiopia, had an estimated population of 3,384,569. The study was conducted at Zewditu memorial hospital, Addis Ababa, which was the first public hospital in the city and had a better patient load. Besides, the availability of repeatedly viral load measurement was better at Zewditu memorial hospital compared to other hospitals in the city. The hospital renders services to all populations of the city and the nation at large. Besides, it has around 6000 adult HIV patients who are currently on ART. In Zewditu memorial hospital viral load measurement was began in 2016.
Population and Sample
In this study, the source population was all adult HIV/AIDS patients at Zewditu memorial hospital. The study population was all HIV/AIDS patients who enrolled between February 2016 and December 2019. All enrolled adult HIV patients during the study period and TB free at the inception of the study with at least two measurements of viral load were included. However, subjects whose date of TB co-infection was unknown were excluded.
The minimum required sample size for this study was calculated using a sample size determination formula for survival analysis using STATA 14 statistical software by considering a history of TB, WHO clinical stage, and hemoglobin level from previous studies. It was calculated under the following statistical assumptions: two-sided significant level (α) of 5%, power 80%, Zα/2 value at 95% CI 1.96, q1: the proportion of subjects that are in group 1 (exposed), q0: the proportion of subjects that are in group 2 (unexposed); 1-q1, HR: hazard ratio, and the probability of an event (E).
From a study conducted in Afar Regional State, northeast Ethiopia.14 Finally, the maximum calculated sample size for this study was 471. To select the study participants, the records of all HIV-positive adults ever started ART and recorded from January 1, 2016, to December 31, 2019, were sorted. Then, the study participants were selected using a simple random sampling technique through computer-generated random numbers (Table 1).
 |
Table 1 Sample Size for Covariates Associated with TB Co-Infection |
Variables of the Study
The dependent variable was the incidence of TB and viral load. The predictor variables were: socio-demographic characteristics (age, sex, marital status, educational status, and occupation), baseline clinical and behavioral characteristics (Baseline CD4 count, history of TB, baseline functional status, past opportunistic infection, baseline hemoglobin, baseline BMI, initial ART regimen, and adherence at baseline) and time-varying endogenous covariates: repeatedly measured viral load recorded as the number of viral copies per milliliters.
Incidence of TB: was defined as a new active TB developed in a given period and patients were followed for four years. For this study censored was defined, as the event of interest may not be observed until the end of the study period, death before developing active TB, and lost follow-up before developing active TB. Event: is defined as the development of active TB during the follow-up period. Also, opportunistic infections (OIs) were diagnosed if HIV-positive adults developed any morbidities after starting ART as documented by the health care professionals. Based on the WHO classification normal weight was between 18.5–24.9 kg/m2, BMI less than 18.5, and greater than 25 is underweight and overweight, respectively.
According to WHO, patients have good adherence when the patients follow the recommendation made by health care professionals such as personal behavior change, taking medication, following a diet, and executing lifestyle change. Furthermore, adherence was classified as good, fair, or poor, according to the percentage of drug dosage calculated from a monthly total dose of ART drugs. Hence, good was reported if equal to or greater than 95% or ≤ 3 doses missing per month, fair if 85–94% or 4–8 dose missing per month, or poor if less than 85% or ≥ 9 doses missing per month.15
Low hemoglobin level was defined as Hgb level less than 10 g/dl. Alcohol consumption: was recorded by asking respondents who have ever drunk alcohol or not. It was dichotomized into drink alcohol or not. Finally, cigarette smoking: was recorded by asking respondents whether they have an ever-smoke cigarette in life history or not. It was dichotomized by 1 (Yes i.e., smoke cigarettes) and 0 (free of smoking cigarettes). A viral load measure measures how much human immunodeficiency is in the blood viral load measurement was done after 6 month of treatment starting date. Viral load is measured using three types of tests: reverse transcriptase chain reaction (RT-PCR) tests, Branched DNA (bDNA) tests, Nucleic acid sequence based implications (NASBA). These test measure amount of the genetic material (RNA) of HIV in the blood.
Ethics Approval and Consent to Participate
The ethical review committee of the College of Medicine and Health Sciences, University of Gondar approved the study protocol. Besides, a support letter was obtained from the medical director of Zewditu memorial hospital to access the medical records of patients. Informed consent was not taken from all respondents enrolled in the study since data were collected from the patient’s follow-up chart. Confidentiality during all phases of research activities was kept and data was held on a secured password-protected system. All the procedures are based on the principles of Helsinki declaration.
Data Collection Procedures and Quality Control
A checklist was prepared and used to collect relevant data by reviewing patient charts. Health professionals working at the ART clinic were assigned as data collectors and data was extracted by reviewing follow-up charts and cards of patients. A two-day training was given for the data collectors on the objective of the study and how to review the documents as per the data extraction checklist.
The health management information system (HMIS) card number was used to identify individual patient cards. The principal investigator supervised the data collectors closely. Before the actual data collection, the preliminary review was conducted among 5% of the sample size on the selected hospital. Then adequacy of the checklist was evaluated and ambiguous questions were modified before the actual data collection. Besides, daily monitoring of data for completeness and consistency was made.
Data Processing and Analysis
The data entry was done using Epi-data version 4.6.0.2 and then exported to R statistical software Version 3.6.1 for further analysis. Descriptive statistics were done and summarized using tables, graphs, and texts. Percentages were used for categorical variables and mean and median were used to summarize the continuous variables. The incidence of TB was calculated. The Kaplan–Meier (KM) curve and Log rank test were used to describe survival experience among different categories. The proportional hazard assumption was checked both graphically and using the Schoenfeld residual test for the survival sub-model. The hazard ratio was used as a measure of association for the survival sub-model. The normality of the data was checked for the longitudinal outcome by using a histogram and a normal quintile Q-Q plot. To handle missing values sensitivity analysis was used.
The generalized linear mixed effect model and the Cox proportional hazard model was fitted for longitudinal and the survival sub-model, respectively. To estimate the effects of the viral load change on the incidence of TB joint models were fitted with time-dependent lagged parameterizations using the JM package. Association parameter (alpha value) from a fitted joint model was used to assess the association between the longitudinal viral load change and the incidence of TB. The significance level of 0.05 was used as a cut-off point for all statistical tests. Cox Snell residual was used to check the overall goodness of fit for the survival sub-model and the marginal residual was used for the longitudinal sub-model. Akaike Information Criteria (AIC) was used for model selection.
Results
Socio-Demographic Characteristics
Data of 482 patients who were on ART between 2016 and 2019 were collected and records of 11 patients were excluded because their date of TB co-infection was not well recorded. The final analysis was done on 471 patients with a response rate of 97.72%. The median age of study subjects was 38 years with an interquartile range (IQR) of 30–46 years (Table 2).
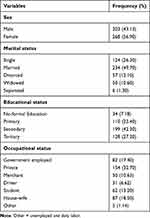 |
Table 2 Baseline Socio-Demographic Characteristics of Patients on ART in Zewditu Memorial Hospital, Addis Ababa, Ethiopia |
Baseline Clinical and Behavioral Characteristics
Two hundred (42.5%) of study participants were in the second WHO stage and 37 (7.9%) were in the fourth WHO stage at the baseline. The median baseline CD4 cell count of the study participants was 325 cells/mm3 with [IQR: 219 to 464]. Of all, 56 (11.9%) of study subjects had developed TB during the follow-up time (Table 3).
 |
Table 3 Frequencies and Percentages of Baseline Clinical and Behavioral Characteristics of Patients on ART in Zewditu Memorial Hospital, Addis Ababa, Ethiopia |
Incidence of TB Co-Infection
The patients were followed for a minimum of 12 and a maximum of 48 months with a median follow-up time of 44 months [IQR, (33, 46)]. Out of 471 study participants, 56 (11.9%) were develop active TB. The incidence rate of TB was 3.08/1000 PY with a 95% CI [2.38–3.99]. From the Kaplan–Meier hazard estimate presented below, we understood that the hazard of TB was higher among patients with the fourth baseline WHO clinical stage and poor baseline adherence (Figures 1 and 2).
 |
Figure 1 Kaplan–Meier survival curve by WHO staging among HIV patients in Zewditu Memorial Hospital, Addis Ababa, Ethiopia. |
 |
Figure 2 Kaplan–Meier survival curve by adherence among HIV patients in Zewditu Memorial Hospital, Addis Ababa, Ethiopia. |
Exploring Viral Load Change
A minimum of three and a maximum of seven measurements were taken during the study period. Individual profile plots showed that there was a variability of viral load within and between patients. A different starting point for patients suggests a random intercept model is reasonable. As the trajectory of viral load over time for patients was not constant it suggests considering a mixed-effect model with random slope is appropriate. The mean profile plot indicates a change in viral load over time follows a linear change. Therefore, the model that includes the linear effect of time was reasonable (Figures 3 and 4).
 |
Figure 3 Individual profile plot of HIV patients in Zewditu Memorial Hospital, Addis ;Ababa, Ethiopia. |
 |
Figure 4 Mean profile plot of HIV patients in Zewditu Memorial Hospital, Addis Ababa, Ethiopia. |
Model Diagnosis and Model Comparisons
The proportional hazard assumption was checked by using the cumulative hazard plot and Schoenfeld residual test. Normality was checked by using the Q-Q plot but it has failed to fulfill and after transformation by log, it fulfills the normality assumptions. The goodness of fit was checked by using Cox Snell residual test for the survival sub-model and the marginal residual was used for the longitudinal sub-model. Since missing value is obvious in longitudinal data, a sensitivity analysis was carried out by using complete case and multiple imputation techniques. After the proportional hazard assumption was checked, both semi-parametric and parametric models were fitted. Based on the assumption of the model with the lowest AIC is the best-fitted model, the Cox proportional hazard model was a parsimonious survival sub-model. For the longitudinal sub-model, the linear mixed effect model with both random intercept and slope was the bested fitted model (Table 4). After all the joint model of Cox proportional hazard model and linear mixed effect model with both random intercept and slope were jointly modeled using time-dependent 6th month lagged parameterizations under Piece wise-PH-GH specifications of the baseline risk function to express the correct relationship between the change in the number of viral load and incidence of TB under both complete case and multiple imputation techniques. Based on the AIC value joint model with time-dependent lagged parameterizations under a complete case analysis approach gives a better fit and is selected as the final model. The reason why the 6th month lagged parameterization was used is the first measurement for the viral load was taken after the sixth month and data were managed by the sixth-month interval (Table 5).
 |
Table 4 Model Comparisons for Survival and Longitudinal Sub-Models |
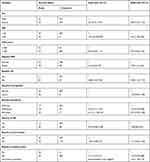 |
Table 5 Survival Sub-Models with Time-Dependent Lagged Parameterizations for Patients on ART in Zewditu Memorial Hospital, Addis Ababa, Ethiopia |
Predictors of Incidence of TB
The result showed that the 6th month lagged parameterization value of the viral load was significantly associated with the incidence of TB. Baseline age, baseline BMI, and past opportunistic infection were significant predictors of time to TB co-infection. The hazard of developing active TB was 2.07 (AHR=2.15, 95% CI [1.06, 4.06]) times higher among patients with age ≥65 years compared to patients with age <65years. The hazard of developing active TB among underweight patients was 2.29 (AHR=2.29, 95% CI [1.20, 4.35]) times higher compared to patients with a normal BMI. Patients with baseline opportunistic infection had 2.98 times higher (AHR=2.76, 95% CI [1.23, 7.17]) hazard of developing active TB compared with patients without baseline opportunistic infection. From the chosen joint model, the past 6th-month value of viral load in the body was significantly associated with today’s risk of developing TB. For a unit increase in the viral load, the incidence of TB was increased by 67% (95% CI 1.67 [1.30, 2.14] (Table 6)).
 |
Table 6 Longitudinal Sub-Models with Time-Dependent Lagged Parameterizations for Patients on ART in Zewditu Memorial Hospital, Addis Ababa, Ethiopia |
Discussion
This study was conducted to determine the incidence of TB and its predictor’s as well as a change in viral load and its effect on the incidence of TB among adult HIV patients on ART at Zewditu memorial hospital, Addis Ababa Ethiopia. In the current study, 56 (11.9%) of patients on ART developed active TB with an incidence rate of 3.08 per 1000 PY observations with a 95% CI [0.0024, 0.004]. This estimate was lower than that of a study done in Ethiopia at Zewditu Memorial Hospital, Addis Ababa (3.1 per 100 PY observation),6 University of Gondar Comprehensive Specialized Hospital (2.2 per 100 PY observation),16 and a study from northeast Ethiopia (8.64 per 100 PY observation).14 The reasons for this discrepancy might be due to the difference in the follow-up period and the exclusion of patients with less than two measurements of the longitudinal outcome. In the former studies, patients were followed for greater than five years whereas in the current study patients were followed for a maximum of four years. Besides, the incidence of the current study was lower than that of a study done in Nigeria and South Africa with an incidence rate of 25.8 per 1000 PY observation, and 4.2100 per PY observation, respectively. This might be due to lifestyle differences: as per WHO reported that the number of individuals who utilize illicit drugs is high in these two countries compared to Ethiopia that could suppress their immunity and increase the incidence of TB.1,17–20
A joint modeling approach was applied to assess the association between longitudinal viral load trajectory and the incidence of TB. Baseline age, baseline BMI, and past opportunistic infection were significant predictors of the incidence of TB. The 6th month lagged parameterization value of the viral load was significantly associated with the incidence of TB. Patients with ≥65 years had a higher hazard of developing TB compared to those patients with <65 years. This finding is in line with the study done in Nigeria.20 The possible explanation could be aged individuals are more susceptible to infections and are frequently less protected by vaccines.21 As a result, HIV patients with aging are more likely to develop TB than their counterparts. Underweight patients had a higher hazard of experiencing active TB compared to patients with normal BMI. This finding is consistent with a study done in northeast Ethiopia.14 The possible explanations could be underweight patients with HIV are associated with an increase in catabolic activity, infection, loss of appetite, and decreased intake which further increases the risk of developing TB.22,23
Another important result that was found to have a significant association with the incidence of TB was baseline opportunistic infection. The hazard of developing the incidence of TB among patients with an opportunistic infection at the baseline was higher compared to patients with no baseline opportunistic infection. This finding is in agreement with the study conducted in northwest Ethiopia16 and South Africa.8 This could be the presence of OIs during ART initiation increases more weakening of the immune system which results in experiencing the incidence of TB. Besides, opportunistic infection diminishes the immediate initiation of ART among HIV-TB co-infected patients with low CD4 count.23
From the results of time-dependent lagged value parameterization joint modeling, we observed that the past 6th month value of viral load in the body is significantly associated with today’s risk of TB. For patients with the same covariates in the model at baseline and who have the same underlying level of viral load. For a unit (log10/mL) increase in the viral load increase the incidence of TB increased by 67%. As per my best knowledge, no study was done to assess the incidence of TB and its association with viral load among adult HIV patients.
Despite the above strength, this study has limitations. As the current study was based on secondary data obtained from patient medical records, important variables like socioeconomic status like income and behavioral variables like substance use status which affected the incidence of TB in the previous studies were not well recorded. Besides, since viral load measurement started recently there is no enough repeatedly measured data that could affect the true effect on the incidence of TB.
The results of this study are a critical knowledge input for policymakers and program planners for designing various TB control programs. Besides, results obtained from this study will be helpful for health care professionals working in the area of TB/HIV control and prevention unit of Zewditu Memorial Hospital.
Conclusion
The Incidence of TB at Zewditu memorial hospital was low. Older age, underweight, and past opportunistic infection were significant predictors of time to TB co-infection. The unobserved true 6th month lagged parameterization value of the viral load had a significant association with the incidence of TB. Besides, baseline CD4 cell count, past opportunistic infection, history of TB, and baseline behavioral factors (alcohol and cigarette consumption) were significantly associated with viral load change. Thus, addressing significant predictors and strengthening continuous follow-ups are highly recommended in the study setting.
Abbreviations
AHR, Adjusted Hazard Ratio; CI, Confidence Interval; CHR, Crude Hazard Ratio; HIV, Human Immunodeficiency Virus; TB, Tuberculosis; WHO, World Health Organization; AIC, Akaike Information Criteria; IQR, Inter Quartile Range; BMI, Body Mass Index; HR, Hazard Ratio; KM, Kaplan–Meier; PHA, Proportional Hazard Assumptions; PLWH, People’s Lives with HIV; PY, Person-Years; RVI, Retroviral Infection.
Acknowledgments
Firstly, we would like to forward our kindest regards to our study participants. We extend our thanks to data collectors and supervisors they are the backbone to finalize the report. In conclusion, the authors would forward great thanks to the University of Gondar for minimal financial support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
1. Kassa A, Teka A, Shewaamare A, Jerene D. Incidence of tuberculosis and early mortality in a large cohort of HIV infected patients receiving antiretroviral therapy in a tertiary hospital in Addis Ababa, Ethiopia. Trans R Soc Trop Med Hyg. 2012;106(6):363–370. doi:10.1016/j.trstmh.2012.03.002
2. WHO. Burden of TB. World Health Organisation report; 2019.
3. WHO. Priority Research Questions for TB/HIV in HIV-Prevalent and Resource-Limited Settings. 2019.
4. F. S. Global HIV Statistics. 2019:2019.
5. F. E. implementation guideline for TB/HIV collaborative activities in Ethiopia. 2007
6. Ahmed A, Mekonnen D, Kindie M. Incidence and predictors of tuberculosis among adult people living with HIV/AIDS in Afar public health facilities, Northeast Ethiopia. AIDS. 2015;1:3–10.
7. Awoke T, Worku A, Kebede Y, et al. Modeling outcomes of first-line antiretroviral therapy and rate of CD4 counts change among a cohort of HIV/AIDS patients in Ethiopia: a Retrospective Cohort Study. PLoS One. 2015;2(4):e0168323. doi:10.1371/journal.pone.0168323
8. Fenner L
9. Badri M, Ehrlich R, Wood R, Pulerwitz T, Maartens G. Association between tuberculosis and HIV disease progression in a high tuberculosis prevalence area. Int J Tuberc Lung Dis. 2001;5(3):225–232.
10. Alene KA, Nega A, Taye BW. Incidence and predictors of tuberculosis among adult people living with human immunodeficiency virus at the University of Gondar Referral Hospital, Northwest Ethiopia. BMC Infect Dis. 2013;13(1):1–9. doi:10.1186/1471-2334-13-1
11. Dalbo M, Tamiso A. Incidence and predictors of tuberculosis among HIV/AIDS infected patients: a five-year retrospective follow-up study. Adv Infect Dis. 2016;6(02):70. doi:10.4236/aid.2016.62010
12. Temesgen B
13. Kibret KT, Yalew AW, Belaineh BG, Asres MM. Determinant factors associated with occurrence of tuberculosis among adult people living with HIV after antiretroviral treatment initiation in Addis Ababa, Ethiopia: a Case Control Study. PLoS One. 2013;8(5):e64488. doi:10.1371/journal.pone.0064488
14. Ahmed A, Mekonnen D, Kindie M. Incidence and predictors of tuberculosis among adult people living with HIV/AIDS in Afar public health facilities, Northeast Ethiopia. BMJ. 2018. doi:10.1136/bmj.k1791
15. Moh E. National Comprehensive HIV Care and Treatment Training for Health care Providers. 2014.
16. Assefa A, Gelaw B, Getnet G, Yitayew G. The effect of incident tuberculosis on immunological response of HIV patients on highly active anti-retroviral therapy at the university of Gondar hospital, northwest Ethiopia: a retrospective follow-up study. BMC Infect Dis. 2014;14(1):1–8. doi:10.1186/1471-2334-14-468
17. Van Rie A
18. Enju LI, Makubi A, Drain P, et al. Tuberculosis incidence rate and risk factors among HIV-infected adults with access to antiretroviral therapy in Tanzania. AIDS. 2015;29(11):1391. doi:10.1097/QAD.0000000000000705
19. Haraka F
20. Chang CA, Meloni ST, Eisen G, et al. Tuberculosis incidence and risk factors among Human Immunodeficiency Virus (HIV)-infected adults receiving antiretroviral therapy in a large HIV program in Nigeria. Open Forum Infect Dis. 2015;2(4). doi:10.1093/ofid/ofv154
21. Kovaiou RD, Herndler-Brandstetter D, Grubeck-Loebenstein B. Age-related changes in immunity: implications for vaccination in the elderly. Expert Rev Mol Med. 2007;9(3):1–17. doi:10.1017/S1462399407000221
22. Feleke BE, Feleke TE, Biadglegne F. Nutritional status of tuberculosis patients, a comparative cross-sectional study. BMC Pulm Med. 2019;19(1):182. doi:10.1186/s12890-019-0953-0
23. Niyongabo T, Henzel D, Idi M, et al. Tuberculosis, human immunodeficiency virus infection, and malnutrition in Burundi. Nutrition. 1999;15(4):289–293. doi:10.1016/S0899-9007(99)00003-9
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
