Back to Journals » OncoTargets and Therapy » Volume 12
JAZF1 Suppresses Papillary Thyroid Carcinoma Cell Proliferation and Facilitates Apoptosis via Regulating TAK1/NF-κB Pathways
Authors Huang L, Cai Y, Luo Y, Xiong D, Hou Z, Lv J, Zeng F, Yang Y, Cheng X
Received 11 September 2019
Accepted for publication 13 November 2019
Published 2 December 2019 Volume 2019:12 Pages 10501—10514
DOI https://doi.org/10.2147/OTT.S230597
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Federico Perche
Liangliang Huang,1,* Yuhuai Cai,1,* Yi Luo,1 Daigang Xiong,1 Zeyu Hou,1 Junyuan Lv,1 Feng Zeng,1 Yan Yang,2,3 Xiaoming Cheng1
1Medical Center of Breast and Thyroid Disease, Affiliated Hospital of ZunYi Medical University, ZunYi, Guizhou 563003, People’s Republic of China; 2Department of Clinical Laboratory, Affiliated Hospital of ZunYi Medical University, ZunYi, Guizhou 563003, People’s Republic of China; 3College of Laboratory Medicine, Zunyi Medical University, Zunyi, Guizhou 563003, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xiaoming Cheng
Medical Center of Breast and Thyroid Disease, Affiliated Hospital of ZunYi Medical University, 149 Dalian Road, ZunYi, Guizhou 563003, People’s Republic of China
Tel +8613985248883
Email [email protected]
Yan Yang
Department of Clinical Laboratory, Affiliated Hospital of ZunYi Medical University, 149 Dalian Road, ZunYi, Guizhou 563003, People’s Republic of China
Tel +8618183468796
Fax +86851-28608316
Email [email protected]
Purpose: Juxtaposed with another zinc finger gene 1 (JAZF1) is involved in gluconeogenesis, insulin sensitivity, cell differentiation, lipid metabolism and inflammation, but its role in carcinoma remains inexplicit.
Patients and methods: We explored the JAZF1 expression in human papillary thyroid cancer (PTC) tissues, adjacent normal thyroid tissues and nodular goitre tissues, as well as Ki67 expression in PTC tissues, using immunohistochemistry staining. Western blotting and RT-qPCR were performed to explore the JAZF1 expression levels in Nthy-ori 3–1, BCPAP and TPC-1 cells. BCPAP cells overexpressing JAZF1 were constructed using an Adv-JAZF1-GFP recombinant adenovirus vector. Next, the cell proliferation assay, colony formation assay, cell cycle analysis, apoptosis and immunofluorescence were performed. The mRNA expression level of nuclear factor-κB p65 (NF-κB p65) was examined using RT-qPCR. The expression of Bcl-2, Bax, transforming growth factor beta-activated kinase 1 (TAK1), NF-κB p65 and NF-κB p-p65 were examined using Western blotting.
Results: The expression of JAZF1 in human PTC tissues was downregulated compared with adjacent thyroid tissues or nodular goitre. Additionally, JAZF1 expression was associated with the location and lymph node metastasis of PTC. The expression level of JAZF1 had a negative correlation with Ki67 labelling index (LI). Compared to Nthy-ori 3–1 cells and TPC-1 cells, BCPAP cells expressed the lowest JAZF1. JAZF1 overexpressed significantly inhibited proliferation, caused G0/G1 cell cycle arrest and promoted apoptosis in BCPAP cells. Furthermore, JAZF1 overexpressed in BCPAP cells clearly upregulated the expression level of Bax protein, whereas decreased the expression of Bcl-2, TAK1, NF-κB but did not affect the mRNA or protein expression level of NF-κB p65.
Conclusion: JAZF1 inhibits proliferation and induces apoptosis in BCPAP cells by suppressing the activation of TAK1/NF-κB signalling pathways, suggesting that JAZF1 may serve as a reliable molecular marker in PTC.
Keywords: Juxtaposed with another zinc finger gene 1, papillary thyroid cancer, TAK1, NF-κB
Introduction
As the most common subtype of thyroid cancer, papillary thyroid cancer (PTC) is accounting for 74–80% of the thyroid malignancy.1 Recently, in China, the 5-year survival of thyroid cancer has demonstrated significant improvement (average change per calendar period 5.4%), but the general mortality of PTC is still higher than that of other endocrine neoplasms. Additionally, in the United States, the data showed that the mortality rates of PTC will be higher than those of lung, ovarian and colorectal cancers in the near future.2,3 Previous findings have indicated that tumour development involves a series of mutation of molecules, including oncogenes and tumour suppressor genes.4–7 Presently, the use of these molecules as markers in the diagnostic and prognostic management of PTC is increasing.8,9 Puli provided evidence suggesting that ETV5 and its putative target CCND1/2 may be proliferative markers of advanced PTC.10 Chen suggested that SDC4 affects PTC cells apoptosis via the Wnt/β-catenin pathways.11 Sun showed that inhibition of E2F8 expression induces G1 phase cell cycle arrest in PTC cells.12 However, the molecular mechanism of PTC pathogenesis remains incompletely understood, and most of these markers still lack accuracy. Hence, the identification of reliable molecular markers of PTC is still in an imperative need.
JAZF1 (Juxtaposed with another zinc finger gene 1), also referred to as TIP27, was first identified as a novel TAK1-interacting protein in 2004.13 Studies have demonstrated that JAZF1 is involved in gluconeogenesis, insulin sensitivity, cell differentiation, lipid metabolism and inflammation.14–17 Furthermore, studies have revealed that JAZF1 is related to tumour progression.18,19 Based on 2014 WHO classification, low-grade endometrial stromal sarcoma is related to gene rearrangement, such as the JAZF1-SUZ12 fusion gene.20 Ueyama showed that JAZF1 influences the development of hepatocellular carcinoma (HCC) among Japanese patients with type 2 diabetes mellitus (T2DM).21 However, the role of JAZF1 in PTC and the molecular mechanism involved are yet to be clarified.
TAK1 (transforming growth factor beta-activated kinase 1), first identified as a mitogen-activated protein kinase kinase kinase (MAP3K), is known to activate the nuclear factor-κB (NF-κB) that has various target genes and plays an essential role in stress responses, immunity, stimulate inflammation and cancer.22,23 Lin revealed an association between TAK1 and the pathogenesis of thyroid cancer via targeting NF-κB.24 As a TAK1-selective co-factor, the role of JAZF1 in thyroid cancer would be by regulating TAK1.13
In this study, we used the human papillary thyroid cancer cell line BCPAP to elucidate the role of JAZF1 in the thyroid cellular activities and the potential molecular mechanisms involved in the proliferation, cell cycle and apoptosis of PTC. Our results indicated that JAZF1 attenuated papillary thyroid carcinoma cell proliferation and facilitated apoptosis by suppressing the activation of NF-κB via regulating TAK1 expression.
Materials and Methods
Thyroid Tissue Specimens
Thyroid tissue specimens were collected from 97 patients underwent thyroidectomy in the Affiliated Hospital of Zunyi Medical University (Guizhou, China) after informed consent was obtained and were stored at −80°C immediately. Patients who received radiotherapy, chemotherapy or other treatments before surgery were excluded. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards (Ethical commission of the Affiliated Hospital of Zunyi Medical University; No. (2017)1-063), and written informed consent was obtained from all individual participants. We used 117 thyroid tissue samples (60 PTC, 20 adjacent thyroid tissues, and 37 nodular goitre samples) after confirmation of the diagnosis by two pathologists (Table 1).
 |
Table 1 Study Cohort Clinical Features |
Immunohistochemistry
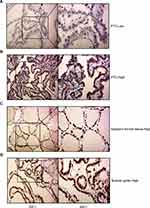
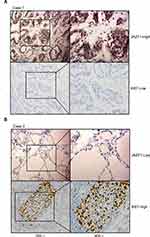
Dissected tissues were embedded in paraffin and then cut at 3-µm thickness for haematoxylin and eosin (H&E) staining and immunohistochemistry (IHC) as previously described.25 The following primary antibodies were used: anti-JAZF1 1:200 (Abcam, Cambridge, UK) and anti-Ki-67 1:4000 (Proteintech, Chicago, USA).
Staining scores for JAZF1 were determined by the intensity distribution (ID) score. Details are as follows: the sum of the percentage of positive cells (0: <5%; 1: 5–25%; 2: 26–50%; 3: 51–75%; 4: 76–100%); the staining intensity graded (0, negative; 1, weak; 2, moderate; and 3, strong). We considered ID scores 6 or greater for high JAZF1 expression, and 4 or lower for low JAZF1 expression.
We used the Ki-67 labelling index (LI) % to evaluate Ki-67 expression. The ratio of the number of Ki-67-positive cells and total number of tumour cells in several representative fields represented the Ki-67 LI. The low or high Ki-67 expression was determined using a cut-off of 4%.25
Two pathologists evaluated the histologic staining, and the scores of histologic staining were determined by two observers.
Cell Lines and Cell Culture
Human normal thyroid follicular epithelial cell line (Nthy-ori 3-1) was purchased from BeNa Chuanglian Biotechnology Research Institute (Beijing, China), and cells were cultured in RPMI 1640 medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum. Human papillary thyroid carcinoma cell lines (BCPAP and TPC-1), were provided by Stem Cell Bank, Chinese Academy of Sciences (Shanghai, China) and BeNa Chuanglian Biotechnology Research Institute (Beijing, China), respectively. BCPAP cells were maintained in RPMI1640 supplemented with 10% fetal bovine serum, non-essential amino acids (Gibco 1114005) and sodium pyruvate solution (Gibco 11360070). TPC-1 cells were maintained in DMEM-H medium (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum. All cells incubated at 37°C in a 5% CO2 atmosphere.
Adenovirus-Mediated JAZF1 Overexpression in BCPAP Cells
The JAZF1-GFP was excised from the original plasmid digested with EcoRI/NotI. Then, the JAZF1-GFP was ligated into the pShuttle-CMV recombinant shuttle vector using T4 DNA ligase to obtain the pShuttle-JAZF1-GFP recombinant shuttle plasmid. Thereafter, the pShuttle-JAZF1-GFP was transformed to pAdxsi vector to obtain pAdxsi-JAZF1-GFP viral plasmid. Finally, a titre of 1.2×1010 PFU/mL recombinant Adv-JAZF1-GFP was obtained.
RNA Extraction and Reverse Transcription-Quantitative PCR Analysis
Total RNA was isolated from BCPAP cells transfected with Adv-JAZF1-GFP or Adv-GFP using Trizol reagent (Takara Bio Inc., Otsu, Japan), according to the manufacturer’s protocol. Next, Prime ScriptTMRT reagent Kit (Takara Bio Inc., Otsu, Japan) was used to reverse transcribe the total RNA to cDNA. Reverse transcription-quantitative PCR analysis was performed using SYBR®Premix Ex TaqTM II (Takara Bio Inc., Otsu, Japan). GAPDH was used as the endogenous control. The levels of JAZF1 and NF-κB p65 were analysed by the 2−∆∆Ct approach, and the levels were compared between the suspected tumour tissue and paraneoplastic tissue in one patient: >1 indicated the expression of JAZF1 and NF-κB p65 was upregulated, and <1 indicated the expression of JAZF1 and NF-κB p65 was down-regulated. The primers for RT-qPCR were as follows: JAZF1: forward, 5’-TGTAGCACCATGACAGGCATC-3’ and reverse, 5’-TTGTCCTCGATGTGCTCGAT-3’; NF-κB p65: forward, 5ʹ-GACGCATTGCTGTGCCTTC-3ʹ and reverse, 5ʹ-TTGATGGTGCTCAGGGATGAC-3ʹ; GAPDH: forward, 5ʹ-GGA GCGAGATCCCTCCAAAAT-3ʹ and reverse, 5ʹ-GGCTGTTGTCATACTTCTCATGG-3ʹ.
Western Blotting
Western blotting analysis was performed as previously described.26 The primary antibodies against the following proteins used in the experiment were from commercial sources: anti-JAZF1 (ab80329; 1:200) and anti-p65 (ab16502; 1:2000), both purchased from Abcam; anti-Bcl-2 (#15071; 1:500) and anti-p-p65, both purchased from CST; anti-Bax (ET1603-34; 1:2000) purchased from Hangzhou HuaAn Biotechnology Co., Ltd.; anti-TAK1 (12330-2-AP; 1:500) purchased from Proteintech; anti-GAPDH (SC-365062; 1:800) purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). The secondary antibodies were as follows: goat anti-rabbit and goat anti-mouse IgG (1:4000 for both).
Immunofluorescence Study
BCPAP cells were plated on coverslips in a 6-well plate and cultured for 24 hrs. Then, they were fixed with 4% paraformaldehyde for 20 mins after washing in PBS. Next, the cells were permeabilized with 0.2% Triton X-100 for 10 mins and blocked with 10% normal goat serum for 20 mins. Using a confocal microscope (LSM510; Carl Zeiss; Germany) to observe the fluorescence from the JAZF1 and NF-κB p65 protein. The nuclei were stained with DAPI according to the manufacturer’s directions. Images were acquired at 200× magnification.
Cell Proliferation and Colony Formation Assay
Cell proliferation ability was assessed using CCK-8 assay. Briefly, 1000 cells were seeded in a 96-well plate for 4 days. For three groups (Adv-JAZF1-GFP transfected cells, Adv-GFP transfected cells and negative control cells), three parallel well detections were set for each group. Every day, three wells from each group were detected. For detection, cells were treated with Cell Counting Kit-8 reagent (Beyotime, Shanghai, China), and the absorbance was measured at 450 nm on a microplate reader at the designated time points after treatment.
For the colony formation assay, 1000 cells were seeded into each well of a 6-well plate and incubated for 14 days. The colonies were stained with crystal violet and photographed. Then, the cell colony formation rate was counted.
Cell Cycle Analysis
Cells (2×106) were harvested 48 hrs after transfection and then resuspended in 4°C 75% cold ethanol to fix overnight, followed by staining with 100 µL of PI (3.8×10−2 sodium citrate, pH 7.0) containing RNase (RNase A; 10 mg/mL) for 30 mins protected from light at 37°C. Flow cytometry (BECKMAN-COULTER, USA) was used to measure the populations in G0-G1, S and G2-M phases. The results were analysed using cell ModFit software.
Apoptosis Assay
For apoptosis detection, Annexin-V APC/7-AAD double staining was used. Cells transfected with Adv-JAZF1-GFP or Adv-GFP were harvested with 0.25% trypsin. To prevent excessive cell digestion, the complete medium was added. After washing twice with PBS (centrifugation at 800 g for 5 mins), cells were resuspended in 500 µL of Binding Buffer. Annexin V-APC and 7-AAD were added, and cells were subsequently incubated for 15 mins at room temperature protected from light. The apoptosis was measured using a flow cytometer (FACSCalibur, Becton-Dickinson, San Diego, CA, USA) and was analysed using CellQuest software.
Statistical Analysis
The data were expressed as means±SD. SPSS 21.0 software (SPSS Version 21.0; IBM Corp., Armonk, NY) was used to conduct statistical analysis. Graphs were constructed using GraphPad Prism 7 (GraphPad Software Inc, San Diego, USA). Student’s t-test was performed to analyse differences between groups. Bivariate correlation was calculated using the Spearman’s rank correlation coefficient. All values represent at least three independent experiments. P<0.05 was considered statistically significant.
Results
Down-regulation of JAZF1 is Associated with Clinicopathological Parameters of PTC
To determine the clinical relevance of JAZF1 expression in PTC, we performed immunohistochemistry in 117 thyroid tissue samples. The detection of JAZF1 immunoreactivity was mainly in the cytoplasm and nucleus (Figure 1). As shown in Figure 1 and Table 2, we observed that JAZF1 was down-regulated in thyroid cancer tissue compared with that in adjacent thyroid tissues or nodular goitre samples (P=0.001).
 |
Table 2 Correlations of JAZF1 Expression with Different Thyroid Tissues (n = 117) |
Subsequently, we explored the association between the expression of JAZF1 and clinicopathological features of PTC (Table 3). The expression levels of JAZF1 were observed related to location (P=0.039) and lymph node metastasis (LNM) (P=0.024). However, there were no associations between JAZF1 expression and patient sex, age, tumour size or TNM stage (all P>0.05).
 |
Table 3 Correlations Between JAZF1 Expression and Clinicopathological Parameters (n = 60) |
Correlations Between JAZF1 and Ki67
To further assess the role of JAZF1 in PTC, we compared its expression with Ki67 based on immunohistochemistry results. As shown in Table 4 and Figure 2, JAZF1 expression had a negative correlation with Ki67 LI. The Spearman’s rank correlation coefficient between JAZF1 and ki67 was −0.27 (P<0.05).
 |
Table 4 Correlation of JAZF1 Expression with Ki67 (n = 60) |
JAZF1 Overexpression Inhibits BCPAP Cells Proliferation
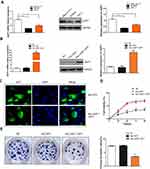
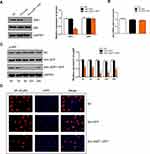
In order to better evaluate the biological function of JAZF1 in thyroid cancer, we detected its expression levels in human normal thyroid follicular epithelial cell line Nthy-ori 3-1, and two PTC cell lines (BCPAP, TPC-1) (Figure 3A). The results showed that, compared to Nthy-ori 3-1 and TPC-1 cells, BCPAP cells expressed the lowest JAZF1. Then, we successfully constructed BCPAP cells overexpressing JAZF1 using adenovirus vectors (Figure 3B), we observed cells under immunofluorescence microscopy. Consistent with IHC results, the subcellular distribution of JAZF1 was mainly in the cytoplasm and nucleus (Figure 3C). Thereafter, we performed cell proliferation and colony formation assays to evaluate the effect of JAZF1 on the proliferation of BCPAP cells. The results demonstrated that JAZF1 overexpression inhibited proliferation and colony formation of BCPAP cells compared with negative control cells or Adv-GFP transfected cells (Figure 3D and E, both P<0.05).
JAZF1 Overexpression Arrests the G0/G1 Phase of BCPAP Cells
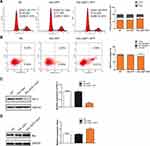
To verify whether the overexpression of JAZF1 affected the cell cycle progression of BCPAP cells, we performed flow cytometry. As shown in Figure 4A, the Adv-JAZF1-GFP-treated BCPAP cells had a high percentage of G0/G1 phase (69.55%) compared with the negative control group (59.77%) or Adv-GFP treated group (61.00%) (P=0.000). The S phase of Adv-JAZF1-GFP-treated BCPAP cells was 14.03%, while the S phase proportions of the control group were 19.00% and 17.05%, respectively. The G2/M phase of Adv-JAZF1-GFP-treated BCPAP cells was 16.42%, while the G2/M phase proportions of the control group were 21.23% and 21.95%, respectively. These findings suggested that overexpression of JAZF1 in BCPAP cells induces G0/G1 cell cycle arrest.
JAZF1 Overexpression Induces the Apoptosis of BCPAP Cells
Next, we examined whether JAZF1 affects the apoptosis of BCPAP cells. The apoptotic cells of three groups (negative control BCPAP cells, Adv-GFP transfected cells and Adv-JAZF1-GFP transfected cells) were analysed by flow cytometry (Figure 4B). The apoptosis rates of the three groups were as follows: 3.35±0.16% (NC), 5.00±0.60% (Adv-GFP treated) and 13.10±0.62% (Adv-JAZF1-GFP treated) (P=0.001). These data suggested that overexpression of JAZF1 increased the apoptosis rate of BCPAP. Thereafter, the expression level of Bcl-2, an anti-apoptotic protein, was examined using Western blotting analysis and was found to be significantly decreased in Adv-JAZF1-GFP-treated cells (Figure 4C; P=0.000), whereas the expression level of Bax protein was upregulated (Figure 4D; P=0.002).
JAZF1 Targets TAK1 Upstream of NF-κB Inhibits the Proliferation and Promotes the Apoptosis of BCPAP Cells
The abovementioned in vitro experiments have initially demonstrated that JAZF1 overexpression inhibited the proliferation of BCPAP cells, caused G0/G1 cell cycle arrest and facilitated apoptosis. Next, we investigated the underlying mechanism. In this study, we focused on JAZF1 as a TAK1-mediated transactivation repressor.13 We demonstrated that overexpression of JAZF1 efficiently reduced the expression level of TAK1 (Figure 5A). TAK1 is a pivotal kinase upstream of NF-κB, a transcription factor known, to regulate both proliferation and apoptosis.27 However, in this study, the protein and mRNA expression level of NF-κB p65, a major subunit of the NF-κB complex, was not found to be significantly changed due to overexpression of JAZF1 (Figure 5A and B, both P>0.05). In order to further clarify the role of NF-κB in JAZF1-mediated regulation, we assessed the phosphorylation status of NF-κB p65 (p-p65). As shown in Figure 5C, a significant decrease in the expression of p-p65 was observed in Adv-JAZF1-GFP-treated cells, suggesting a reduction in the activation of NF-κB.
The immunofluorescence study demonstrated that NF-κB p65 was located in both the cytoplasm and nuclei of BCPAP. Overexpression of JAZF1 blocked NF-κB p65 translocation, and the proportion of nuclear expression of NF-κB p65 was the lowest in BCPAP cells overexpressing JAZF1 (Figure 5D). These results demonstrated that JAZF1-mediate BCPAP cells proliferation inhibition the and apoptosis facilitation may targeting TAK1 upstream of NF-κB.
Discussion
As a novel repressor of TAK1, a type of nuclear receptor that regulates gluconeogenesis, previous studies on JAZF1 mainly focused on diabetes and lipid metabolism.28,29 Recently, studies have reported that JAZF1 also regulates the biological functions of human tumours, especially endometrial stromal sarcomas and prostate cancer.30,31 JAZF1 has been hypothesized as a tumour suppressor, Hazelett found that, in JAZF1 intron, the G allele creates an NKX3-1-binding site while making a FOXA1-binding site destruction in line with the DHT-dependent is associated with the risk in decreasing its enhancer activity.32 Presumably, this enhancer influenced its role in tumour suppression. Many studies have reported the important role of tumor suppressor genes in cell biological activities such as proliferation, apoptosis and cell cycle.33–36 Our study is the first to explore the role of JAZF1 as a tumour suppressor gene in PTC.
Our study characterized the expression of JAZF1 in PTC, adjacent thyroid tissues and nodular goitre samples using IHC and analysed the correlation of JAZF1 expression with clinicopathological parameters and Ki67. We found that JAZF1 expression was decreased in PTC compared with that in adjacent thyroid tissues or nodular goitre samples. Additionally, there was a significant association between its expression with location and lymph node metastasis in PTC. Ki67 is a common proliferation marker.37,38 Tang et al have demonstrated that Ki67 may be an important indicator to determine tumour aggressiveness in PTC.39 Our study verified the negative correlation between JAZF1 and Ki67, suggesting the potential role of JAZF1 as a proliferation-related protein in PTC. However, in this study, we drew conclusions based on the analysis of a small sample size, and we will expand the sample size in a further study to clarify the correlation between JAZF1 and Ki67.
Furthermore, we detected the expression levels of JAZF1 in human normal thyroid follicular epithelial cell line Nthy-ori 3-1 and two PTC cell lines (BCPAP, TPC-1). The results showed that BCPAP cells expressed the lowest JAZF1, compared to Nthy-ori 3-1 and TPC-1 cells. According to the literature data, the lower JAZF1 expression in thyroid cancer cell lines than that of human normal thyroid follicular epithelial cell line was not unexpected. However, the expression difference of JAZF1 between BCPAP and TPC-1 remains to be explored. The human papillary thyroid carcinoma cell line BCPAP expresses mutated BRAF V600E gene, while TPC-1 expresses the rearranged form of RET.40 This may be responsible for their different expressions of JAZF1. Our results are in line with the study that demonstrated that the basal secretion of CXCL8 was highest in BCPAP cells compared to NHT and TPC-1 cells.41 However, whether the mechanism is the same remains to be further studied.
We first evaluated the role of JAZF1 in the PTC cell line BCPAP. Adenovirus vectors were used to construct BCPAP cells overexpressing JAZF1. Consistent with IHC results, we observed that JAZF1 is mainly localized in the cytoplasm and nucleus. In BCPAP cells, JAZF1 overexpression inhibited cell proliferation and colony formation. In the previous section, we analyzed the relationship between JAZF1 and Ki67 expression, indicating that JAZF1 may exert its tumour suppression effect by negatively regulating cell proliferation. Ki67 is a cell proliferation–associated factor, presented in all active phases in cell cycle progression.42 We speculated the role of JAZF1 in cell cycle distribution. Our results from flow cytometry demonstrated that overexpression of JAZF1 caused G0/G1 cell cycle arrest of BCPAP cells. Previous studies demonstrated that the G1 phase is the critical period for determining cell fate.43 We speculated that overexpression of JAZF1 may facilitate BCPAP cells to make the decision to exit the cell cycle early during cell division. The potential mechanism involved needs further study. However, Yuasa et al have revealed that overexpression of JAZF1 facilitates the cell proliferation and cell cycle progression of C2C12 cells, indicating that overexpressed JAZF1 may contribute to oncogenesis in muscle.44 This paradoxical phenomenon may be explained by the following statement. The primary function of JAZF1 is to inhibit TAK1-mediated transactivation as a co-repressor of TAK1. Interestingly, the transcriptional activity of TAK1 depends on cell type. In certain cell types, TAK1 causes DR1-dependent transcription; however, in other cell types, it inhibits transcription, acting as both positive regulator and negative regulator of transcription.13 There are no studies to report whether the JAZF1/TAK1 complex induces TAK1 transactivation. Apoptosis is important in cells, and Bae et al have demonstrated that overexpression of JAZF1 may upregulate pro-apoptotic genes, thereby inducing heart failure symptoms in cardiomyocytes.45 Besides the proapoptotic members like Bax, the Bcl-2 family involves the antiapoptotic members like Bcl-2.46 Our experiments did show that overexpression of JAZF1 downregulated anti-apoptotic protein Bcl-2, upregulated pro-apoptotic protein Bax and induced apoptosis of BCPAP cells.
Based on the abovementioned experimental results, we further explore the molecular mechanisms involved in JAZF1-mediated BCPAP cell regulation. JAZF1 contains a Glu/Asp-rich region and three putative zinc finger motifs. We have repeatedly mentioned that JAZF1 is a co-suppressor of TAK1, a nuclear orphan receptor, and researchers have speculated that the TAK1-JAZF1 complex recruits other nuclear proteins that may depend on the Glu/Asp-rich region and zinc finger domains within JAZF1.13 Nuclear factor of κB (NF-κB) is a nuclear transcription factor that is known to be involved in the control of numerous cellular processes, such as cellular proliferation and apoptosis.47,48 TAK1 is a pivotal kinase upstream of the NF-κB signalling pathway.24 Zou et al revealed that α-mangostin-mediated inflammation inhibition may through the inhibition of TAK1-NF-κB pathways activation.49 Wang et al found that CXC195 exerts antiproliferation of human hepatocellular cancer cells via regulating the TAK1-mediated NF-κB pathway.50 Our results showed that JAZF1 inhibited the expression of TAK1. This would be consistent with the previous demonstration that JAZF1 decreased the expressions of TAK1.51 The specific mechanism has not been reported yet. Furthermore, we found the inhibition of phosphorylation of NF-κB subunits p65 due to overexpression of JAZF1; however, the mRNA and protein level of NF-κB p65 was not found to be significantly changed. In immunofluorescence experiments, we found that JAZF1 blocked NF-κB p65 nuclear translocation. Bcl-2 is an anti-apoptotic protein that regulated mainly at the transcription level.52 Catz and Johnson have demonstrated that Bcl-2 is transcriptionally regulated by NF-κB.53 Western blotting showed that overexpression of JAZF1 induced apoptosis of BCPAP cells through the downregulation of anti-apoptotic protein Bcl-2. These indicated that JAZF1 may promote proliferation and induce apoptosis in BCPAP cells through TAK1/NF-κB. However, whether JAZF1 regulates cell cycle progression via regulating TAK1/NF-κB needs to be further explored. Apart from functions as co-modulator of TAK1, JAZF1 may function as a transcription factor itself. The zinc finger motifs within JAZF1 might play a role in protein–protein and protein–DNA interactions.13 The zinc finger domain is the mandatory signalling domain for NF-κB activation.54 We speculate that not only the JAZF1-TAK1 complex but also JAZF1 itself activates the NF-κB signal pathway directly. Recently, Johnson identified JAZF1 as a novel regulator in the process of ciliated cell differentiation and might exert its role downstream of IL6 and upstream of FOXJ1.14 However, whether this signalling pathway plays a role in tumour cells remains unknown. Future studies must determine the function of JAZF1 in cancer development.
Conclusion
These findings, taken together, have revealed that JAZF1 inhibits proliferation and facilitates apoptosis in BCPAP cells by suppressing the TAK1/NF-κB signalling pathways activation, suggesting that JAZF1 may serve as a reliable molecular marker in PTC.
Acknowledgments
This work was supported by the National Natural Science Foundation of China project #1 under grant number 81960494; HongHuaGang Bureau Science and Technology project #2 under grant number[2017]19; GuiZhou Province Science and Technology project #3 under grant number LH[2015]7489; and Guizhou Province University Engineering Technology Research Center project #4 under grant number KY[2012]022.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Barollo S, Pezzani R, Cristiani A, et al. Prevalence, tumorigenic role, and biochemical implications of rare BRAF alterations. Thyroid. 2014;24(5):809–819. doi:10.1089/thy.2013.0403
2. Zeng H, Chen W, Zheng R, et al. Changing cancer survival in China during 2003–15: a pooled analysis of 17 population-based cancer registries. Lancet Glob Health. 2018;6(5):e555–e567. doi:10.1016/S2214-109X(18)30127-X
3. Aschebrook-Kilfoy B, Kaplan EL, Chiu BC, Angelos P, Grogan RH. The acceleration in papillary thyroid cancer incidence rates is similar among racial and ethnic groups in the United States. Ann Surg Oncol. 2013;20(8):2746–2753. doi:10.1245/s10434-013-2892-y
4. Xing M. BRAF mutation in thyroid cancer. Endocr Relat Cancer. 2005;12(2):245–262. doi:10.1677/erc.1.0978
5. Howell GM, Hodak SP, Yip L. RAS mutations in thyroid cancer. Oncologist. 2013;18(8):926–932. doi:10.1634/theoncologist.2013-0072
6. Rahbari R, Kitano M, Zhang L, Bommareddi S, Kebebew E. RTN4IP1 is down-regulated in thyroid cancer and has tumor-suppressive function. J Clin Endocrinol Metab. 2013;98(3):E446–E454. doi:10.1210/jc.2012-3180
7. Liu D, Yang C, Bojdani E, Murugan AK, Xing M. Identification of RASAL1 as a major tumor suppressor gene in thyroid cancer. J Natl Cancer Inst. 2013;105(21):1617–1627. doi:10.1093/jnci/djt249
8. Shahebrahimi K, Madani SH, Fazaeli AR, Khazaei S, Kanani M, Keshavarz A. Diagnostic value of CD56 and nm23 markers in papillary thyroid carcinoma. Indian J Pathol Microbiol. 2013;56(1):2–5. doi:10.4103/0377-4929.116139
9. Marcello MA, Morari EC, Cunha LL, et al. P53 and expression of immunological markers may identify early stage thyroid tumors. Clin Dev Immunol. 2013;2013:846584. doi:10.1155/2013/846584
10. Puli OR, Danysh BP, McBeath E, et al. The transcription factor ETV5 mediates BRAFV600E-induced proliferation and TWIST1 expression in papillary thyroid cancer cells. Neoplasia. 2018;20(11):1121–1134. doi:10.1016/j.neo.2018.09.003
11. Chen LL, Gao GX, Shen FX, Chen X, Gong XH, Wu WJ. SDC4 gene silencing favors human papillary thyroid carcinoma cell apoptosis and inhibits epithelial mesenchymal transition via Wnt/β-catenin pathway. Mol Cells. 2018;41(9):853–867. doi:10.14348/molcells.2018.0103
12. Sun J, Shi R, Zhao S, et al. E2F8, a direct target of miR-144, promotes papillary thyroid cancer progression via regulating cell cycle. J Exp Clin Cancer Res. 2017;36(1):40. doi:10.1186/s13046-017-0504-6
13. Nakajima T, Fujino S, Nakanishi G, Kim YS, Jetten AM. TIP27: a novel repressor of the nuclear orphan receptor TAK1/TR4. Nucleic Acids Res. 2004;32(14):4194–4204. doi:10.1093/nar/gkh741
14. Johnson JA, Watson JK, Nikolić MZ, Rawlins EL. Fank1 and Jazf1 promote multiciliated cell differentiation in the mouse airway epithelium. Biol Open. 2018;7:4. doi:10.1242/bio.033944
15. Meng F, Lin Y, Yang M, et al. JAZF1 inhibits adipose tissue macrophages and adipose tissue inflammation in diet-induced diabetic mice. Biomed Res Int. 2018;2018:4507659. doi:10.1155/2018/4507659
16. Ming GF, Xiao D, Gong WJ, et al. JAZF1 can regulate the expression of lipid metabolic genes and inhibit lipid accumulation in adipocytes. Biochem Biophys Res Commun. 2014;445(3):673–680. doi:10.1016/j.bbrc.2014.02.088
17. Yuan L, Luo X, Zeng M, et al. Transcription factor TIP27 regulates glucose homeostasis and insulin sensitivity in a PI3-kinase/Akt-dependent manner in mice. Int J Obes (Lond). 2015;39(6):949–958. doi:10.1038/ijo.2015.5
18. Amador-Ortiz C, Roma AA, Huettner PC, Becker N, Pfeifer JD. JAZF1 and JJAZ1 gene fusion in primary extrauterine endometrial stromal sarcoma. Hum Pathol. 2011;42(7):939–946. doi:10.1016/j.humpath.2010.11.001
19. Thomas G, Jacobs KB, Yeager M, et al. Multiple loci identified in a genome-wide association study of prostate cancer. Nat Genet. 2008;40(3):310–315. doi:10.1038/ng.91
20. Tsuyoshi H, Yoshida Y. Molecular biomarkers for uterine leiomyosarcoma and endometrial stromal sarcoma. Cancer Sci. 2018;109(6):1743–1752. doi:10.1111/cas.2018.109.issue-6
21. Ueyama M, Nishida N, Korenaga M, et al. The impact of PNPLA3 and JAZF1 on hepatocellular carcinoma in non-viral hepatitis patients with type 2 diabetes mellitus. J Gastroenterol. 2016;51(4):370–379. doi:10.1007/s00535-015-1116-6
22. Wang Z, Zhao S, Song L, et al. Natural cyclopeptide RA-V inhibits the NF-κB signaling pathway by targeting TAK1. Cell Death Dis. 2018;9(7):715. doi:10.1038/s41419-018-0743-2
23. Sakurai H. Targeting of TAK1 in inflammatory disorders and cancer. Trends Pharmacol Sci. 2012;33(10):522–530. doi:10.1016/j.tips.2012.06.007
24. Lin P, Niu W, Peng C, Zhang Z, Niu J. The role of TAK1 expression in thyroid cancer. Int J Clin Exp Pathol. 2015;8(11):14449–14456.
25. Ke RH, Wang Y, Mao Y, Zhang J, Xiong J. Decreased expression of LASS2 is associated with worse prognosis in meningiomas. J Neurooncol. 2014;118(2):369–376. doi:10.1007/s11060-014-1441-2
26. Zeng F, Huang L, Cheng X, et al. Overexpression of LASS2 inhibits proliferation and causes G0/G1 cell cycle arrest in papillary thyroid cancer. Cancer Cell Int. 2018;18:151. doi:10.1186/s12935-018-0649-1
27. He A, Ji R, Shao J, He C, Jin M, Xu Y. TLR4-MyD88-TRAF6-TAK1 complex-mediated NF-κB activation contribute to the anti-inflammatory effect of V8 in LPS-induced human cervical cancer SiHa cells. Inflammation. 2016;39(1):172–181. doi:10.1007/s10753-015-0236-8
28. Wei Q, Zhou B, Yang G, et al. JAZF1 ameliorates age and diet-associated hepatic steatosis through SREBP-1c -dependent mechanism. Cell Death Dis. 2018;9(9):859. doi:10.1038/s41419-018-0923-0
29. Jang WY, Bae KB, Kim SH, et al. Overexpression of Jazf1 reduces body weight gain and regulates lipid metabolism in high fat diet. Biochem Biophys Res Commun. 2014;444(3):296–301. doi:10.1016/j.bbrc.2013.12.094
30. Hodge JC, Bedroske PP, Pearce KE, Sukov WR. Molecular cytogenetic analysis of JAZF1, PHF1, and YWHAE in endometrial stromal tumors: discovery of genetic complexity by fluorescence in situ hybridization. J Mol Diagn. 2016;18(4):516–526. doi:10.1016/j.jmoldx.2016.02.001
31. Luo Z, Rhie SK, Lay FD, Farnham PJ. A prostate cancer risk element functions as a repressive loop that regulates HOXA13. Cell Rep. 2017;21(6):1411–1417. doi:10.1016/j.celrep.2017.10.048
32. Hazelett DJ, Rhie SK, Gaddis M, et al. Comprehensive functional annotation of 77 prostate cancer risk loci. PLoS Genet. 2014;10(1):e1004102. doi:10.1371/journal.pgen.1004102
33. Pospiech K, Płuciennik E, Bednarek AK. WWOX tumor suppressor gene in breast cancer, a historical perspective and future directions. Front Oncol. 2018;8:345. doi:10.3389/fonc.2018.00345
34. Qiu J, Li X, He Y, Sun D, Li W, Xin Y. Distinct subgroup of the Ras family member 3 (DIRAS3) expression impairs metastasis and induces autophagy of gastric cancer cells in mice. J Cancer Res Clin Oncol. 2018;144(10):1869–1886. doi:10.1007/s00432-018-2708-3
35. Nikbakht DM, Azarnezhad A, Hashemibeni B, Salehi M, Kazemi M, Babazadeh Z. An effective concentration of 5-Aza-CdR to induce cell death and apoptosis in human pancreatic cancer cell line through reactivating RASSF1A and up-regulation of bax genes. Iran J Med Sci. 2018;43(5):533–540.
36. Bai YH, Zhan YB, Yu B, et al. A novel tumor-suppressor, CDH18, inhibits glioma cell invasiveness via UQCRC2 and correlates with the prognosis of glioma patients. Cell Physiol Biochem. 2018;48(4):1755–1770. doi:10.1159/000492317
37. Li LT, Jiang G, Chen Q, Zheng JN. Ki67 is a promising molecular target in the diagnosis of cancer (review). Mol Med Rep. 2015;11(3):1566–1572. doi:10.3892/mmr.2014.2914
38. Miller I, Min M, Yang C, et al. Ki67 is a graded rather than a binary marker of proliferation versus quiescence. Cell Rep. 2018;24(5):1105–1112.e5. doi:10.1016/j.celrep.2018.06.110
39. Tang J, Gui C, Qiu S, Wang M. The clinicopathological significance of Ki67 in papillary thyroid carcinoma: a suitable indicator. World J Surg Oncol. 2018;16(1):100. doi:10.1186/s12957-018-1384-8
40. Cui D, Zhao Y, Xu J. Activation of CXCL5-CXCR2 axis promotes proliferation and accelerates G1 to S phase transition of papillary thyroid carcinoma cells and activates JNK and p38 pathways. Cancer Biol Ther. 2019;20(5):608–616. doi:10.1080/15384047.2018.1539289
41. Coperchini F, Pignatti P, Leporati P, et al. Normal human thyroid cells, BCPAP, and TPC-1 thyroid tumor cell lines display different profile in both basal and TNF-α-induced CXCL8 secretion. Endocrine. 2016;54(1):123–128. doi:10.1007/s12020-015-0764-x
42. Bruno S, Darzynkiewicz Z. Cell cycle dependent expression and stability of the nuclear protein detected by Ki-67 antibody in HL-60 cells. Cell Prolif. 1992;25(1):31–40. doi:10.1111/j.1365-2184.1992.tb01435.x
43. Dalton S. Linking the cell cycle to cell fate decisions. Trends Cell Biol. 2015;25(10):592–600. doi:10.1016/j.tcb.2015.07.007
44. Yuasa K, Aoki N, Hijikata T. JAZF1 promotes proliferation of C2C12 cells, but retards their myogenic differentiation through transcriptional repression of MEF2C and MRF4-implications for the role of Jazf1 variants in oncogenesis and type 2 diabetes. Exp Cell Res. 2015;336(2):287–297. doi:10.1016/j.yexcr.2015.06.009
45. Bae KB, Kim MO, Yu DH, et al. Overexpression of Jazf1 induces cardiac malformation through the upregulation of pro-apoptotic genes in mice. Transgenic Res. 2011;20(5):1019–1031. doi:10.1007/s11248-010-9476-4
46. Wang YN, Zhang LL, Fan XY, Wu SS, Zhang SQ. Poly-L-arginine induces apoptosis of NCI-H292 cells via ERK1/2 signaling pathway. J Immunol Res. 2018;2018:3651743. doi:10.1155/2018/3651743
47. Xia Y, Shen S, Verma IM. NF-κB, an active player in human cancers. Cancer Immunol Res. 2014;2(9):823–830. doi:10.1158/2326-6066.CIR-14-0112
48. Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. doi:10.1038/nrc780
49. Zou W, Yin P, Shi Y, et al. A novel biological role of α-mangostin via TAK1-NF-κB pathway against inflammatory. Inflammation. 2019;42(1):103–112. doi:10.1007/s10753-018-0876-6
50. Wang Y, Tu Q, Yan W, et al. CXC195 suppresses proliferation and inflammatory response in LPS-induced human hepatocellular carcinoma cells via regulating TLR4-MyD88-TAK1-mediated NF-κB and MAPK pathway. Biochem Biophys Res Commun. 2015;456(1):373–379. doi:10.1016/j.bbrc.2014.11.090
51. Ming GF, Li X, Yin JY, et al. JAZF1 regulates visfatin expression in adipocytes via PPARα and PPARβ/δ signaling. Metabolism. 2014;63(8):1012–1021. doi:10.1016/j.metabol.2014.05.006
52. Alam M, Kashyap T, Pramanik KK, Singh AK, Nagini S, Mishra R. The elevated activation of NFκB and AP-1 is correlated with differential regulation of Bcl-2 and associated with oral squamous cell carcinoma progression and resistance. Clin Oral Investig. 2017;21(9):2721–2731. doi:10.1007/s00784-017-2074-6
53. Catz SD, Johnson JL. Transcriptional regulation of bcl-2 by nuclear factor kappa B and its significance in prostate cancer. Oncogene. 2001;20(50):7342–7351. doi:10.1038/sj.onc.1204926
54. Kanayama A, Seth RB, Sun L, et al. TAB2 and TAB3 activate the NF-kappaB pathway through binding to polyubiquitin chains. Mol Cell. 2004;15(4):535–548. doi:10.1016/j.molcel.2004.08.008
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.