Back to Journals » Neuropsychiatric Disease and Treatment » Volume 19
Jiedu Yizhi Formula Improves Cognitive Function by Regulating the Gut Dysbiosis and TLR4/NF-κB Signaling Pathway
Authors Zhang P , Wang T, Zhu X, Feng L , Wang J, Li Y, Zhang X, Cui T, Li M
Received 17 October 2022
Accepted for publication 7 December 2022
Published 4 January 2023 Volume 2023:19 Pages 49—62
DOI https://doi.org/10.2147/NDT.S393773
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Pengqi Zhang,1 Tianye Wang,1 Xiaoting Zhu,2 Lina Feng,1 Jiale Wang,1 Yunqiang Li,1 Xinyue Zhang,1 Tingting Cui,1 Mingquan Li2
1College of Traditional Chinese Medicine, Changchun University of Chinese Medicine, Changchun, Jilin Province, People’s Republic of China; 2Neurology Department, Third Affiliated Clinical Hospital of Changchun University of Traditional Chinese Medicine, Changchun, Jilin Province, People’s Republic of China
Correspondence: Mingquan Li, Neurology Department, Third Affiliated Clinical Hospital of Changchun University of Traditional Chinese Medicine, Changchun, 130033, People’s Republic of China, Tel +86-15543120222, Email [email protected]
Objective: The objective of this study was to explore the neuroprotective mechanism of JDYZF in treating AD from the perspective of inflammation and intestinal microflora.
Methods: A total of 24 APP/PS1 mice were randomly divided into four groups: model (n = 6), JDYZF low-dose (n = 6), JDYZF high-dose (n = 6), and positive drug (n = 6), six C57 mice were used as the control group. The body weights and diets of all mice were examined daily. After 8 weeks of administration, the learning and memory of mice were evaluated by the Morris water maze test. The histopathological changes of hippocampus, liver and kidney in mice were observed by HE staining after being euthanized. The expression of p-tau in hippocampus tissue was detected by immunohistochemistry. After that, 16S rDNA sequencing was used to investigate the relationship between JDYZF and intestinal microbiota. Finally, a comparison of TLR4, p65, p-p65, iκB, p-iκB, and IL-1β protein expression in the hippocampus tissue of mice in each group was measured by Western blot.
Results: The results showed that APP/PS1 mice taking JDYZF orally were generally in good condition. Compared with the control group, JDYZF significantly improved learning and memory ability in ethology. Histology showed that JDYZF improved the hippocampal structure of mice and inhibited the deposition of p-tau. JDYZF treatment could regulate the gut microbiota of APP/PS1 mice by increasing the richness of Lachnospiraceae, Ruminococcaceae, and Actinobacteria and reducing that of Alistipes and Muribaculaceae. It also significantly inhibited the activation of the TLR4/NF-κB signaling pathway in the brain. In addition, no obvious toxic reactions were found in the liver and kidney of APP/PS1 mice after taking JDYZF for 8 weeks.
Conclusion: The findings revealed that JDYZF improved cognitive ability and alleviated the TLR4/NF-κB signaling pathway in APP/PS1 mice, and the modulating the gut microbiota presented here may help illuminate its activation mechanism.
Keywords: Jiedu Yizhi Formula, Alzheimer’s disease, fecal microbial diversity, NF-κB, APP/PS1 mice
Introduction
Background
Alzheimer’s disease (AD) is a neurodegenerative disease characterized by cognitive impairment and behavioral abnormalities progressively.1 Compared with the 2010 census figures, the AD population of China grew faster in 2022.2 However, the country’s diagnosis and treatment rate for AD remains low.3 Therefore, improving the prevention and treatment of AD is urgent.4 So far, Aβ protein accumulation and tau protein hyperphosphorylation are the main neuropathological of AD.5 However, contrary to expectations, treatment options for AD are unsatisfactory.6,7 Therefore, elucidating the molecular mechanism of AD and developing potent agents are urgent for improving patient functional status and quality of life.
Gut microbes are the largest organ in the human body, and regulating the microbes in the gut has been sought as a promising strategy to treat AD. Studies have shown that the noncanonical nuclear factor-κB (NF-κB) signaling pathway is closely related to tau protein formation and neuroinflammation in Alzheimer’s disease.8,9 After activation, it can lead to anti-apoptotic genes and cytokines IL-1, IL-6, TNF-α, and IL-8.10 NF-κB signaling pathway plays a key role in inflammation. Moreover, NF-κB is known to be an important modifier in the microbiome to AD, gut microbial diversity affected the TLR4/NF-κB signaling pathway in the inflammatory response. On the contrary, many studies show that TLR4/NF-κB signaling pathway can be inhibited by shifting the composition and the relative abundance of the intestinal microbiota.
Herbal remedies, which are very promising candidates for treatment, have a long history of use in a treat or preventing AD.11 The main therapeutic principle of TCM is a holistic view of patients. In other words, TCM could treat AD progression through multiple targets and multiple channels. A gut-brain axis perspective is consistent with traditional medical thinking. Therefore, NF-κB signaling may be an important pathway to treat AD by regulating the Gut-brain axis by TCM.
Purpose
Professor Ren Jixue, a master of Chinese medicine, put forward the marrow theory. We use the standard model of marrow theory as a reference and further established that marrow deficiency and poison damage have been identified as key mechanisms of AD-induced pathogenesis. In particular, Jiedu Yizhi Formula (JDYZF) is a classical TCM prescription for AD and has been applied over 10 years in clinical. Specifically, JDYZF fills kidney essence and clears away heat and toxic material, promoting gastrointestinal motility, and effectively purging and clearing the viscera. JDYZF includes Golden thread (Latin name: Coptidis Rhizoma), Sharpleaf Glangal Fruit (Latin name: Alpiniae Oxyphyllae Fructus), Glue of tortoise shell (Latin name: Carapax et Plastrum Testudinis Colla), Rhubarb (Latin name: Rheum palmatum L), Earthworm (Latin name: Pheretima), Asiatic Cornelian Cherry Fruit (Latin name: Corni Fructus), Szechwan Lovage Rhizome (Latin name: Chuanxiong Rhizoma). Using JDYZF to treat AD has a long history of demonstrated effective clinical therapeutic effects.12 Our previous research found that JDYZF can regulate the PI3K-Akt/Gsk3β/p53 signaling pathway, reduces the expression of inflammatory factors, and plays a protective role in the neurotoxicity of AD model cells.13 A central signaling component of the PI3K-Akt pathway is NF-κB, which functions together with the downstream kinase, to participate in the transmission and expression of various signals.14 However, its specific mechanism of protecting and modulating the brain-intestine axis in mice remains unclear.
In this study, we used JDYZF administration and behavioral testing in an APP/PS1 mouse model of AD, as well as standard immunohistochemical measures in the brain tissue. Next, we examined the effect of JDYZF on the gut microbacteria phenotypes and the involvement of TLR4/NF-κB signaling pathway in this process, to study the effect of JDYZF in alleviating memory deficits, and attempted to elucidate the underlying mechanisms.
Materials and Methods
Preparation of the JDYZF
JDYZF was purchased from Jilin Hongjian Pharmacy Co., Ltd. (Jilin, China), including Coptidis Rhizoma, Alpiniae Oxyphyllae Fructus, Carapax et Plastrum Testudinis Colla, Rhei Radix et Rhizoma, Pheretima, Corni Fructus and Chuanxiong Rhizoma. These herbs were mixed at a ratio of 1:2:1:1:1:1:1. The quality met the requirements of Pharmacopoeia of the People’s Republic of China 2015 edition. JDYZF was boiled and then prepared into freeze-dried powder through the freeze-drying mechanism, sealed, and stored at −20°C.
Experimental Animals
Twenty-four male APP/PS1 mice and 6 male C57 mice (SPF level; 4 months age-old; weigh 25±5 g) were purchased from Nanjing Junke Bioengineering Co., Ltd. (Nanjing, China; certification no. SCXK-SU 2020–0009). All the mice had access to water and food in freedom and were housed in a feeding room at a temperature of 22 ± 1°C and relative humidity of 60 ± 5% on a 12-hour light/dark cycle.
Seven days after in the SPF room, the APP/PS1 mice were randomly divided into four groups. Six mice each in the low-dose group (LG), and high-dose group (HG), respectively, received 10.536 g/kg/d and 20.268 g/kg/d JDYZF by gavage. Meanwhile, six mice in the positive drug group (PG) were administered donepezil hydrochloride (0.45 g/kg) by gavage. Furthermore, six APP/PS1 mice in the model group (MG) and six C57 mice in the control group (CG) were administered normal saline (0.1 mL/10 g) by gavage. The treatment period is 8 weeks. We weighed the weights and diets of mice at a fixed time every day and observed the general state and appearance characteristics of each mouse.
Morris Water Maze Test
After 8 weeks of intervention, the Morris water maze (MWM) test was used for evaluation.15 Briefly, the navigation experiment lasted for 6 days. After 6 days, the platform was removed and the mice were placed in the quadrant opposite the platform for the probe trial. Behavior Analysis System EthoVison XTVersion 11.0 (Noldus, the Netherlands) was used for recording and analysis.
Haematoxylin and Eosin (HE) Staining
After the behavioral experiment, the mice were euthanized. Brain tissues, liver, and kidney samples of 3 mice in each group were randomly taken quickly and fixed in 4% paraformaldehyde. The specimens were dehydrated, paraffin embedded, sectioned, stained in hematoxylin and eosin, dehydrated with gradient alcohol, transpired with xylene, and sealed with neutral gum. The basic pathological changes of brain tissue, liver, and kidney were observed under an optical microscope.16
Immunohistochemistry
Paraffin sections of brain tissue were dewaxed and washed, antigen repaired, serum blocked, DAB chromogenic after incubation with p-tau (1:5000, servicebio, China) antibody and secondary antibodies, restained nuclei, and microscopic examination after dehydration and sealing. The Eclipse Ci-L (NIKON, Japan) photographic microscope was used to select the target area of the tissue for 400 times imaging, to ensure that the background light of each photo was consistent. After the completion of imaging, the immunohistochemical method was processed by Image Pro Plus 6.0 analysis, ImageJ software was used to analyze the cumulative optical density (IOD) value and positive cell count, and calculate the area density = IOD value/area of the tissue.
16S rRNA Microbial Community Analysis
At the end of the treatment period, two fresh feces in the middle piece were collected from each mouse and frozen at −80°C for further analysis. The microbiome was analyzed on the HiSeq2500 PE250 sequencing platform of Novogene Bioinformatics Technology Co., Ltd. In short, the sample DNA was extracted with QIAamp Fast DNA Stool Mini Kit (Qiagen), diluted to 1 ng/µL for PCR amplification, and the V3-V4 region of 16S rDNA gene was targeted with Barcoded primers (515F and 806R) for sequencing analysis. Cluster OTU according to 97% sequence similarity, and then analyze the intra-group abundance and difference. α-Diversity was selected Chao diversity index by using Qiime v1.7.0., and evaluated the sample reliability by plotting the rarefaction curve with R v2.15.3. These calculations were analyzed by the Tukey’s test.17 β-Diversity was assessed using PCoA. Then, MetaStat was used to analyze differential microbiota by using R v2.15.3.
Western Blotting
Homogenize the fresh mouse hippocampal and put it into the lysis buffer. Extract the total protein from the brain tissue, after quantitative determination of protein, SDS-PAGE electrophoresis, membrane transferred, blocked, added primary antibody (including anti-iκB-α (1:500, cell signaling technology, USA), anti-NF-κB p65 (1:1000, cell signaling technology, UK), anti-p-NF-κB p65 (1:1000, Santa Cruz Biotechnology, USA), anti-IL-1β (1:1000, Santa Cruz Biotechnology, USA), anti-TLR4 (1:1000, Santa Cruz Biotechnology, USA) and anti-p-iκB-α (1:200, Santa Cruz Biotechnology, USA)) and then used the appropriate secondary antibody to detect the protein.18 After imaging, the protein of TLR4 and IL-1β expression was expressed by the ratio of the gray value of the target protein to the gray value of GAPDH. The expression of other proteins was expressed by the ratio of the gray value of phosphorylated protein to the gray value of total protein. The Western blot results were analyzed by image lab and Image J, and the experimental data were processed by GraphPad prism 8.
Statistical Analysis
GraphPad Prism 8.0 (GraphPad, San Diego, CA, USA) was used to analyze data. All experiments were repeated at least three times and the obtained data were presented as the mean ± standard deviation (x ± s). One-way ANOVA was applied to analyze statistical significance. P-value less than 0.05 indicated a statistically significant difference.
Results
Effects of JDYZF for Weight and General Status in APP/PS1 Mice
Some studies have shown that under the intervention of lipopolysaccharide, the neuroinflammation of AD mice is negatively correlated with weight.19 As shown in Figure 1, the weight of mice in the CG increased steadily within 8 weeks, which was in line with the normal growth law of mice. Compared with the CG, APP/PS1 mice have a high initial weight generally. However, with the standard feeding and treatment, the food intakes and body weights of mice in each group have changed significantly. LG was closer to CG, while PG intakes were more than MG, but the weight change was not obvious. HG intakes were similar to MG, and body weight fluctuate greatly. Further, the mice in the MG had obvious domain occupation consciousness and had fights and biting behaviors. These basic informations showed that LG is developing in a more favorable direction for AD mice.
 |
Figure 1 Effects of JDYZF on the levels of weight and diet. (A) Weight; (B) Diet. *p < 0.05, ****p < 0.01 compared with the CG; #p < 0.05, ##p < 0.01 compared with the LG, (B) F = 12.12, N=6. |
JDYZF Can Improve Cognitive Impairment in APP/PS1 Mice
We used the Morris water maze test to evaluate and compare the learning ability and long-term and short-term memory ability of mice in each group. The experiment was divided into two parts: the escape latency and the probe trial. The results showed that in the escape latency, the CG had a good learning ability. The learning and memory of mice in the MG decreased significantly. Compared with the CG, the escape latency was prolonged on the 4th and 5th days (p < 0.05, p < 0.01). Compared with the MG. LG, HG, and PG showed various degrees of cognitive improvement on the 4th day (p < 0.05) (shown in Figure 2A). During the probe trial, the CG looked for the quadrant where the platform was located, which was targeted and tended to be a linear strategy. The search strategy of the LG and the PG were close to the CG, which could cross the platform many times, showing a trend strategy. The search strategy of the HG randomly crossed the quadrant with the platform, and the memory was improved. The mice in the MG always showed marginal exploration, learning, and memory has not improved (shown in Figure 2D). In addition, compared with the MG, the time of spending in the platform quadrant in PG, LG and HG were prolonged (p < 0.05), and the number of crossing the platform were increased (p < 0.01) (shown in Figure 2B and C). This showed that APP/PS1 mice have neurological damage at the age of 6 months. A drug intervention can reduce this damage to varying degrees, and the effect of LG seemed to be more significant.
JDYZF Attenuates Hippocampal Neuron Damage in APP/PS1 Mice
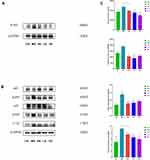
JDYZF can improve the learning and memory of APP/PS1 mice and alleviate hippocampal neuronal damage. Hyperphosphorylation of p-tau protein is the early pathological basis of AD, resulting in a large number of neuroinflammation and degeneration. HE staining showed that the nuclei of the hippocampal CA1 area in the CG were large and round, with distinct layers, clear structure, complete membrane and nuclear membrane, normal staining of nuclei, complete morphology of organelles, and neat arrangement of nerve fibers. Additionally, the hippocampal tissues of MG showed pyknotic nuclei, some neurons were damaged, the nerve cells in the CA1 area were disordered, the normal nerve cells decreased, and the neuronal gap increased. JDYZF could recover the number and morphology of nerve cells in the hippocampal area and showed good neurons and nucleoli (shown in Figure 3A). This finding indicated that JDYZF has a protective effect on neurons and can delay the pathological progress of AD in the early stage.
The immunohistochemical method showed that the cumulative optical density of p-tau protein in mice brain tissues from high to low was the MG, HG, PG, LG, and CG (p < 0.01) (shown in Figure 3B and C). APP/PS1 mice have more p-tau protein deposition in the hippocampus, which was consistent with the pathological changes of AD mice. JDYZF can clear the excessive p-tau protein deposition in the hippocampus of APP/PS1 mice.
JDYZF Improved the Fecal Microbiota of APP/PS1 Mice
Next, we examined the effect of APP/PS1 mice on the gut microbacteria phenotypes and the involvement of JDYZF in this process. OTUs with 97% similarity were obtained using cluster tags and plotted Petal diagram (shown in Figure 4). Four hundred and sixty-two OTUs are in common use by all groups. As shown in Figure 5A and B, the alpha diversity analysis in the Chao1 index indicated that the whole microbial assortments significantly decreased in the MG (p < 0.05). However, JDYZFL treatment significantly increased in response to APP/PS1 mice (p < 0.05). Furthermore, PCoA analysis showed that the gut microbial clusters in the MG were significantly separated from those in the CG, whereas JDYZFL treatment inhibited the segregation and demonstrated a marked shift close to those in the CG (shown in Figure 5C).
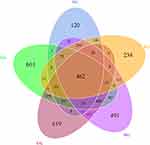 |
Figure 4 OTUs-based Venn diagram. |
 |
Figure 5 Alpha and beta diversity. (A) Observed_species; (B) chaos analysis (Wilcoxon test); (C) PCoA (weighted). |
Then, one-way ANOVA was used to identify the microbial multivariate. The results showed that JDYZF influenced species abundance of Alzheimer’s disease. We performed the MetaStat analysis to screen different microbiota in each group (shown in Figure 6). Lachnospiraceae, Ruminococcaceae, Actinobacteria, and Muribaculaceae, were the most representative.
JDYZF Inhibits the Expression of Proteins Related to the Proinflammatory Pathway
Finally, the interaction between JDYZF and TLR4/NF-κB signaling pathway was investigated due to the important role of this pathway in inflammation and oxidative stress of hippocampus. Quantitative analysis revealed that the expression of TLR4, p-p65/p65, and p-iκB/iκB in the hippocampus were enhanced (p < 0.01), and the expression of these proteins was down-regulated in the hippocampus of PG (p < 0.01). The LG seemed to be more effective. Notably, IL-1β as a star protein downstream in the NF-κB signaling pathway is positively correlated with LPS and APOE4. LPS and APOE4 are the most closely related inflammatory indicators with AD.20–22 Therefore, we evaluated the expression of IL-1β. Surprisingly, the results were consistent with the upstream protein level (shown in Figure 7). These data showed that JDYZF can down-regulation the protein expression related to the NF-κB pathway, reduced neuroinflammatory response, and improve cognitive function.
JDYZF Has No Hepatotoxicity and Nephrotoxicity
AD is a chronic disease of the nervous system, which needs to be treated with long-term oral Chinese medicine in the clinic. Therefore, we analyzed the HE staining of the liver and kidney for mice. We found that the hepatocytes were scattered outward with the interlobular artery as the center through liver slices, and the hepatocytes were arranged regularly. The morphology of the hepatic lobules was normal, and no obvious abnormalities were found (shown in Figure 8A). In the kidney tissue, the kidney medulla were oval, the tube wall was thin, and no obvious abnormalities were found in the glomerulus (shown in Figure 8B). It showed that oral administration of JDYZF for 8 weeks will not cause significant changes in the structure of the liver and kidney in mice. This finding indicated a preliminary basis for the safety of oral JDYZF.
Discussion
Currently, most drugs developed to treat AD remain in Phase III clinical trials, and there are no innovative drugs approved for the specific treatment of AD. The drugs already on the market include Acetylcholinesterase and N-methyl-Daspartate, etc., which only moderately improve symptomatic in Alzheimer’s patients but do not slow disease progression.23 Therefore, there is an urgent unmet need for the development of new therapeutics for this unfortunate disease. Herbal medicine has been widely used in Asian medicine, notably used in the treatment of AD.24 At the same time, TCM also advocates the principle of preventing disease, which is also necessary for the treatment of AD. Prior studies indicate that patients have prodromal symptoms such as constipation and psychiatric symptoms for approximately ten years ago.25–27 An early-stage and timely management of the patients may be a promising therapeutic strategy in the management of AD. A previous study reported that JDYZF effectively improved memory deficits and reduced AD-like pathological changes in Aβ-injected AD rats, attenuated the expression of NLRP3/caspase-1/GSDMD, and reduce the content of IL-1β and IL-18 in hippocampus and cortex,28 improved chronic cognitive impairment. These findings indicated that JDYZF may represent a promising candidate that could be used for neurodegeneration treatment and reduction of the risk of AD. However, it is unknown whether JDYZF beneficially affects p-tau protein and attenuates inflammatory reactions in APP/PS1 mice.
APP/PS1 mice are a forceful model for evaluating AD, which can evaluate the learning and memory function, and detect the pathological level of p-tau protein. Multiple neurological disorders reveal the importance of individualized differences, including gender, and age.29 Existing studies in humans and other animals of AD reported the importance of gender.30,31 Therefore, we chose male mice that were used as recipients. We paid attention to the weight changes and diet of mice. Prior studies that have noted the change in body weight are related to AD.32 Being overweight is still one of the predisposing factors of AD.33 However, AD patients are negatively correlated with body weight.34 In this experiment, APP/PS1 mice lost weight and diets significantly. Although the specific reasons are not clear, we can speculate that the reasons include enriching pro-inflammatory bacteria and suppressing probiotics in APP/PS1 mice gut,35,36 low dietary intake,37 nutrient deficiency,38 high metabolic consumption, and regular irregular physical exercise.39,40 Among them, dietary restriction is not only related to decreased vigilance and memory deterioration, but also related to Aβ protein and p-tau protein deposited and atrophy in the medial temporal cortex and other locations.41,42 Higher metabolic consumption may be related to neurotoxicity, which consumes nutrients in the body, thus oxidizing and damaging brain tissue.43 Therefore, we believe that appropriate weight gain has a protective effect on AD patients. Interestingly, JDYZF can achieve this result.44
Tau protein is the core factor of Alzheimer’s disease and can predict disease,45,46 which may affect cognition in any regulation.47 Dr. Arne Ittner said, “the toxic effect of tau protein for brain cells is necessary, which leads to the impairment of memory”.48 Therefore, p-tau is the main target of AD diagnosis and treatment,49 down-regulation of endogenous p-tau protein helps to slow down the development of AD and its inflammatory response.50 In this study, JDYZF could inhibit p-tau protein, alleviate axonal neuron damage caused by p-tau protein. Compared with JDYZF groups, the hippocampal formation in MG include a trisynaptic loop from the entorhinal cortex to the dentate gyrus, from the dentate gyrus to CA3 showed pyknotic nuclei, some neurons were damaged, the nerve cells in the CA1 area were disordered, the normal nerve cells decreased, and the neuronal gap increased.
The use of beneficial microorganisms has been proven to have one of the most beneficial effects on AD. As the intestinal flora is the largest receiving organ in the body, we hypothesized that the intestinal environment may be a valuable strategy for developing novel AD therapeutics. Preclinical studies provide direct evidence for a link between disorders in flora and AD.51 Most studies found that probiotic administration conferred neuroprotective benefits and could attenuate cognitive deficits. Meanwhile, gut microbiota imbalance may regulate the TLR4/NF-κB signaling pathway in the brain immune system.52,53 In this study, we identified that JDYZF treatment could regulate gut microbiota by increasing the richness of Ruminococcaceae and Actinobacteria and reducing that of Alistipes, Lachnospiraceae, Deferribacteres, and Muribaculaceae. The Ruminococcaceae abundance is associated with short-chain fatty acids production and APOE genotypes. This suggests that Ruminococcaceae is worth further investigation as a potential target to mitigate cognitive decline.54,55 Similarly, Actinobacteria as potential therapeutic options for AD in the same way.56 Research has shown that the content of Deferribacteres is a negative correlation with cognitive parameters.57 Deferriactors, as harmful Gram-negative bacteria, are closely related to the inflammatory response and obesity.58 Several studies have shown that Deferribacteres abundance is positively correlated with LPS, TNF-α, and NF-κB-p65 levels.59 It can regulate TLR4/MyD88/NF-κB signal path in inflammatory response by inhibiting Deferribacteres.60 Lachnospiraceae is less abundant in AD,61 and it is positively correlated with TLR4/NF-κB.62,63 Preliminary studies have shown that the administration of berberine fumarate significantly ameliorated metabolic disorders, and reduced the populations of Lachnospiraceae. In addition, it reduced inflammation, inhibiting the overexpression of TLR4.64 Furthermore, Ginkgolide B extracts against cognitive impairment with neuroprotective activity and can reverse the increased abundance of Muribaculaceae.65 Muribaculaceae was associated with cognitive impairment and depressive mood and participates in the TNF-α/NF-κB signaling pathway.66
NF-κB and other members of its family are important regulators in the inflammatory process and participate in the inflammatory and stress process of the organisms.67,68 TLR4, an upstream member of the NF-κB pathway, widely exists on the surface of microglia. TLR4 activates the NF-κB pathway and its downstream events after p-tau protein deposition and makes p65 and p50 in its downstream change from the inactive state to the phosphorylated active state, and binding to specific DNA common sequences, thereby enhancing the transcription of inflammation-related proteins. In addition, TLR4 is a specific receptor for LPS and has an important role in gram-negative infections.69 It connects with intestinal flora, neuroinflammation, and nutrients.70
As a traditional Chinese medicine compound, JDYZF has complex components, involving alkaloids, nucleosides, flavonoids, and other compounds. Modern pharmacological research showed that the main component of Coptis Chinensis is berberine. Berberine has a good neuroprotective effect.71 It down-regulates NF-κB and activates iκB, IL-1β and TNF-α by blocking PI3K-Akt and MAPK signaling pathways,72 and regulating apoptosis and oxidative stress.73 Therefore, berberine can alleviate cognitive impairment, and this potential therapeutic mechanism is related to inhibiting p-tau hyperphosphorylation and NF-κB signal.74 Yan found that bitter cardamom has an anti-inflammatory effect and can reduce the damage of lipopolysaccharide for nerve cells through PI3K-Akt/NF-κB pathway,75 and improve the learning and memory of model mice. In order to clarify the potential mechanism of this traditional prescription, we further determined whether JDYZF affected the NF-κB signal in APP/PS1 mice through Western blot. We found that TLR4 was activated in APP/PS1 mice and contributed to the NF-κB signal downstream, which is characterized by iκBα and p65 expression. In terms of dose, the HG did not bring more benefits to the mice’s cognition. On the contrary, the LG significantly improved the gut microbiota diversity and composition, inhibited the up-regulated expression of TLR4, suppression iκBα phosphorylation and degradation, and inhibit subsequent nuclear translocation of NF-κB p65. Suppress p-iκBα and p-p65 can regulate neuroinflammation and oxidative stress, reduce the activation of neuroglia cells and reverse p-tau protein, thereby improving cognition. Based on these findings, we believe that the effect of JDYZF on the activation of inflammatory corpuscles in APP/PS1 mice is through the down-regulation of TLR4/NF-κB signal mediated.
In summary, this thesis has provided that JDYZF can save cognition, inhibit microglia activation, and reduce neuroinflammation and neuron loss. The anti-inflammatory mechanism of JDYZF may involve regulating intestinal microecology and blocking TLR4/NF-κB pathway. Moreover, the pathological changes in the liver and kidney were not observed after 8 weeks of treatment, which provided a basis for the safety of the clinical medication. These findings further reveal the understanding of a part of the mechanism for JDYZF and provide new evidence for the beneficial role of traditional medicine in the prevention and treatment of AD.
Conclusion
In summary, this study verified the mechanism of JDYZF on AD across the TLR4/NF-κB signal pathway. JDYZF can regulate gut microbiota imbalance, negatively regulate TLR4/NF-κB signaling pathway in the brain immune system, clear the phosphorylation of p-tau protein, inhibit the vicious cycle of cognitive damage caused by an inflammatory reaction, and thus treat Alzheimer’s disease. Based on the current research, the inflammatory reaction and apoptosis may be the tip of the iceberg in the treatment of AD,76 and other pathways may also be involved in the mechanism of JDYZF. Developing new drugs based solely on the pathological formation of AD is not satisfactory.77 The human body is a complex system. It is better to look at AD from the perspective of a holistic view than as an independent individual. Traditional Chinese medicine has the characteristics of multi-channel and multi-target. Based on the existing research, clarifying the mechanism of JDYZF ulteriorly will provide a basis and new ideas for the treatment of Alzheimer’s disease.
Abbreviations
AD, Alzheimer’s disease; PG, the positive drug group; p-tau, Phosphorylated Tau, MG, the model group; JDYZF, Jiedu Yizhi Formula, CG, the control group; NF-κB, Nuclear factor-κB, HE staining, Haematoxylin and Eosin staining; LG, the low-dose JDYZF group, MWM, Morris water maze; HG, the high-dose JDYZF group.
Ethics Approval
The whole process of animal experiments passed the ethical review of the Ethics Committee of Changchun University of Traditional Chinese Medicine (No.2021195 Changchun, China).
Acknowledgments
This work was supported by [Science and Technology Department of Jilin Province of China] (Grant numbers [20200404065YY]; [Natural Science Foundation] (YDZJ202201ZYTS232)).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by grants from the Science and Technology Department of Jilin Province of China [No. 20200404065YY] and the Jilin Provincial Science and Technology Development Plan Project [No. YDZJ202201ZYTS232].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hernandez C, Shukla S. Liposome based drug delivery as a potential treatment option for Alzheimer’s disease. Neural Regen Res. 2022;17(6):1190–1198. doi:10.4103/1673-5374.327328
2. Zhou M, Wang H, Zeng X, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2019;394(10204):1145–1158. doi:10.1016/S0140-6736(19)30427-1
3. Jia J, Wei C, Chen S, et al. The cost of Alzheimer’s disease in China and re-estimation of costs worldwide. Alzheimers Dement. 2018;14(4):483–491. doi:10.1016/j.jalz.2017.12.006
4. Ren R, Qi J, Lin S, et al. The China Alzheimer report 2022. Gen Psychiatr. 2022;35(1):e100751. doi:10.1136/gpsych-2022-100751
5. Lee PJ, Tsai CL, Liang CS, et al. Biomarkers with plasma amyloid β and tau protein assayed by immunomagnetic reduction in patients with amnestic mild cognitive impairment and Alzheimer’s disease. Acta Neurol Taiwan. 2022;31(2):53–60.
6. Srivastava P, Tripathi PN, Sharma P, et al. Design and development of some phenyl benzoxazole derivatives as a potent acetylcholinesterase inhibitor with antioxidant property to enhance learning and memory. Eur J Med Chem. 2019;163:116–135. doi:10.1016/j.ejmech.2018.11.049
7. Tripathi PN, Srivastava P, Sharma P, et al. Biphenyl-3-oxo-1,2,4-triazine linked piperazine derivatives as potential cholinesterase inhibitors with anti-oxidant property to improve the learning and memory. Bioorg Chem. 2019;85:82–96. doi:10.1016/j.bioorg.2018.12.017
8. Rai SN, Tiwari N, Singh P, et al. Therapeutic potential of vital transcription factors in alzheimer’s and parkinson’s disease with particular emphasis on transcription factor EB mediated autophagy. Front Neurosci. 2021;15:777347. doi:10.3389/fnins.2021.777347
9. Hou J, Jeon B, Baek J, et al. High fat diet-induced brain damaging effects through autophagy-mediated senescence, inflammation and apoptosis mitigated by ginsenoside F1-enhanced mixture. J Ginseng Res. 2022;46(1):79–90. doi:10.1016/j.jgr.2021.04.002
10. Abdel Moneim AE. The neuroprotective effect of berberine in mercury-induced neurotoxicity in rats. Metab Brain Dis. 2015;30(4):935–942. doi:10.1007/s11011-015-9652-6
11. Singh AK, Rai SN, Maurya A, et al. Therapeutic potential of phytoconstituents in management of Alzheimer’s disease. Evid Based Complement Alternat Med. 2021;2021:5578574. doi:10.1155/2021/5578574
12. Peng C, Han J, Ye X, et al. IL-33 treatment attenuates the systemic inflammation reaction in acinetobacter baumannii pneumonia by suppressing TLR4/NF-κB signaling. Inflammation. 2018;41(3):870–877. doi:10.1007/s10753-018-0741-7
13. Li Y, Wang B, Liu C, et al. Inhibiting c-Jun N-terminal kinase (JNK)-mediated apoptotic signaling pathway in PC12 cells by a polysaccharide (CCP) from Coptis chinensis against Amyloid-β (Aβ)-induced neurotoxicity. Int J Biol Macromol. 2019;134:565–574. doi:10.1016/j.ijbiomac.2019.05.041
14. Lou Y, Wang C, Tang Q, et al. Paeonol inhibits IL-1β-induced inflammation via PI3K/Akt/NF-κB pathways: in vivo and vitro studies. Inflammation. 2017;40(5):1698–1706. doi:10.1007/s10753-017-0611-8
15. Tian H, Ding N, Guo M, et al. Analysis of learning and memory ability in an Alzheimer’s disease mouse model using the Morris water maze. J Vis Exp. 2019;152. doi:10.3791/60055
16. Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc. 2014;2014(6):655–658. doi:10.1101/pdb.prot073411
17. Lundberg DS, Yourstone S, Mieczkowski P, et al. Practical innovations for high-throughput amplicon sequencing. Nat Methods. 2013;10(10):999–1002. doi:10.1038/nmeth.2634
18. Ni D, Xu P, Gallagher S. Immunoblotting and immunodetection. Curr Protoc Cell Biol. 2017;74. doi:10.1002/cpcb.18
19. Knopp RC, Baumann KK, Wilson ML, et al. Amyloid beta pathology exacerbates weight loss and brain cytokine responses following low-dose lipopolysaccharide in aged female Tg2576 mice. Int J Mol Sci. 2022;23(4):2377. doi:10.3390/ijms23042377
20. Patrick RP. Role of phosphatidylcholine-DHA in preventing APOE4-associated Alzheimer’s disease. FASEB J. 2019;33(2):1554–1564. doi:10.1096/fj.201801412R
21. Yang W, Liu Y, Xu QQ, et al. Sulforaphene ameliorates neuroinflammation and hyperphosphorylated tau protein via regulating the PI3K/Akt/GSK-3 β pathway in experimental models of alzheimer’s disease. Oxid Med Cell Longev. 2020;2020:4754195. doi:10.1155/2020/4754195
22. Zhou J, Yu W, Zhang M, et al. Imbalance of microglial TLR4/TREM2 in LPS-Treated APP/ps1 transgenic mice: a potential link between alzheimer’s disease and systemic inflammation. Neurochem Res. 2019;44(5):1138–1151. doi:10.1007/s11064-019-02748-x
23. Thu Thuy Nguyen V, Endres K. Targeting gut microbiota to alleviate neuroinflammation in Alzheimer’s disease. Adv Drug Deliv Rev. 2022;188:114418. doi:10.1016/j.addr.2022.114418
24. Jin X, Liu MY, Zhang DF, et al. Baicalin mitigates cognitive impairment and protects neurons from microglia-mediated neuroinflammation via suppressing NLRP3 inflammasomes and TLR4/NF-κB signaling pathway. CNS Neurosci Ther. 2019;25(5):575–590. doi:10.1111/cns.13086
25. Khan S, Barve KH, Kumar MS. Recent advancements in pathogenesis, diagnostics and treatment of Alzheimer’s Disease. Curr Neuropharmacol. 2020;18(11):1106–1125. doi:10.2174/1570159X18666200528142429
26. Nedelec T, Couvy-Duchesne B, Monnet F, et al. Identifying health conditions associated with Alzheimer’s disease up to 15 years before diagnosis: an agnostic study of French and British health records. Lancet Digit Health. 2022;4(3):e169–e178. doi:10.1016/S2589-7500(21)00275-2
27. Fu P, Gao M, Yung KKL. Association of intestinal disorders with parkinson’s disease and alzheimer’s disease: a systematic review and meta-analysis. ACS Chem Neurosci. 2020;11(3):395–405. doi:10.1021/acschemneuro.9b00607
28. Wang L, Zhang P, Li C, et al. A polysaccharide from Rosa roxburghii Tratt fruit attenuates high-fat diet-induced intestinal barrier dysfunction and inflammation in mice by modulating the gut microbiota. Food Funct. 2022;13(2):530–547. doi:10.1039/d1fo03190b
29. Sacks D, Baxter B, Campbell BCV, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13(6):612–632. doi:10.1177/1747493018778713
30. Rai SN, Chaturvedi VK, Singh BK, et al. Commentary: trem2 deletion reduces late-stage amyloid plaque accumulation, elevates the Aβ42: aβ40Ratio, and exacerbates axonal dystrophy and dendritic Spine Loss in the PS2APP Alzheimer’s mouse model. Front Aging Neurosci. 2020;12:219. doi:10.3389/fnagi.2020.00219
31. Chung J, Das A, Sun X, et al. Genome-wide association and multi-omics studies identify MGMT as a novel risk gene for Alzheimer’s disease among women. Alzheimers Dement. 2022. doi:10.1002/alz.12719
32. Gao S, Nguyen JT, Hendrie HC, et al. Accelerated weight loss and incident dementia in an elderly African-American cohort. J Am Geriatr Soc. 2011;59(1):18–25. doi:10.1111/j.1532-5415.2010.03169.x
33. Vamanu E, Rai SN. The link between obesity, microbiota dysbiosis, and neurodegenerative pathogenesis. Diseases. 2021;9(3):45. doi:10.3390/diseases9030045
34. Sergi G, De Rui M, Coin A, et al. Weight loss and Alzheimer’s disease: temporal and aetiologic connections. Proc Nutr Soc. 2013;72(1):160–165. doi:10.1017/S0029665112002753
35. Hersoug LG, Møller P, Loft S. Role of microbiota-derived lipopolysaccharide in adipose tissue inflammation, adipocyte size and pyroptosis during obesity. Nutr Res Rev. 2018;31(2):153–163. doi:10.1017/S0954422417000269
36. Wang J, Zhu X, Li Y, et al. Jiedu-Yizhi formula improves cognitive impairment in an Aβ25-35-induced rat model of alzheimer’s disease by inhibiting pyroptosis. Evid Based Complement Alternat Med. 2022;2022:6091671. doi:10.1155/2022/6091671
37. Mattson MP. Emerging neuroprotective strategies for Alzheimer’s disease: dietary restriction, telomerase activation, and stem cell therapy. Exp Gerontol. 2000;35(4):489–502. doi:10.1016/s0531-5565(00)00115-7
38. Luchsinger JA, Mayeux R. Dietary factors and Alzheimer’s disease. Lancet Neurol. 2004;3(10):579–587. doi:10.1016/S1474-4422(04)00878-6
39. Rogers NT, Steptoe A, Cadar D. Frailty is an independent predictor of incident dementia: evidence from the English longitudinal study of ageing. Sci Rep. 2017;7(1):15746. doi:10.1038/s41598-017-16104-y
40. Hu X, Okamura N, Arai H, et al. Neuroanatomical correlates of low body weight in Alzheimer’s disease: a PET study. Prog Neuropsychopharmacol Biol Psychiatry. 2002;26(7–8):1285–1289. doi:10.1016/s0278-5846(02)00291-9
41. Val-Laillet D, Aarts E, Weber B, et al. Neuroimaging and neuromodulation approaches to study eating behavior and prevent and treat eating disorders and obesity. Neuroimage Clin. 2015;8:1–31. doi:10.1016/j.nicl.2015.03.016
42. Zhang Q, Jin K, Chen B, et al. Overnutrition induced cognitive impairment: insulin resistance, gut-brain axis, and neuroinflammation. Front Neurosci. 2022;16:884579. doi:10.3389/fnins.2022.884579
43. Mun YS, Park HK, Kim J, et al. Association between body mass index and cognitive function in mild cognitive impairment regardless of APOE ε4 status. Dement Neurocogn Disord. 2022;21(1):30–41. doi:10.12779/dnd.2022.21.1.30
44. Tamura BK, Masaki KH, Blanchette P. Weight loss in patients with Alzheimer’s disease. J Nutr Elder. 2007;26(3–4):21–38. doi:10.1300/j052v26n03_02
45. Rai SN, Singh C, Singh A, et al. Mitochondrial dysfunction: a potential therapeutic target to treat alzheimer’s disease. Mol Neurobiol. 2020;57(7):3075–3088. doi:10.1007/s12035-020-01945-y
46. La Joie R, Visani AV, Baker SL, et al. Prospective longitudinal atrophy in Alzheimer’s disease correlates with the intensity and topography of baseline tau-PET. Sci Transl Med. 2020;12(524):eaau5732. doi:10.1126/scitranslmed.aau5732
47. Blum D, Buée L. Alzheimer’s disease risk, obesity and tau: is insulin resistance guilty? Expert Rev Neurother. 2013;13(5):461–463. doi:10.1586/ern.13.35
48. Stefanoska K, Gajwani M, Tan ARP, et al. Alzheimer’s disease: ablating single master site abolishes tau hyperphosphorylation. Sci Adv. 2022;8(27):eabl8809. doi:10.1126/sciadv.abl8809
49. Chang CW, Shao E, Mucke L. Tau: enabler of diverse brain disorders and target of rapidly evolving therapeutic strategies. Science. 2021;371(6532):eabb8255. doi:10.1126/science.abb8255
50. Bloom GS. Amyloid-β and tau: the trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014;71(4):505–508. doi:10.1001/jamaneurol.2013.5847
51. Bonfili L, Cecarini V, Berardi S, et al. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci Rep. 2017;7(1):2426. doi:10.1038/s41598-017-02587-2
52. Bai X, Fu R, Duan Z, et al. Ginsenoside Rh4 alleviates antibiotic-induced intestinal inflammation by regulating the TLR4-MyD88-MAPK pathway and gut microbiota composition. Food Funct. 2021;12(7):2874–2885. doi:10.1039/d1fo00242b
53. Tang J, Xu L, Zeng Y, et al. Effect of gut microbiota on LPS-induced acute lung injury by regulating the TLR4/NF-kB signaling pathway. Int Immunopharmacol. 2021;91:107272. doi:10.1016/j.intimp.2020.107272
54. D’Amato A, Di Cesare Mannelli L, Lucarini E, et al. Faecal microbiota transplant from aged donor mice affects spatial learning and memory via modulating hippocampal synaptic plasticity- and neurotransmission-related proteins in young recipients. Microbiome. 2020;8(1):140. doi:10.1186/s40168-020-00914-w
55. Tran TTT, Corsini S, Kellingray L, et al. APOE genotype influences the gut microbiome structure and function in humans and mice: relevance for Alzheimer’s disease pathophysiology. FASEB J. 2019;33(7):8221–8231. doi:10.1096/fj.201900071R
56. Binda C, Lopetuso LR, Rizzatti G, et al. Actinobacteria: a relevant minority for the maintenance of gut homeostasis. Dig Liver Dis. 2018;50(5):421–428. doi:10.1016/j.dld.2018.02.012
57. Gureev AP, Syromyatnikov MY, Ignatyeva DA, et al. Effect of long-term methylene blue treatment on the composition of mouse gut microbiome and its relationship with the cognitive abilities of mice. PLoS One. 2020;15(11):e0241784. doi:10.1371/journal.pone.0241784
58. Wang S, Yao J, Zhou B, et al. Bacteriostatic effect of quercetin as an antibiotic alternative in vivo and its antibacterial mechanism in vitro. J Food Prot. 2018;81(1):68–78. doi:10.4315/0362-028X.JFP-17-214
59. Li L, Li R, Zhu R, et al. Salvianolic acid B prevents body weight gain and regulates gut microbiota and LPS/TLR4 signaling pathway in high-fat diet-induced obese mice. Food Funct. 2020;11(10):8743–8756. doi:10.1039/d0fo01116a
60. Mao B, Guo W, Tang X, et al. Inosine pretreatment attenuates LPS-induced lung injury through regulating the TLR4/MyD88/NF-κB signaling pathway in vivo. Nutrients. 2022;14(14):2830. doi:10.3390/nu14142830
61. Verhaar BJH, Hendriksen HMA, de Leeuw FA, et al. Gut microbiota composition is related to AD Pathology. Front Immunol. 2022;12:794519. doi:10.3389/fimmu.2021.794519
62. Wang X, Yu N, Wang Z, et al. Akebia trifoliata pericarp extract ameliorates inflammation through NF-κB/MAPK signaling pathways and modifies gut microbiota. Food Funct. 2020;11(5):4682–4696. doi:10.1039/c9fo02917f
63. Zhou Y, Zhang M, Zhao X, et al. Ammonia exposure induced intestinal inflammation injury mediated by intestinal microbiota in broiler chickens via TLR4/TNF-α signaling pathway. Ecotoxicol Environ Saf. 2021;226:112832. doi:10.1016/j.ecoenv.2021.112832
64. Cui HX, Hu YN, Li JW, et al. Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxid Med Cell Longev. 2018;2018:8930374. doi:10.1155/2018/8930374
65. Liu J, Ye T, Zhang Y, et al. Protective effect of ginkgolide B against cognitive impairment in mice via regulation of gut microbiota. J Agric Food Chem. 2021;69(41):12230–12240. doi:10.1021/acs.jafc.1c05038
66. Zhang DD, Li HJ, Zhang HR, et al. Poria cocos water-soluble polysaccharide modulates anxiety-like behavior induced by sleep deprivation by regulating the gut dysbiosis, metabolic disorders and TNF-α/NF-κB signaling pathway. Food Funct. 2022;13(12):6648–6664. doi:10.1039/d2fo00811d
67. Davies DA, Adlimoghaddam A, Albensi BC. Role of Nrf2 in plasticity s and memory in alzheimer’s disease. Cells. 2021;10(8):1884. doi:10.3390/cells10081884
68. Zhang Q, Lenardo MJ, Baltimore D. 30 Years of NF-κB: a blossoming of relevance to human pathobiology. Cell. 2017;168(1–2):37–57. doi:10.1016/j.cell.2016.12.012
69. Wan H, Han J, Tang S, et al. Comparisons of protective effects between two sea cucumber hydrolysates against diet induced hyperuricemia and renal inflammation in mice. Food Funct. 2020;11(1):1074–1086. doi:10.1039/c9fo02425e
70. Velloso LA, Folli F, Saad MJ. TLR4 at the crossroads of nutrients, gut microbiota, and metabolic inflammation. Endocr Rev. 2015;36(3):245–271. doi:10.1210/er.2014-1100
71. Akbar M, Shabbir A, Rehman K, et al. Neuroprotective potential of berberine in modulating Alzheimer’s disease via multiple signaling pathways. J Food Biochem. 2021;45(10):e13936. doi:10.1111/jfbc.13936
72. He W, Wang C, Chen Y, et al. Berberine attenuates cognitive impairment and ameliorates tau hyperphosphorylation by limiting the self-perpetuating pathogenic cycle between NF-κB signaling, oxidative stress and neuroinflammation. Pharmacol Rep. 2017;69(6):1341–1348. doi:10.1016/j.pharep.2017.06.006
73. Živančević K, Baralić K, Bozic D, et al. Involvement of environmentally relevant toxic metal mixture in Alzheimer’s disease pathway alteration and protective role of berberine: bioinformatics analysis and toxicogenomic screening. Food Chem Toxicol. 2022;161:112839. doi:10.1016/j.fct.2022.112839
74. Lin X, Zhang N. Berberine: pathways to protect neurons. Phytother Res. 2018;32(8):1501–1510. doi:10.1002/ptr.6107
75. Yan T, Zhang X, Mao Q, et al. Alpinae Oxyphyllae Fructus alleviated LPS-induced cognitive impairments via PI3K/AKT/NF-κB signaling pathway. Environ Toxicol. 2022;37(3):489–503. doi:10.1002/tox.23415
76. Yin Z, Gao D, Du K, et al. Rhein Ameliorates Cognitive Impairment in an APP/PS1 transgenic mouse model of Alzheimer’s disease by relieving oxidative stress through activating the SIRT1/PGC-1α pathway. Oxid Med Cell Longev. 2022;2022:2524832. doi:10.1155/2022/2524832
77. Mank A, Rijnhart JJM, van Maurik IS, et al. A longitudinal study on quality of life along the spectrum of Alzheimer’s disease. Alzheimers Res Ther. 2022;14(1):132. doi:10.1186/s13195-022-01075-8
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.