Back to Journals » Therapeutics and Clinical Risk Management » Volume 19
Italian Real-World Analysis of the Impact of Polypharmacy and Aging on the Risk of Multiple Drug–Drug Interactions (DDIs) in HCV Patients Treated with Pangenotypic Direct-Acting Antivirals (pDAA)
Authors Fagiuoli S , Toniutto P , Coppola N , Ancona DD, Andretta M, Bartolini F, Ferrante F, Lupi A , Palcic S, Rizzi FV, Re D, Alvarez Nieto G, Hernandez C, Frigerio F , Perrone V, Degli Esposti L , Mangia A
Received 1 November 2022
Accepted for publication 29 December 2022
Published 18 January 2023 Volume 2023:19 Pages 57—65
DOI https://doi.org/10.2147/TCRM.S394467
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Stefano Fagiuoli,1 Pierluigi Toniutto,2 Nicola Coppola,3 Domenica Daniela Ancona,4 Margherita Andretta,5 Fausto Bartolini,6 Fulvio Ferrante,7 Alessandro Lupi,8 Stefano Palcic,9 Francesca Vittoria Rizzi,10 Davide Re,11 Gema Alvarez Nieto,12 Candido Hernandez,13 Francesca Frigerio,14 Valentina Perrone,14 Luca Degli Esposti,14 Alessandra Mangia15
1Department of Medicine and Surgery, University of Milan Bicocca & Gastroenterology Hepatology and Transplantation Unit, ASST Papa Giovanni XXIII, Bergamo, Italy; 2Hepatology and Liver Transplantation Unit, Azienda Ospedaliero Universitaria, Udine, Italy; 3Infectious Diseases Unit, University of Campania L. Vanvitelli, Naples, Italy; 4Dipartimento Farmaceutico ASL BAT, Trani, Italy; 5UOC Assistenza Farmaceutica Territoriale, Azienda Ulss 8 Berica, Vicenza, Italy; 6Dipartimento Farmaceutico-Usl Umbria 2, Terni, Italy; 7Dipartimento Diagnostica Ed Assistenza Farmaceutica – ASL Frosinone, Frosinone, Italy; 8Struttura Complessa Di Cardiologia – ASL VCO, Omegna, Italy; 9Farmaceutica Territoriale- Azienda Sanitaria Universitaria Integrata Giuliano-Isontina (ASUGI), Trieste, Italy; 10UOS Farmacovigilanza e Monitoraggio Spesa Farmaceutica- ASL BAT, Trani, Italy; 11Servizio Farmaceutico Territoriale ASL Teramo, Teramo, Italy; 12Gilead Sciences, Medical Affairs Italy, Milan, Italy; 13Gilead Sciences, Global Medical Affairs, London, UK; 14Clicon S.r.l., Health Economics and Outcomes Research, Bologna, Italy; 15Gastroenterology and Transplant Hepatology, Papa Giovanni XXIII Hospital, Bergamo, 24127, Italy
Correspondence: Luca Degli Esposti, CliCon S.r.l. Società Benefit, Health, Economics & Outcomes Research, Via Murri, 9, Bologna, 40137, Italy, Tel +390544 38393, Email [email protected]
Purpose: The study aims at investigating the impact of polymedication and aging in the prevalence of multiple drug-drug interactions (DDIs) on HCV patients treated with sofosbuvir/velpatasvir (SOF/VEL) or glecaprevir/pibrentasvir (GLE/PIB).
Patients and Methods: This is a retrospective analysis based on administrative data covering around 6.9 million individuals. Patients treated with SOF/VEL or GLE/PIB over November 2017–March 2020 were included. Index date corresponded to SOF/VEL or GLE/PIB first prescription during such period; patients were followed up for treatment duration. Analyses were then focused on patients with ≥ 2 comedications at risk of multiple DDIs. The severity and the effect of multiple DDI were identified using the Liverpool University tool.
Results: A total of 2057 patients with SOF/VEL and 2128 with GLE/PIB were selected. Mean age of SOF/VEL patients was 58.5 years, higher than GLE/PIB ones (52.5 years) (p < 0.001), and patients > 50 years were more present in SOF/VEL vs GLE/PIB cohorts: 72% vs 58%, (p < 0.001). Most prescribed co-medications were cardiovascular, alimentary and nervous system drugs. Proportion of patients with ≥ 2 comedications was higher in SOF/VEL compared to GLE/PIB cohort (56.5% vs 32.3%, p < 0.001). Those at high-risk of multiple DDIs accounted for 11.6% (N = 135) of SOF/VEL and 19.6% (N = 135) of GLE/PIB (p < 0.001) patients with ≥ 2 comedications. Among them, the potential effect of DDI was a decrease of DAA serum levels (11% of SOF/VEL and GLE/PIB patients) and an increased concentration of comedication serum levels (14% of SOF/VEL and 42% of GLE/PIB patients).
Conclusion: This real-world analysis provided a thorough characterization on the burden of polymedication regimens in HCV patients treated with SOF/VEL or GLE/PIB that expose such patients to an increased risk of DDIs. In our sample population, SOF/VEL regimen was more frequently detected on elderly patients and on those with ≥ 2 comedications at risk of multi-DDI, ie, among patients characterized by higher rates of comorbidities and polypharmacy.
Keywords: administrative database, glecaprevir/pibrentasvir, hepatitis C, sofosbuvir/velpatasvir, polymedication
Introduction
Hepatitis C is a chronic viral infection of the liver due to the hepatitis C virus (HCV), which represents the main cause for cirrhosis development, hepatocellular carcinoma (HCC) and need for liver transplantation.1 HCV infection is regarded as one of the global healthcare challenges: in 2016, the World Health Organization (WHO) drafted the Global Viral Hepatitis Strategy, with the ambitious target to eradicate HCV by 2030.2 As for Italy, recent epidemiologic analysis estimated around 443,491 patients currently living with HCV.3
Until the last 8 years, HCV therapeutic management relied on long-term interferon-based therapies, while starting from 2014 the development of short course oral direct-acting antivirals (DAAs) and of pangenotypic DAAs (pDAAs) revolutionized the HCV treatment landscape.4 DAA therapies proved to be able to achieve sustained virological response (SVR) within weeks in the vast majority of patients.4,5 Nevertheless, the common presence of comedications in HCV patients can make pDAA treatments challenging for the arising potential of drug–drug interactions (DDIs).6 Depending on the mechanism of such interactions, DDIs can result in either an increased serum concentration of pDAA to toxic levels or in a reduction to ineffective ones. In addition, pDAAs may also decrease or increment the serum concentration of comedications with subsequent sub-therapeutic or toxic effects, respectively.7
Chronic HCV infection usually presents with a persistent inflammation status, responsible of diverse extra-hepatic diseases.8 Patients often present with multiple comorbidities requiring polypharmacy regimens, thus enhancing the risk of DDIs that in turn could significantly alter pDAA or comedication exposure.9–11 This trend may be even more exacerbated in the elderly population that usually bears a multimorbid profile with the intrinsic higher risk of DDIs among concomitant medications.12 The European Association for the Study of the Liver (EASL) guidelines indicate that the assessment of DDIs should be undertaken prior starting a DAA treatment, with full details on all prescribed therapies, over-the-counter (OTC) drugs, vitamin supplementation and illicit drugs.13
To date, several studies in real-life settings reported significant proportions of HCV patients with concomitant morbidities and related medications, mostly related to cardiovascular (CV) or central nervous system (CNS) conditions.14–16 A previous real-world study by our group conducted in Italy on two cohorts of patients treated with sofosbuvir/velpatasvir (SOF/VEL) or glecaprevir/pibrentasvir (GLE/PIB) provided insights on their characteristics and DDI assessment based on the presence of at least one comedication.16 The present analysis was aimed at an in-depth exploration of the potential impact of polypharmacy regimens and aging on the prevalence of multiple DDIs among patients treated with SOF/VEL or GLE/PIB in settings of daily clinical practice in Italy.
Materials and Methods
Data Source
The data used for the analysis were collected from administrative databases, as previously described.16 Briefly, the data were extracted from administrative flows from a pool of Italian Healthcare Entities from Piedmont, Veneto, Friuli Venezia Giulia, Umbria, Abruzzo, Lazio and Puglia regions covering a total of 6.9 million individuals (around 11.4% of the Italian population), and specifically on health-assisted subjects with at least one CV risk factor (detected from 2010 to 2018). In a feasibility analysis performed on a sample of the Healthcare Entities included, approximately 99% of the patients responding to the inclusion criteria listed below had at least a cardiovascular record; therefore, the included patients were representative of the overall sample analysed. The databases used for the analysis were the demographic database, the pharmaceutical database (containing the Anatomical–Therapeutic Chemical (ATC) code, number of packages, number of units per package, prescription date), and the hospitalization database (including all hospitalization data with discharge diagnosis codes classified according to the International Classification of Diseases, Ninth Revision, Clinical Modification, ICD–9–CM).
To guarantee patient privacy, an anonymous univocal numeric code (Patient ID) was assigned to each health-assisted subject by the Healthcare Entities. The Patient ID code allowed electronic linkage between databases. The anonymous code of the patient ensures the anonymity of the extracted data in full compliance with UE Data Privacy Regulation 2016/679 (“GDPR”) and Italian D.lgs. n. 196/2003, as amended by D.lgs. n. 101/2018. All the results of the analyses were produced as aggregated summaries, which could not be connected, either directly or indirectly, to individual patients.
Study Population and Study Variables
A retrospective observational analysis has been performed. Patients included had at least one prescription for the pDAAs SOF/VEL (ATC code: J05AP55) or GLE/PIB (ATC code: J05AP57) between November 2017 and March 2020 (inclusion period). The first prescription of pDAAs detected during the inclusion period was considered as the index date and marked the beginning of follow-up, which corresponded to the treatment duration. A 12-month period before the index date was used to describe patients’ characteristics (characterization period). Treatment duration was evaluated considering the therapeutic coverage of all the packages dispensed. Patients were included in two cohorts mutually exclusive based on the type of pDAAs prescribed at index date, namely SOF/VEL cohort and GLE/PIB cohort.
Age and gender were collected at index date. Clinical characteristics were investigated by the Charlson Comorbidity Index (CCI)17 reported as mean with standard deviation (SD), and median with interquartile range. Patients were further stratified considering the cut-off of 50 years of age.
The presence of comedications was analyzed throughout the follow-up by retrieving from the pharmaceutical database all the drug prescriptions based on the first-level ATC code, and, whenever possible, the detail on the specific drug prescribed was also recorded. Specifically, the comedications of interest were those related to CV system (ATC code C), CNS system (ATC code N) and alimentary tract and metabolism (ATC code A).
Potential DDIs, together with their severity level and possible related effects were assessed by the Liverpool University HEP Drug Interaction Checker tool, which represents an international resource recommended by EASL.13,18 Based on the type of comedications prescribed, the tool identifies four categories indicating the severity of each interaction: i) no interaction expected; ii) potential weak interaction; iii) potential interaction; iv) contraindicated drugs. Potential outcomes of DDIs were related to increase in comedication concentration, decrease or increase in DAA concentration as reported in the Liverpool University tool. If a patient had multiple co-treatments with different interaction profiles, the most severe interaction was considered. Demographics, comedications, and DDIs were evaluated in the overall population to focus on patients that received at least two comedications with potential interaction or contraindication and therefore at risk of multiple DDIs.
Statistical Analysis
Categorical variables were expressed as numbers and percentages, whereas continuous variables were reported as mean with SD, and median with minimum, maximum and interquartile (Q1-Q3) ranges. Student’s t-test was used to compare continuous variables for normally distributions and Mann–Whitney U-test for skewed distributions, as appropriate. Categorical were analysed through chi-square test. Statistical significance was accepted at P<0.05. Following the “Opinion 05/2014 on Anonymization Techniques” drafted by the “European Commission Article 29 Working Party”, the analyses involving less than 4 patients were not reported (NR) for data privacy, as they were potentially traceable to single individuals. All analyses have been performed using STATA SE version 12.0.
Results
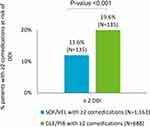
The analysis included 4185 HCV patients: 2057 treated with SOF/VEL and 2128 with GLE/PIB. The demographic and clinical characteristics of the two groups are reported in Table 1. SOF/VEL patients were older than GLE/PIB ones (mean age: 58.5 vs 52.5 years, p<0.001), with a higher percentage of patients over 50 years in SOF/VEL vs GLE/PIB (72% vs 58%, p<0.001). Figure 1 details the distribution of patients by age ranges in SOF/VEL and GLE/PIB cohorts. Younger patients were more represented in the GLE/PIB cohort (18.3% for age 0–39 and 24.1% for age 40–49 vs 10.5% and 17.1% for age 0–39 and 40–49, respectively, in SOF/VEL cohort). The proportion of male gender was comparable between the groups. Mean CCI was higher in SOF/VEL than in GLE/PIB patients (0.8 vs 0.6, p<0.001). During follow-up, the drugs most frequently prescribed were those related to CV (43.4% SOF/VEL, 23.6% GLE/PIB), alimentary (36.5% SOF/VEL, 21.3% GLE/PIB) and CNS system (24.7% SOF/VEL, 15.2% GLE/PIB). Specifically, as reported in Figure 2, while on pDAAs treatment, the use of comedications was higher in SOF/VEL vs GLE/PIB: (71.6% vs 50.4%, p<0.001) cohorts, as well as the number of patients receiving at least two comedications (56.5% vs 32.3%, p<0.001). Among the latter, HCV patients with two or more comedications at risk of multi-DDIs with pDAAs were more frequent in the GLE/PIB groups (19.6%, N=135) than among SOF/VEL (11.6%, N=135) (p<0.001) (Figure 3). The demographic and clinical characteristics of these patients are reported in Table 2. In both groups, the majority of the patients included were over 50 years. A higher mean age was observed in SOF/VEL cohort with at least two comedications at risk of multiple DDIs (69.9 vs 63.0 years, p<0.001), together with higher proportion of patients over 50 years (94% vs 79%, p<0.001), confirming the same trend observed in the overall population. Interestingly, the number of patients under 50 years with multiple DDIs was three times higher in GLE/PIB vs SOF/VEL (21% vs 6%, p<0.05). No statistically significant differences were observed for CCI.
 |
Table 1 Demographic and Clinical Characteristics of SOF/VEL and GLE/PIB Patients |
 |
Table 2 Demographic and Clinical Characteristics at Baseline of Patients with ≥2 Comedications and at Risk of Multiple DDIs |
 |
Figure 1 Patients distribution by age ranges, comparison between SOF/VEL and GLE/PIB cohort. Abbreviations: GLE/PIB, glecaprevir/pibrentasvir; SOF/VEL, sofosbuvir/velpatasvir. |
 |
Figure 2 Proportion of patients with comedications. Abbreviations: GLE/PIB, glecaprevir/pibrentasvir; SOF/VEL, sofosbuvir/velpatasvir. |
In the SOF/VEL cohort, the most represented comedications were those leading to potential interactions: pantoprazole/atorvastatin (N=11 patients), pantoprazole/simvastatin (N=7 patients), lansoprazole/atorvastatin, pantoprazole/warfarin, pantoprazole/silodosin (N=5 patients each) and omeprazole/atorvastatin (N=4 patients). In GLE/PIB patients, four patients received pantoprazole and atorvastatin, the latter contraindicated with this pDAA. Other combinations were not reported for data privacy (≤3 patients involved).
The distribution of the most frequent drug classes in patients under and over 50 years are reported in Supplementary Table 1. In the SOF/VEL cohort (Supplementary Table 1A), all patients under 50 years received drugs for acid-related disorders and five patients used CV drugs. In patients aged over 50 years, 87% were also prescribed with drugs for acid-related disorders, while 62.2% received CV drugs (mainly lipid modifying drugs, prescribed in over half of the patients). In the GLE/PIB cohort (Supplementary Table 1B), 78.6% and 84.1% of the patients respectively aged below and over 50 years had a drug for acid-related disorders as comedication. CV drugs were reported in 42.9% of those under 50 years and in 64.5% in patients aged 50 years or over. In this case, the proportion of patients with lipid modifying agents was lower compared to that of the SOF/VEL cohort and accounted for 39.3% of the GLE/PIB cohort.
As Figure 4 shows, among patients with at least two comedications at risk of multiple DDIs, the proportion of those receiving therapeutic combinations known to potentially increase comedication serum levels was three times higher in GLE/PIB compared to SOF/VEL treatment group (42% vs 14%). Besides, no differences were noticed for comedications referred as able to decrease DAA serum levels (11% in both GLE/PIB and SOF/VEL groups). None of the patients was administrated with drugs known to increase DAA serum levels.
Discussion
In 2014, the introduction of DAAs has revolutionised the therapy of HCV infections, formerly based on interferon therapy.19 A further step onward was made in 2017, with the introduction of SOF/VEL, the first pangenotypic combination with a wider treatment spectrum on HCV genotypes 1, 2, 3, 4, 5, and 6, followed shortly after by GLE/PIB.4,20–22
However, in view of the clinical complexity of HCV-infected population and the subsequent need of polypharmacy regimens, DDIs represent a hot topic of current drug research and an open challenge for clinicians, preventing a more extensive utilization of DAAs, especially in particularly comorbid subjects (ie, the elderly).11,12
This real-world analysis in settings of daily clinical practice in Italy evaluated HCV+ patients treated with pDAA to assess the potential DDIs related to polypharmacy regimens, namely drugs targeted to alimentary tract and metabolism, CV and nervous systems. In particular, we analysed HCV patients treated with SOF/VEL and GLE/PIB pDAA combinations to characterize the demographic and clinical characteristics at baseline of patients with two or more comedications who have an elevated risk of multiple DDIs.
Here, we observed that SOF/VEL group consisted of a generally older population, with a greater representativeness of over 50 subjects and a worse comorbidity profile, documented by the significantly higher CCI. These findings are consistent with our previous study on 3181 HCV+ Italian subjects, among whom SOF/VEL treated patients were older and affected by more severe cardiovascular and CNS clinical conditions compared to those under GLE/PIB therapy.16 Specifically, the comedications of interest were those related to CV system (ATC code C), CNS system (ATC code N) and alimentary tract and metabolism (ATC code A). In the SOF/VEL treatment group, characterized by older age with worse comorbidity profile, the percentage of drug prescriptions and the number of patients receiving more than two comedications was higher, regardless of comedication drug category. Nevertheless, restricting the analysis to HCV patients with at least two comedications at increased DDI risk, a higher frequency of patients with polypharmacy therapies (≥2) was observed in the GLE/PIB group. Furthermore, the proportion of younger subjects (under 50 years) with multiple DDIs was more than threefold increased in GLE/PIB compared to SOF/VEL (21% vs 6%) treated patients. Taken together, these data seem to suggest that SOF/VEL is the pDAA combination more commonly prescribed in the elderly clinically complex patients already under polypharmacy regimens. SOF/VEL scheme has been extensively studied in a series of Phase III clinical trials named ASTRAL (ASTRAL-1, ASTRAL-2, ASTRAL-3, ASTRAL-4, and ASTRAL-5) that indicated this therapeutic regimen as a good option for multi-treated comorbid patients.4,23
A point that deserves attention is the dual effect of DDIs on the blood levels of both DAAs and comedications. Depending on the underlying mechanism of interactions, DDIs can lead to an increased toxic serum concentration of pDAA or to a reduced ineffective level. Likewise, pDAAs can also alter, increasing or decreasing, the circulating levels of comedications, with subsequent toxic or sub-therapeutic effects, respectively.7 In the patients at risk of multiple DDIs, drug schemes known to result in increased comedication serum levels were found three times more frequently in the GLE/PIB compared to the SOF/VEL treatment group. In contrast, the proportion of patients receiving pDAAs/comedications combinations known to decrease circulating pDAAs was comparable between GLE/PIB or SOF/VEL treatments. In this regard, a Spanish study on HCV patients treated with DAA observed around 25% of the patients with clinically significant DDI, the majority of which affecting the concomitant medication thus requiring action as temporary withdrawal of DAA, administration schedule or dose adjustment or therapeutic alternative prescription.11 In another real-world study, patients with potential clinically significant interaction (53.1%) or contraindicated (15.2%) were also often managed by appropriate dose adjustments or temporary withdraw/changing of comedications.24 Ultimately, an US study on real-world care management of HCV patients treated with SOF/VEL or GLE/PIB reported that pharmacists recommendations concerning DDI were not followed by approximately 40% of the patients.25
This analysis has some limitations mainly related to its observational design, based on data extraction from administrative databases, and the small sample size of some sub-groups. Thus, detailed information on the clinical picture and previous history of HCV patients is missing. Moreover, data on drug prescriptions were retrieved from pharmaceutical databases, thus there is a lacking information regarding the concomitant use of OTC products and vitamin supplementation on one hand, and drugs administered during hospitalizations on the other hand. For this reason, the impact on DDIs of medications not recorded in the pharmaceutical databases might be underestimated.
Conclusion
This real-world analysis of the Italian clinical setting confirmed that HCV patients are clinically vulnerable and burdened by a complex comorbidity profile. The resulting necessity of multi-therapy regimens exposes these patients to an increased risk of DDIs with pDAA therapy. Here we evaluated the most used comedications in HCV+ population, specifically drugs for the treatment of alimentary tract, CV and CNS disturbances, that are likely to elicit DDIs. In line with available evidence, SOF/VEL seems to be more frequently prescribed in elderly subjects, characterized by higher rates of comorbidities and polypharmacy regimens, and bearing a more favourable DDI profile.
In conclusion, in front of the benefits brought by the advent of DAAs, especially pDDAs after 2017, DDIs still remain an open issue that deserves further efforts to fully optimize the therapeutic management of HCV patients.
Ethics Approval and Informed Consent
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the following Ethics Committees: Comitato Etico Interprovinciale Area 1 (A.O.U. Foggia, ASL Foggia, ASL BAT) (Prot. N 27/CE/2019, 20/04/2019); Comitato Etico per le Sperimentazioni Cliniche (CESC) della Provincia di Vicenza (Prot. N 1627, 28/10/2020); Comitato Etico per le province di L’Aquila e Teramo (Prot N. 08, 16/05/2019); Comitato Etico Lazio 2 (Prot N 0199138/2018, 5/12/2018); Comitato Etico per la Sperimentazione Clinica della provincia di Venezia e IRCCS S.Camillo (28/07/2020); Comitato Etico Unico Regionale (Prot N 5.17, 5/02/2019); Comitato Etico Aziende Sanitarie Umbria (Prot N 14657/18/ON, 25/10/2018); Comitato Etico Interaziendale A.O.U. “Maggiore della Carità” (Prot N 364/CE, 12/04/2019); Comitato Etico per la Sperimentazione Clinica delle province di Verona e Rovigo (Prot N 64198, 03/10/2018). Each LHU is the owner of its data. CliCon s.r.l. Società Benefit receives the data necessary to carry out the analysis from the LHUs, that independently proceed to extrapolate the data from their administrative databases and give the anonymized data to CliCon S.r.l. Società Benefit.
Informed Consent Statement
Informed consent was not required since obtaining it is impossible for organizational reasons (pronouncement of the Data Privacy Guarantor Authority, General Authorization for personal data treatment for scientific research purposes – n.9/2014).
Funding
Gilead purchased the study report that is the basis for this manuscript. This manuscript was developed with Gilead and CliCon S.r.l. Società Benefit. The views expressed here are those of the authors and not necessarily those of the supporters. The agreement signed by CliCon S.r.l. Società Benefit and Gilead does not create any entityship, joint venture, or any similar relationship between parties. CliCon S.r.l. Società Benefit is an independent company. Neither CliCon S.r.l. Società Benefit nor any of their representatives are employees of Gilead for any purpose.
Disclosure
SF reports speaking/consulting/research for AbbVie, Astellas, Bayer, Gilead, Intercept, Roche, Eisai, Kedrion, MSD, Novartis. PT reports speaking/consulting/research for AbbVie, Bayer, Gilead. NC reports speaking/consulting/research for AbbVie, Bristol, Gilead, Janssen, Merck, ViiV Healthcare, MSD. CH, GAN, FF are employees of Gilead. LDE, VP are employees of Clicon S.r.l. Società Benefit, Health Economics and Outcomes Research. AM reports speaking/consulting/research for Angelini, Gilead, Intercept, MSD, Spring Bank. All the remaining authors reports no conflicts of interest in this work.
References
1. Khullar V, Firpi RJ. Hepatitis C cirrhosis: new perspectives for diagnosis and treatment. World J Hepatol. 2015;7(14):1843–1855. doi:10.4254/wjh.v7.i14.1843
2. World Health Organization. Global health sector strategy on viral hepatitis 2016–2021. Towards ending viral hepatitis. World Health Organization; 2016. Available from: https://apps.who.int/iris/handle/10665/246177.
3. Gardini I, Bartoli M, Conforti M, Mennini FS, Marcellusi A. Estimation of the number of HCV-positive patients in Italy. PLoS One. 2019;14(10):e0223668. doi:10.1371/journal.pone.0223668
4. Zoratti MJ, Siddiqua A, Morassut RE, et al. Pangenotypic direct acting antivirals for the treatment of chronic hepatitis C virus infection: a systematic literature review and meta-analysis. EClinicalMedicine. 2020;18:100237. doi:10.1016/j.eclinm.2019.12.007
5. Schulte B, Wübbolding M, Marra F, et al. Frequency of potential drug-drug interactions in the changing field of HCV therapy. Open Forum Infect Dis. 2020;7(2):ofaa040. doi:10.1093/ofid/ofaa040
6. Maasoumy B, Port K, Calle Serrano B, et al. The clinical significance of drug-drug interactions in the era of direct-acting anti-viral agents against chronic hepatitis C. Aliment Pharmacol Ther. 2013;38(11–12):1365–1372. doi:10.1111/apt.12523
7. Ahmed A, Lutchman GA, Kwo PY. Drug‐drug interactions in hepatitis C virus treatment: do they really matter? Clin Liver Dis. 2017;10(5):111–115. doi:10.1002/cld.668
8. Tang L, Marcell L, Kottilil S. Systemic manifestations of hepatitis C infection. Infect Agent Cancer. 2016;11:29. doi:10.1186/s13027-016-0076-7
9. Mangia A, Piazzolla V, Giannelli A, et al. SVR12 rates higher than 99% after sofosbuvir/velpatasvir combination in HCV infected patients with F0-F1 fibrosis stage: a real world experience. PLoS One. 2020;14(5):1.
10. Kondili LA, Gaeta GB, Ieluzzi D, et al. Real-life data on potential drug-drug interactions in patients with chronic hepatitis C viral infection undergoing antiviral therapy with interferon-free DAAs in the PITER cohort study. PLoS One. 2017;12(2):e0172159. doi:10.1371/journal.pone.0172159
11. Margusino-Framiñán L, Cid-Silva P, Giménez-Arufe V, et al. Influence of drug–drug interactions on effectiveness and safety of direct-acting antivirals against hepatitis C virus. Eur J Hosp Pharm. 2021;28(1):16–21. doi:10.1136/ejhpharm-2019-001889
12. Nobili A, Garattini S, Mannucci PM. Multiple diseases and polypharmacy in the elderly: challenges for the internist of the third millennium. J Comorb. 2011;1:28–44. doi:10.15256/joc.2011.1.4
13. Pawlotsky JM, Negro F, Aghemo A, et al. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018;69(2):461–511.
14. Sicras-Mainar A, Morillo-Verdugo R. Potential interactions between pangenotypic direct-acting antivirals and concomitant cardiovascular therapies in patients with chronic hepatitis C virus infection. J Int Med Res. 2020;48(10):300060520964659. doi:10.1177/0300060520964659
15. Sicras-Mainar A, Morillo-Verdugo R. Concomitant use of direct-acting antivirals (DAA) and central nervous system drugs in patients with hepatitis C virus infection. Adicciones. 2020;1(1):1551.
16. Mangia A, Scaglione F, Toniutto P, et al. Drug–drug interactions in Italian patients with chronic hepatitis C treated with pangenotypic direct acting agents: insights from a real-world study. IJERPH. 2021;18(13):7144. doi:10.3390/ijerph18137144
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
18. Liverpool HEP Interactions; 2020. Available from: https://www.hep-druginteractions.org/.
19. Fried M, Hoofnagle J. Therapy of Hepatitis C. Semin Liver Dis. 1995;15(1):82–91. doi:10.1055/s-2007-1007265
20. Chahine EB, Sucher AJ, Hemstreet BA. Sofosbuvir/velpatasvir: the first pangenotypic direct-acting antiviral combination for hepatitis C. Ann Pharmacother. 2017;51(1):44–53. doi:10.1177/1060028016668897
21. Yen HH, Chen YY, Lai JH, et al. Pan-genotypic direct-acting antiviral agents for undetermined or mixed-genotype hepatitis C infection: a real-world multi-center effectiveness analysis. JCM. 2022;11(7):1853. doi:10.3390/jcm11071853
22. Viganò M, Andreoni M, Perno CF, et al. Real life experiences in HCV management in 2018. Expert Rev Anti Infect Ther. 2019;17(2):117–128. doi:10.1080/14787210.2019.1563755
23. Lee R, Kottilil S, Wilson E. Sofosbuvir/velpatasvir: a pangenotypic drug to simplify HCV therapy. Hepatol Int. 2017;11(2):161–170. doi:10.1007/s12072-016-9776-8
24. Hui VWK, Au CL, Lam ASM, et al. Drug–drug interactions between direct-acting antivirals and co-medications: a territory-wide cohort study. Hepatol Int. 2022;16(6):1318–1329. doi:10.1007/s12072-022-10402-y
25. Curry MP, Flamm SL, Milligan S, et al. Prevalence of drug-drug interactions with pangenotypic direct-acting antivirals for hepatitis C and real-world care management in the United States: a retrospective observational study. J Manag Care Spec Pharm. 2021;27(9):1239–1248. doi:10.18553/jmcp.2021.20550
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.