Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Italian Real-World Analysis of a Tyrosine Kinase Inhibitor Administration as First- or Second-Line of Therapy in Patients with Chronic Myeloid Leukemia
Authors Perrone V, Giacomini E, Andretta M, Arenare L, Cillo MR, Latini M, Mecozzi A, Pagliaro R, Vercellone A, Degli Esposti L
Received 5 March 2021
Accepted for publication 17 May 2021
Published 8 June 2021 Volume 2021:17 Pages 617—622
DOI https://doi.org/10.2147/TCRM.S309342
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Valentina Perrone,1 Elisa Giacomini,1 Margherita Andretta,2 Loredana Arenare,3 Maria Rosaria Cillo,4 Marisa Latini,5 Alessandra Mecozzi,6 Romina Pagliaro,5 Adriano Vercellone,7 Luca Degli Esposti1
1CliCon S.r.l. Health, Economics & Outcomes Research, Bologna, Italy; 2UOC Assistenza Farmaceutica Territoriale, Azienda ULSS 8 Berica, Vicenza, Italy; 3UOC Farmaceutica e Territoriale e Integrativa– Asl Latina, Latina, Italy; 4Pharmaceutical Department Local Health Unit (LHU) Salerno, Salerno, Italy; 5U.O.C. Farmaceutica Territoriale – Asl Roma 5, Roma, Italy; 6UOC Farmacia SE/CTO Asl Roma 2, Roma, Italy; 7Department of Pharmacy, Local Health Unit (LHU) Naples 3 South, Napoli, Italy
Correspondence: Valentina Perrone
CliCon S.r.l. Health, Economics & Outcomes Research, via Murri, Bologna, 9 - 40137, Italy
Tel +39 0544 38393
Fax +39 0544 212699
Email [email protected]
Purpose: To date, litte evidence is reported about the real-life dosage of tyrosine kinase inhibitors prescribed in Italy. The present observational retrospective study aimed to evaluate the mean daily dose of nilotinib prescribed as first- and second-line therapy among patients suffering from chronic myeloid leukemia (CML) in settings of clinical practice in Italy.
Patients and Methods: Data were obtained from the administrative databases of a sample of Italian entities. All adult patients prescribed nilotinib were included from January 2013 to December 2016 if they were using it as first-line and from January 2015 to December 2018 as second-line therapy. The mean daily dose was calculated considering the dosage between first and last nilotinib prescription date or last BCR/ABL test date.
Results: Among CML patients treated with nilotinib as first-line (N=87), the mean daily dose of nilotinib was 500.5 mg during a mean treatment duration of 798.9 days and of 498.54 mg considering the last determination of BCR/ABL test (mean duration of 811 days). A total of 103 CML patients were prescribed nilotinib as second-line therapy; of them, 80.6% had previously received imatinib, 17.5% dasatinib. The mean daily dose of nilotinib was found to be 566.3 mg with a mean time duration of 302.8 days, while when the last BCR/ABL test was taken into account (mean duration of 323.1 days), a mean daily dose of 565.2 mg was detected.
Conclusion: The study reported on the real-world dosage pattern of a TKI for CML management. Our results compared with the dosage of nilotinib reported in datasheet (600 mg and 800 mg for first- and second-line, respectively) showed a trend of mean daily dose prescribed in clinical practice settings lower than the dosage currently indicated.
Keywords: BCR/ABL, dosage, nilotinib, real-world
Introduction
Chronic myeloid leukemia (CML) is a condition initiating in the hematopoietic stem cells, and it is characterized by the “Philadelphia chromosome”, a reciprocal translocation between chromosomes 9 and 221 that leads to the constitutive expression of fusion tyrosine kinase BCR-ABL1, an oncoprotein with deregulated tyrosine kinase activity.2 Overall incidence is in the range of 10–15 cases per million persons each year, with no major ethnic or geographic differences.1
The treatment landscape of CML experienced a breakthrough with the development of tyrosine kinase inhibitors (TKIs) targeting BCR-ABL kinase activity, that are now regarded as the mainstay of CML treatment.3 Nowadays CML is regarded as a chronic disease requiring long-term treatments that aim to achieve a stable deep molecular response (DMR) yielding to therapy discontinuation and treatment-free remission.4 It has been reported a small but constant decrement of conditional probability of failure-free survival independent from response or duration of TKI therapy, as around 5% of patients in remission continue to fail TKI therapy in an additional year.5 Most patients (in Western countries) newly diagnosed with CML in chronic phase have now reached a near normal life expectancy:6 an US study showed indeed a 5-year survival only slightly lower than that of the general population.7
According to the latest “European LeukemiaNet 2020 recommendations” for treating CML,6 the goal of CML therapies is to ensure a good quality of life and a normal survival without life-long treatment.
First-line treatment in newly diagnosed CML patients should belong to TKI class. Specifically, the Food and Drug Administration (FDA) and the European Medicine Agency (EMA) have given approval to four TKIs as first-line treatment: the first-generation TKI imatinib and the second generation TKIs nilotinib, dasatinib and bosutinib.6,8–10 All these drugs can be used as second-line therapy while ponatinib, a third generation TKI, is approved for second and later lines and for patients with T315I mutation.4
Given that each TKI is recommended at a standard dose for the respective line of therapies, dose adjustment/modification of TKIs is currently investigated in clinical studies and real-life settings.11 Indeed, there is increasing evidence towards therapy personalization with dose modifications throughout CML treatment to prevent and/or manage side effects while achieving and maintaining the cytogenetic and molecular responses.11 In this direction, clinical trials showed dose reduction as a safe strategy and raise the question if dose reductions should be considered prior to treatment-free remission attempts.11 However, to date the recommendations are towards a proper utilization of TKI according to the dosage reported in the datasheet. To date, few studies have considered the dosage pattern of TKI in Italy, and are mainly focused on the first-generation TKI12–14 or to second-generation drugs among CML elderly patients.15,16 In this context, the present study aims to evaluate the mean daily dose of nilotinib as frontline and second-line therapy among patients with CML in Italian clinical practice settings.
Patients and Methods
Data Source
Data were collected from administrative databases of a sample of Italian Healthcare Entities geographically distributed throughout the national territory and including approximately 8.2 million health-assisted subjects. The databases queried were: beneficiaries database to collect information such as age and sex; pharmaceuticals database to get data on drug prescribed as Anatomical-Therapeutic Chemical (ATC) code, prescription date, marketing authorization code, number of packages and units per package; hospitalization database in which diagnosis and procedure codes classified based on the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) are reported; outpatient specialist services database that contains date, type and description activity of diagnostic tests and specialist visits. The patient code in each database allowed data-linkage across all different databases. All the data presented were produced as aggregated summaries, therefore individual patients cannot be identified either directly or indirectly. The study has been approved by the following local Ethics Committees of the Entities involved: Comitato Etico Lazio 1, ref. N. 47, approval date 10/01/2018; Comitato Etico Lazio 2, ref.N 0087354, approval date 15/05/2019; Comitato Etico Interaziendale Campania Sud. ref N 0031971 approval date 26/02/2020; Comitato Etico Interaziendale Campania Sud. ref. N 0069997 approval date 06/05/2020; Comitato Etico per la sperimentazione clinica delle province di Verona e Rovigo. ref N 52048, approval date 25/07/2018.
Study Design
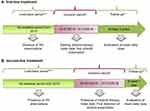
This retrospective observational study included all adult patients with a prescription of nilotinib (ATC code: L01XE08). Two sets of analyses were designed to evaluate nilotinib as first- and second-line (Figure 1A and B, respectively).
First-Line Treatment Analysis
All adult patients with a first prescription for nilotinib as first-line during the inclusion period from January 2013 to December 2016 (starting and ending dates of inclusion periods were different in some cases, depending on data availability for each healthcare entity) were analysed. The index date corresponded to the first prescription date and marked the start of the follow-up period, which lasted at least 3 years afterwards. Patients were looked-back before the index date to verify the absence of previous TKI treatments: the data were available starting from 2012 for one entity, from 2015 for another one and from 2010 for the others.
Second-Line Treatment Analysis
All patients with nilotinib prescription between January 2015 and December 2018 were analysed (starting and ending dates of inclusion periods were different in some cases, depending on data availability for each healthcare entity). Within this period, the index date was the first detection of nilotinib prescription. Patients were looked-back during all available period before index date. In this period, the presence of other TKIs such as imatinib (ATC code: L01XE01), dasatinib (ATC code: L01XE06) and ponatinib (ATC code: L01XE24) was investigated. Follow-up period was of at least one year after index date. For this set of analysis, the sample population were included among the entities that allow to cover sufficient look-back and follow-up period.
In both first-line and second-line treatment analysis, the presence of BCR/ABL test was identified by ICD-9-CM procedure codes: 91.36.5, 91.29.4_0, 91.29.3.
Mean Daily Dose Evaluation
The approved dose of nilotinib reported in the summary of product characteristics (SmPC) is 600 mg daily as first-line treatment and 800 mg daily as second-line.6 To evaluate the mean daily dose, all nilotinib prescriptions during follow-up were considered. Patients with less than two prescriptions during follow-up were excluded from the analyses.
The mean daily dose was calculated as the total dosage between first and penultimate prescription divided per the total number of days between first and last nilotinib prescriptions. In patients that discontinued nilotinib during follow-up, the mean daily dose was calculated either until last nilotinib prescription date or last BCR/ABL test date. The latter time point was considered since presence of BCR/ABL determination ensure the response of therapy is monitored after the treatment is discontinued.17,18 A representative scheme of the mean daily dose evaluation is shown in Figure 2. Median daily dose was also reported with interquartile (IQR range).
 |
Figure 2 Mean daily dose evaluation. Abbreviation: PX, prescription. |
Results
First-Line Treatment Analysis
In the first set of analyses, a total of 87 CML patients with nilotinib as first-line were included; 50.6% were male and mean age was 51.8 years. Mean daily dose of nilotinib calculated considering the last nilotinib prescription date was 500.5 mg and mean treatment duration was 798.9 days. When the last determination of BCR/ABL test was taken into account, the mean daily dose was 498.54 mg with a mean treatment duration of 811 days (Table 1). Most patients (N= 54, mean age 51.6 years, 48.1% male) remain with nilotinib during all follow-up, and their mean daily dose was of 530.2 mg during a mean treatment duration of 1066.3 days. In patients that discontinued nilotinib (N=19, mean age 50.3 years, 47.4% male) the mean daily dose was 262.1 mg during a mean treatment duration of 260.9 days, while the mean dose to the last BRC/ABL test was 243.1 mg considering a mean time of 316.3 days. Among patients that switched to another TKI (N=14, mean age 54.3, 64.3% male), mean daily dose of nilotinib was 522.5 mg during a mean of 497.7 days of treatment duration (Table 1). Median daily doses are reported in Supplementary Table 1.
 |
Table 1 Mean Daily Dose of Nilotinib as First-Line Therapy |
Second-Line Treatment Analysis
A total of 103 CML patients with nilotinib as second-line therapy was identified. Mean age was 58.5 years and 30.6% were male. Most patients (80.6%) were previously treated with imatinib or with dasatinib (17.5%). Mean daily dose was 566.3 mg, and patients were treated with nilotinib for a mean time of 302.8 days (Table 2). A mean daily dose of 565.2 mg was observed when considering the last BCR/ABL test (mean duration of 323.1 days). Patients prescribed nilotinib during all follow-up (N=80, mean age 58.6 years, 53.8% male) had a mean daily dose of 580 mg, and mean treatment duration of 340.5 days. Patients that discontinued nilotinib (N=10, mean age 62.3 years, 50% male) had a mean daily dose of 329.0 mg and were treated for a mean of 113.3 days. Ultimately, among patients switching to a third line TKI (N=13, mean age 54.9 years, 30.8% male), mean daily dose was found to be of 614.3 mg, with a mean treatment duration of 216.7 days. Median daily doses are reported in Supplementary Table 1.
 |
Table 2 Mean Daily Dose of Nilotinib as Second-Line Therapy |
Discussion and Conclusion
The regulatory approved dosage regimens of drugs are usually derived from clinical trials results, however, post-marketing dosing variations are often observed in clinical practice.19
Dose modifications (as in reducing the dose) of TKI is reported in literature as a potential approach before treatment-free remission (TFR) attempts.11,20 Evidence gained from real-world settings may be useful to obtain insight on modification of dosing patterns in clinical practice. In this context, the present report focused on the mean daily dose of nilotinib taken by CML patients, as our aim was to provide a photograph of nilotinib dosage pattern in clinical practice setting. Our results highlighted that, in clinical practice settings, the daily dose was generally lower than the one established for nilotinib for newly treated or experienced users. Specifically, mean daily dose of all treatment duration was observed to be 498.5 mg vs 600 mg (300 mg twice daily) reported in SmPC for first-line therapy and 565.2 vs 800 mg (400 mg twice daily) reported in the SmPC for second-line therapy when the time point was set to the last detection of BCR/ABL tests, indicating that patients were still monitored after stopping the treatment. A similar trend was noted when the mean daily dose considering last nilotinib prescription resulted lower than SmPC (500.5 mg and 566.3 mg for first- and second-line, respectively).
The study has limitations, mainly related to the observational nature of the study and on the data source, ie administrative databases. The first limitation is represented by the lack of clinical information, particularly those related to the actual state of CML, and the presence of undetectable confounders that might potentially influence our results. Therefore, it was not possible to report if the dose observed was related to pre-existing conditions or emerging comorbidities after starting TKI therapy.21–23 Nor was it possible to assess patient outcomes or the reasons behind treatment interruption or dose modification. The data availability periods provided by some entities were different in time and duration. The results of this study referred to the population analysed and could not be generalizable at a national level. Ultimately, the analyses focused on nilotinib dosage pattern and further analyses will be performed on dose evaluation of other TKIs as first and second therapy in settings of clinical practice.
Acknowledgments
Novartis Farma S.p.A purchased the study report that is the basis for this manuscript and funded the publication. The views expressed here reflect those of the authors and not necessarily those of the supporters. Clicon S.r.l. is an independent company and neither CliCon S.r.l. nor any of their representatives are employees of Novartis for any purpose.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hochhaus A, Saussele S, Rosti G, et al. Chronic myeloid leukaemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28:iv41–51. doi:10.1093/annonc/mdx219
2. Holyoake TL, Vetrie D. The chronic myeloid leukemia stem cell: stemming the tide of persistence. Blood. 2017;129(12):1595–1606. doi:10.1182/blood-2016-09-696013
3. Li N, Yang X, Fan L, et al. Nilotinib versus dasatinib as second-line therapy in patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase who are resistant or intolerant to imatinib: a cost-effectiveness analysis based on real-world data. J Med Econ. 2017;20(4):328–336. doi:10.1080/13696998.2016.1261032
4. Annunziata M, Bonifacio M, Breccia M, et al. Current strategies and future directions to achieve deep molecular response and treatment-free remission in chronic myeloid leukemia. Front Oncol. 2020;10. doi:10.3389/fonc.2020.00883
5. Sasaki K, Kantarjian HM, Jain P, et al. Conditional survival in patients with chronic myeloid leukemia in chronic phase in the era of tyrosine kinase inhibitors. Cancer. 2016;122(2):238–248. doi:10.1002/cncr.29745
6. Hochhaus A, Baccarani M, Silver RT, et al. European LeukemiaNet 2020 recommendations for treating chronic myeloid leukemia. Leukemia. 2020;34(4):966–984. doi:10.1038/s41375-020-0776-2
7. Sasaki K, Strom SS, O’Brien S, et al. Prospective analysis: relative survival in patients with chronic myeloid leukemia in chronic Phase in the era of tyrosine kinase inhibitors. Lancet Haematol. 2015;2(5):e186–93. doi:10.1016/S2352-3026(15)00048-4
8. Jain P, Kantarjian H, Alattar ML, et al. Analysis of long term responses and their impact on outcomes in patients with chronic phase CML treated with four different TKI modalities – analysis of 5 prospective clinical trials. Lancet Haematol. 2015;2(3):e118–28. doi:10.1016/S2352-3026(15)00021-6
9. Jain P, Kantarjian HM, Ghorab A, et al. Prognostic factors and survival outcomes in patients with chronic myeloid leukemia in blast phase in the tyrosine kinase inhibitor era: cohort study of 477 patients. Cancer. 2017;123(22):4391–4402. doi:10.1002/cncr.30864
10. Jain P, Kantarjian H, Alattar ML, et al. Long-term molecular and cytogenetic response and survival outcomes with imatinib 400 mg, imatinib 800 mg, dasatinib, and nilotinib in patients with chronic-phase chronic myeloid leukaemia: retrospective analysis of patient data from five clinical trials. Lancet Haematol. 2015;2(3):e118–128.
11. Copland M. Is there a role for dose modification of TKI therapy in CML? Curr Hematol Malig Rep. 2019;14(4):337–345. doi:10.1007/s11899-019-00524-w
12. Latagliata R, Ferrero D, Iurlo A, et al. Imatinib in very elderly patients with chronic myeloid leukemia in chronic Phase: a Retrospective Study. Drugs Aging. 2013;30(8):629–637. doi:10.1007/s40266-013-0088-6
13. Crugnola M, Castagnetti F, Breccia M, et al. Outcome of very elderly chronic myeloid leukaemia patients treated with imatinib frontline. Ann Hematol. 2019;98(10):2329–2338. doi:10.1007/s00277-019-03767-y
14. Breccia M, Luciano L, Latagliata R, et al. Age influences initial dose and compliance to imatinib in chronic myeloid leukemia elderly patients but concomitant comorbidities appear to influence overall and event-free survival. Leuk Res. 2014;38(10):1173–1176. doi:10.1016/j.leukres.2014.06.020
15. Latagliata R, Stagno F, Annunziata M, et al. Frontline dasatinib treatment in a “Real-Life” Cohort of patients older than 65 years with chronic myeloid leukemia. Neoplasia. 2016;18(9):536–540. doi:10.1016/j.neo.2016.07.005
16. Latagliata R, Attolico I, Trawinska MM, et al. Bosutinib in the real-life treatment of chronic Phase Chronic Myeloid Leukemia (CML) patients aged > 65 years resistant/intolerant to frontline tyrosine-kynase inhibitors. Blood. 2019;134(Supplement_1):1649. doi:10.1182/blood-2019-127029
17. Rousselot P, Charbonnier A, Cony-Makhoul P, et al. Loss of major molecular response as a trigger for restarting tyrosine kinase inhibitor therapy in patients with chronic-phase chronic myelogenous leukemia who have stopped imatinib after durable undetectable disease. J Clin Oncol. 2014;32(5):424–430. doi:10.1200/JCO.2012.48.5797
18. Cross NCP, White HE, Colomer D, et al. Laboratory recommendations for scoring deep molecular responses following treatment for chronic myeloid leukemia. Leukemia. 2015;29(5):999–1003. doi:10.1038/leu.2015.29
19. Ogata A, Kaneko M, Narukawa M. Lower-dose prescriptions in the post-marketing situation and the influencing factors thereon. PLoS One. 2019;14(6):e0218534. doi:10.1371/journal.pone.0218534
20. Rea D, Cayuela J-M, Dulucq S, Etienne G. Molecular responses after switching from a standard-dose twice-daily nilotinib regimen to a reduced-dose once-daily schedule in patients with chronic myeloid leukemia: a Real Life Observational Study (NILO-RED). Blood. 2017;130(Supplement 1):318.
21. Sasaki K, Kantarjian HM, O’Brien S, et al. Incidence of second malignancies in patients with chronic myeloid leukemia in the era of tyrosine kinase inhibitors. Int J Hematol. 2019;109(5):545–552. doi:10.1007/s12185-019-02620-2
22. Sasaki K, Lahoti A, Jabbour E, et al. Clinical safety and efficacy of nilotinib or dasatinib in patients with newly diagnosed chronic-Phase chronic myelogenous leukemia and pre-existing liver and/or renal dysfunction. Clin Lymphoma Myeloma Leuk. 2016;16(3):152–162. doi:10.1016/j.clml.2015.12.003
23. Jain P, Kantarjian H, Boddu PC, et al. Analysis of cardiovascular and arteriothrombotic adverse events in chronic-phase CML patients after frontline TKIs. Blood Adv. 2019;3(6):851–861. doi:10.1182/bloodadvances.2018025874
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.