Back to Journals » Journal of Blood Medicine » Volume 6
Issues in assessing products for the treatment of hemophilia – the intersection between efficacy, economics, and ethics
Authors Farrugia A, Noone D, Schlenkrich U, Schlenkrich S, O'Mahony B, Cassar J
Received 6 March 2015
Accepted for publication 21 April 2015
Published 15 June 2015 Volume 2015:6 Pages 185—195
DOI https://doi.org/10.2147/JBM.S79091
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Martin Bluth
Video abstract presented by Albert Farrugia
Views: 189
Albert Farrugia,1,2 Declan Noone,3 Uwe Schlenkrich,4 Steffen Schlenkrich,5 Brian O'Mahony,3 Josephine Cassar6
1School of Surgery, QEII Medical Centre, The University of Western Australia (M509), Crawley, WA, Australia; 2College of Medicine, Medicine and Environment, Australian National University, Canberra, WA, Australia; 3Irish Haemophilia Society, Dublin, Ireland; 4Deutsche Hämophiliegesellschaft (DHG), Hamburg, Germany; 5Am Holzbiel, Erfurt-Töttleben, Germany; 6Faculty of Health, University of Canberra, Canberra, WA, Australia
Abstract: Following the obviation of the pathogen safety threats posed by previous generations of clotting factor concentrates for the treatment of hemophilia, the principal issue facing the patient community is timely access to adequate supplies of continuously improving therapies. The application of evidence-based medicine has enhanced the basis of hemophilia therapy, while resulting in some challenges to patient care. Increasingly, the criteria used for the approval and payment of treatment products by regulatory and reimbursement agencies, respectively, are becoming inflexible and unrealistic. This is occurring particularly in the requirements for demonstrating product efficacy. Concurrently, emerging evidence of the interpatient variability in the clinical response to therapy has led to the proposed personalization of therapeutic regimens. Possible impediments to optimal care include competitive tensions among suppliers who seek to gain label claims for reimbursement purposes, which result in clinical trial designs of, arguably, unethical design, carried out in poor countries. We synthesize these converging developments to suggest some changes to the current hemophilia treatment paradigm, which should make it more patient-centric and enable speedier access to new therapies.
Keywords: hemophilia, treatment products, clinical trials, standards, reimbursement
Unresolved (and irresolvable) issues in hemophilia therapy access
Fifty years of development and progress have led to people with hemophilia having access to potentially unlimited supplies of therapeutic products, in the form of coagulation factor concentrates (CFCs) safe from historical hazards. Despite this, emerging evidence of subclinical bleeding with current prophylactic regimens indicates, arguably, that all patients are undertreated.1 The conventional classification of hemophilia is also under question, with evidence that levels of 15%–20% are needed to protect against all bleeds.2 Acquiring this level of protection requires access to more CFCs until the distant promise of a cure attained through gene therapy.3 The first era of treatment products limited access to Factor VIII for hemophilia A in particular, due to the difficulty in extracting Factor VIII from limited plasma supplies. This resulted in chronic insufficiency and undertreatment in all environments. The current era in the established economies is dominated by the provision of recombinant products. These promise to eliminate the access problem, as their supply is, hypothetically, unlimited as enhancements in the technology are applied.4 The issues pertaining to hemophilic therapeutic access currently include:
- Treatment costs
- Approval processes for authorization and reimbursement
- Treatment hazards.
These intertwined issues, and the continuing pressures on health care costs, are influencing the current paradigms for these areas. The traditional delineation between market authorization and reimbursement agencies is under pressure in the European Union (EU) through processes seeking to establish common paths for the approval and funding of products.5 This has succeeded an ongoing body of literature assessing the pharmacoeconomics of treatment choices, including the treatment of hazards such as inhibitors to CFCs.6 Concurrently, the market approval processes for CFCs have been more closely aligned to the traditional assessment methods for medicines. We will review these developments and attempt to synthesize proposals for the continued progression of access to care for hemophilia.
The evidence-based medicine era and hemophilia
Over the past 20 years, a paradigm of evidence-based medicine (EBM) has become established in the approval and oversight of medical interventions, primarily through a recognition, through the work of Archie Cochrane, that the adoption of therapeutic interventions was historically detached from evidence of their efficacy.7 A key feature of EBM has been the acceptance of a hierarchy of measures contributing to evidence, viewed as a pyramid and headed by the randomized clinical trial (RCT). RCTs had been developed, primarily through the work of Hill8 in previous decades, but it was only following the inception of EBM that they became the yardstick/standard for the assessment of efficacy and safety.
Product authorization agencies initially charged with ensuring the safety of therapies rapidly absorbed the EBM paradigm as their remit was widened to include the assessment of efficacy and therapeutic claims. In some landscapes, particularly the EU, the regulatory oversight of blood and plasma products was excluded from the mainstream framework until relatively recently. As governments in the social-market economies have detached from the direct ownership of plasma fractionation activity, the relevant marketing authorization (MA) agencies have assumed higher levels of oversight over their activities, including the assessment of efficacy. In this regard, MAs such as the US Food and Drug Administration (FDA) and the EU’s Medicines Agency (EMA) have attempted to implement the tenets of EBM. Recognizing the limitations imposed by a small population of patients in the rare blood disorder congenital fibrinogen deficiency, the FDA granted approval for a treatment product based on a study on 15 patients in which clinical efficacy was assessed through a surrogate endpoint.9 Similar flexibility was shown by the EMA in approving activated Factor VII for the treatment of Glanzmann’s thrombasthenia through various overlapping inputs of a patient population of less than 10.10
This has been tempered by overzealous assessments from the Cochrane Collaboration, which did not endorse prophylaxis as a preferred modality to treatment on demand for hemophilia11 until demonstrated through a RCT.12 This also demonstrated progressive joint disease in the control population, a regrettable outcome given the 30 years of experience of the benefits of prophylaxis and one confirmed by follow-up of the patient population.13 The continued use of these randomized studies is leading to undertreatment of patients and permanent injury14 as patients are randomized into on-demand treatment with all its consequences. We will discuss this issue in detail.
From evidence-based to individualized medicine – the position of hemophilia
EBM may be viewed as the predominant Kuhnian paradigm15 in therapeutics. As in all such paradigms, tensions are evident, which may lead to its replacement. It is dubious if the originators of the EBM hierarchy envisaged the requirement for RCTs as the yardstick by which efficacy would be established. This traditional paradigm provides a comfort zone for regulators, while evident tensions arise, such as the issues around the removal and subsequent restoration of the MA of aprotinin for minimizing blood loss during cardiac surgery.16,17 The brisk controversy around this issue shows that the interpretation of RCT data is still as subjective as any other human endeavor. The recent Boldt scandal18 in which clinical trials used in MA and reimbursement process were retracted from the literature because of fraudulent practices demonstrates the vulnerability of the paradigm. Depending on which version of the EBM pyramid is examined, systematic reviews and their quantification through meta-analyses are often pivotal in guiding practice, including therapeutic choices in hemophilia, and in MA procedures.19 They also influence hypotheses around which RCTs are constructed to answer a specific question, eg, “Does prophylaxis produce better long-term outcomes in hemophilia patients than on-demand therapy?” As mentioned earlier, the particular Cochrane Review on this issue continued to assert that this was an open question until the late 2000s, against a background of 40 years of clinical experience. This type of Cochrane Question also seems counter to traditional health principles that “Prevention (prophylaxis) is better than cure (on-demand therapy)”. The discrepancies between hypothesis-generating meta-analyses and subsequent RCTs20 also contribute to reservations about the conventional EBM hierarchy.
In many RCTs/meta-analyses addressing important questions in hemophilia treatment, a key assumption has been equivalence in the treatment effects of different CFCs and homogeneity in the treated population. The first assumption is viewed with reservation on the basis that biological medicines such as CFCs cannot be considered as generic or biosimilar.21 Some investigators have addressed the issue by stratifying patients according to the type of CFC administered,22 demonstrating heterogeneity in treatment effects analyzed a posteriori which may be a reflection of differences between products. In addition, differences in the pharmacokinetic profiles of infused CFCs suggest that homogeneity in the patient population cannot be assumed.23,24 Overall, the limitation of RCTs to providing information only about averages has fuelled the thrust toward incorporating the assessment of efficacy into a new paradigm of personalized medicine for hemophilia.25 A striking example of the application of personalized prophylaxis with the achievement of improved outcomes at lower costs shows the potential of this approach.26
A convergence between the EBM paradigm of clinical trials and personalized medicine may be possible in the product authorization area through the use of N – of – 1 trials. N – of – 1 trials are considered to provide the strongest level of evidence about the existence of a causal relationship between a treatment and an outcome.27 The N – of – 1 trial is an RCT carried out in one patient, who undergoes pairs of treatment periods where one period includes the experimental treatment under investigation and the other period includes a placebo/comparator treatment. The treatment periods are randomly assigned for each cycle, eg, Pair 1, Placebo, Treatment; Pair 2, Treatment, Placebo; Pair 3, Treatment, Placebo.
Kravitz et al28 have estimated a hypothetical 33% enhancement in therapeutic precision when using N – of – 1 trials versus the conventional clinical trial structure. In an Australian study, significant cost-savings relative to conventional designs were reported.29 A similar outcome has been estimated by Kravitz et al30 in the United States. Given the variability in pharmacokinetic23,31 and bleeding32 profiles in hemophilia, a similar analysis would be relevant for the possible use of N – of – 1 trials in this and other rare bleeding disorders. It would contribute to the growing literature on the pharmacoeconomics of hemophilia and widen the perspective to include personalized treatment, as we shall discuss further. The criteria for eligibility for N – of – 1 trials make them especially suited for rare, chronic conditions33 such as hemophilia. In practical terms, trials for CFCs include basic pharmacokinetic measurements and comparison to approved products. Trials also include assessment of efficacy, generally through a clinical endpoint such as joint bleeds. We suggest that both these parameters may be studied through the basic type of N – of – 1 design described earlier. It should be noted that crossover trial designs, of which N – of – 1 trials are an example, have been applied to hemophilia investigations in a number of settings.34–36 The ability to combine N – of – 1 trials to estimate population treatment effects37 demonstrates that they can also constitute a tool for evidence-based clinical guidelines as with conventional RCTs and meta-analyses, while influencing such guidelines through a patient-centric and individualized approach. In the current era of patient-centeredness and comparative effectiveness research, N – of – 1 trials are proposed as important parts of the methodological armamentarium, facilitating individualized care and improving patient outcomes.38 The new era of personalized medicine stands to supplant the old EBM paradigm, and although some consider a synthesis of EBM and PM to be possible and natural,39 we concur with De Leon’s view40 that the differences between these approaches make them incommensurable.15
The FDA41 and the EMA42 have committed to considering N – of – 1 trials and other approaches for assessing health technologies for rare chronic disorders43 but at least one peer health technology assessment (HTA) body in Europe has expressed reservations regarding their applicability.44 Hence, tensions are evident between the MA and HTA bodies, which we will now review.
EBM and costs
The fathers of EBM have stated that “practicing evidence based medicine will identify and apply the most efficacious interventions to maximise the quality and quantity of life for individual patients; this may raise rather than lower the cost of their care”.45 Despite this, it is difficult not to conclude that, over the past decades of its dominance, the EBM paradigm has been absorbed as a major tool for cost-containment in health reimbursement systems. A major shaper of this development has been the Cochrane Collaboration, which has included in its goals “Making Cochrane the home of evidence to enable informed decision making”.46 The authors’ conclusions in Cochrane Reviews of interventions for rare bleeding disorders47–49 are peppered with references to cost issues and the need to demonstrate cost-effectiveness.
Concurrently, the establishment of RCTs as the yardstick for MA for specific indications has increased the cost of drug development, and the unfortunate withdrawal of most governments from the area of clinical trials has shifted the costs entirely to industry. This has generated a huge global industry for the production of trials. One necessary feature for carrying out such trials is the free provision of the drugs under investigation, as well as any added comparator drugs, to the trial subjects. Some funding agencies have noted how this leads to considerable savings to the health systems involved.50,51 We ponder on the necessary conflict of interest, which must arise as MAs recognize the implications of their measures on the provision of free medicines in the health systems they serve, noting that as of February 2015, 260 studies were registered for investigations on hemophilia.52
MAs and/versus HTA bodies – ongoing processes in the EU
This gradual convergence between the historically distinct regulatory and reimbursement components of pharmaceutical provision is particularly discernible in the ongoing process of developing common evaluation pathways for the approval and HTA processes in the EU.53 This convergence is being pursued solely in the area of clinical efficacy. The various HTA bodies established to advise government payers in Europe have different approaches to assessing effectiveness. This is a broader and more pragmatic concept than efficacy as measured by an RCT,54 the methodology favored by MAs. An important divide involves the way in which health interventions are prioritized. Agencies such as the UK National Institute for Health and Clinical Excellence and the Australian Pharmaceutical Benefits Advisory Committee use the Quality-Adjusted Life Year as a metric to allow all interventions to be directly related through a preestablished threshold for cost-effectiveness and reimbursement.55 The German Institute for Quality and Efficiency in Health Care (Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen – IQWIG), on the other hand, uses an efficiency frontier approach that compares all possible interventions for one specific indication, divorced from any prioritization with other issues. The IQWIG’s choice of methodology is based on the agency’s position that prioritization is a political, not a scientific choice.56 The EU HTA bodies’ attempt at convergence has resulted in a HTA Core Model,57 still unavailable publicly, which is informed through a detailed questionnaire supplied to product sponsors.58 It is difficult to discern the basis of a harmonized approach to HTA evaluation through this document, particularly in the crucial areas around assessing therapeutic benefits and effectiveness measures. It has been noted that HTA bodies employ a wider range of inputs in their evaluation than a narrow efficacy assessment as used by most MAs,59 but a wide body of convergence is evident, with bodies like IQWIG giving primacy to the RCT as the first level of evidence.44 Concurrently, the use of health-related quality of life measures utilized by HTA bodies is also the subject of regulatory guidance.60 This poses the question as to why MAs do not widen their assessments to include such measures, as recent policy documents suggest they are committed to do.41,42 It would seem more logical for HTA bodies to assess the extent to which products should be reimbursed, through a synthesis of cost and effectiveness, using the same effectiveness measures that resulted in the approval, by MAs, of a MA application. While some merit exists for a parallel track in order to abbreviate the overall market access process, the most time-consuming phase is the drug development process. The subsequent approval process for efficacy and effectiveness should not be hindered through bureaucratic inefficiency, as is clearly the case with some agencies experiencing considerably longer approval times than others with comparable resources.61
The impact on hemophilia CFCs
Two recent case studies in the EU demonstrate the potential effect of HTA processes on the position of CFCs in national markets. In the first case, a systematic review of available evidence of most aspects of hemophilia care was performed to inform the Swedish Council on HTA.62 An expert group of eminent Swedish treaters concluded that, for all 12 clinical areas reviewed, the scientific evidence to determine practice was lacking and relevant studies were mostly absent. The group employed a mainstream EBM paradigm in selecting studies for the review, according a primacy to RCTs and a consideration to prospective controlled studies. Nonrandomized studies were only considered if the other kinds were not available. The Swedish HTA was carried out but the results of the analysis were not made public, while Sweden’s generous provision of treatment for hemophilia continues.63 The price of recombinant Factor VIII, the dominant modality for hemophilia care in Sweden, has been stable over the past 3 years, while government reimbursement agencies in similar social market health systems have developed mechanisms for significant reductions in price.64 In the second case, IQWIG in Germany has been commissioned by the Federal Ministry of Health to produce a Rapid Report on the treatment of hemophilia patients.65 The process for the generation of such a report is described on page 17 of General Methods 4.1.44 and may include input from patients. It must be presumed that the EBM hierarchy favored by IQWIG will influence this product. The Report will inform the process whereby reimbursement of CFCs through insurance occurs in Germany. In this process, a maximum reimbursable price requested for new novel/innovative products is granted only if IQWIG’s assessment agrees that the product provides an added benefit relative to currently reimbursed CFCs as described on page 142 of General Methods 4.1.44 As of February 2015, this process has been tested with two CFCs for hemophilia. The sponsor of the recombinant Factor VIII Turoctocog alfa claimed added benefits in the form of additional safety from infectious agents and enhanced provision and convenience to patients through validated storage at room temperature. These claims were not accepted by the IQWIG and the request for a maximum price on these bases was rejected by the Gemeinsamer Bundesausschuss (G-B) (German Federal Committee),66 which is the actual decision maker in the German Government. Scrutiny of the G-B’s reasons reveals that, acting on the IWIG’s advice, this body considers all Factor VIII CFCs to be equivalent in safety and efficacy, and therefore similarly appropriate to act as comparators to Turoctocog alfa.67 The G-B also did not consider the sponsor’s representation that the inclusion of an additional product, reimbursable at the maximum price, would improve the security of supply, on the basis that this did not constitute a therapeutic advantage as required by law. In the recently announced decision regarding Simoctocog alfa, a recombinant Factor VIII differentiated through generation in human cell lines, IQWIG also turned in a negative assessment,68 asserting that the sponsor had only performed single-dose studies, over a relatively short time frame in a crossover design. This decision does not augur well for IQWIG’s consideration of possible N – of – 1 trials as these would be similarly configured. This decision by IQWIG appears to consider that the MA for the agent did not cover effectiveness adequately, another example of where HTA and MA bodies diverge on critical issues. Analysis of this decision is important for the future of access to hemophilia care in Germany, Europe’s largest market for CFCs. The price of products in Germany is still relatively high compared to that obtained in centralized, competitive tender processes, as may be seen on page 7 of the study by G-B,66 while traditionally, prescribers are free to treat patients with their product of choice. Strong lobbying by industry directly to prescribers ensures high visibility of products, including the new generation of innovative, value-adding CFCs. Furthermore, the high reference CFC reimbursement price, even at the maximum limit allowed by the insurance process, has a strong influence in those countries that reference Germany for their drug prices.69,70 Hence, events in Germany are influential on the hemophilia community within and outside its borders. The IQWIG’s criteria for added benefit leading to extra reimbursement will be scrutinized strongly as other cases, offering more discernible benefits than were apparent with the first CFC submissions, are subjected to the process. In particular, the outcomes for innovative products such as CFCs modified to result in longer half-lives will be assessed with great interest to see whether outcomes recorded primarily through pharmacokinetics will be accepted as additionally beneficial by IQWIG. An appropriate balance between cost-containment and the reward of clinically beneficial innovation is desirable. We note that, while IQWIG’s dismissal of the arguments around Turoctocog alfa’s benefits regarding safety and convenience may be valid, their apparent requirement for direct comparison with a comparator – ie, trials – is troubling, given IQWIG’s preference for RCTs. We consider the use of historical controls in lieu of subjects in control arms to be acceptable, as we shall discuss further.
A question of comparison
The attention of the approval process for CFCs is currently focused on new, innovative products manufactured by biotechnology. Over the past few years, several of these products have been developed and studies of their use in patients, subsequently used to acquire market authorization and access, have been published (Table 1).
  | Table 1 Some recent studies on novel clotting factor concentrates |
The requirements of mainstream EBM and other aspects required by HTA bodies such as Health Related Quality of Life are visible in several of these studies. In particular, the establishment of a therapeutic claim for prophylaxis has been justified, in some trials, by randomizing patients to either prophylaxis or on-demand treatment with the CFC under investigation. This approach merits scrutiny. It appears that the sponsoring manufacturers in these trials are seeking a label therapeutic claim, specifically, for prophylaxis. We would propose that, in the context of an RCT for prophylaxis versus on-demand therapy, on-demand therapy has the status of a placebo. Clearly, the question of the effectiveness of prophylaxis, or any aspect of aspect of CFC replacement therapy, cannot be assessed with a true placebo, as this would constitute an example of the parachute scenario.84 The assignment of on-demand treatment as a control is not as drastic, but we would pose the following:
- A scrutiny of Table 1 indicates that not all applications approved by the FDA included randomized trials. At least one application was approved with historical controls. We note that this was NovoEight (Turoctocog alfa), which was subsequently approved by the EMA with the same data but rejected by the G-B on the basis, among other reasons, that there were no comparative data with an acceptable comparator.
- The efficacy and superiority of prophylaxis versus on-demand therapy had been established for several decades before it was demonstrated, to the satisfaction of the Cochrane Collaboration, with a RCT in children – the Joint Outcome Study.12 This study showed increased and progressive13 joint deterioration in children who bleed more frequently due to being randomized to the on-demand arm. The same investigators are now conducting a similar study – the SPINART trial – in adults.85 The first year’s results of a 3-year randomization shows that on-demand treatment is highly inferior to prophylaxis, begging the question as to why this trial is not discontinued on ethical grounds. All trials including the SPINART trial continue to confirm the benefit of prophylaxis under all clinical circumstances.
- The basis for any RCT is the existence of equipoise, defined as “a state of genuine uncertainty about the value of a treatment course within the expert medical community”.86 In this instance, against the background of evidence from the published RCTs,12,74,87 the question arises “Is there genuine uncertainty in the minds of the clinical investigators performing RCTs on new CFCs that these will be inferior in preventing bleeds when they are shown to be effective in treating bleeds?” we would contend that this is not the case. Although it is a regulatory/industry mantra that bioequivalence cannot be presumed for CFCs, there is no evidence, and no biological–pathological hypothesis, that can give rise to such uncertainty.
- In an instance where a trial for an antiplatelet antithrombotic was placed on hold by the FDA because of concerns about the choice of placebo as comparator,88 the agency did not accept concerns about the relatively high cost of a therapeutically similar comparator as justification for a placebo-controlled trial. Subsequently, the trial design was pursued by the investigators who persuaded the FDA to allow a placebo-controlled design, only to discontinue enrollment after the committee overseeing the trial found a 40% reduction in treatment versus placebo effects.89 This indicates that it may be investigators, and companies sponsoring the trials, who are influencing trial design.
- We note that the father of equipoise, Benjamin Freedman proposes that placebo controls may be justified in a number of circumstances, including when validated optimal treatments are not available to the treatment population because of cost-constraints.90 Freedman hastens to add that this principle may only be applied when background conditions of justice exist within the health system where the trial is occurring and that when the system does not establish entitlement to even a minimum care level, the principle must not be used to justify the use of placebos on the poor.
- This brings us back to the case of new CFCs. Scrutiny of the studies in Table 1, and others, which have employed a RCT design to support a prophylaxis claim have included investigators and patient populations drawn from underprivileged countries where the level of hemophilia care is minimal. While it is not possible to assess which patients were used for the specific prophylaxis versus on-demand comparison, it is valid to assume that in most of the resourced environments this would be refuted on ethical grounds. We note the requirements for biomedical research in human subjects which are encapsulated in the Declaration of Helsinki.91 This Declaration is unfortunately no longer supported by the FDA for trials carried outside the United States,92 on the basis that adherence to the International Conference Guideline of Good Clinical Practice.93 We would suggest that the International Conference on Harmonisation (ICH) Good Clinical Practice Guideline is not an ethical code but a procedural regulatory manual based on a synthesis of the regulatory frameworks of the United States, Japan, and Europe, the major pharmaceutical markets. It does not include any of the Declaration of Helsinki’s adjurations regarding the protection of subjects from unnecessary allocation to placebos. The Declaration’s intent is evident. We have scrutinized the requirements of both MAs and HTA bodies, and we see no indication that, even in the event of a prophylaxis versus on-demand therapy RCT being demanded, such a trial cannot be done with two prophylaxis arms, one for the investigative treatment and one for an approved comparator. It is clear, for example, that the IQWIG’s current posture would allow a comparator to be used from all the hemophilia CFCs approved in Germany. As required by the Declaration of Helsinki, any subjects on these clinical trials, including those in resource-poor countries, are to be kept on the treatment after the trial has ended, an issue of clear significance when comparator products are used.
Summary and conclusion
The processes for the approval to market and provide access for medicines, including CFCs for hemophilia, have been traditionally distinct and separate. Over the past decade, the continuing pressures on health care budgets, irrespective of their source, have contributed toward an ongoing convergence for some of these processes. Concurrently, the implantation of the EBM paradigm for both these areas has revealed inadequacies which impede access to care and increased treatment costs. The increasingly diverse and competitive landscape of CFC products is leading to clinical trials that exhibit some doubts regarding their ethical basis. We propose that MA should continue to be detached from the process of reimbursement. The criteria for approval of both authorization and reimbursement should be wider than the conventional RCT framework. They should include the assessment of effectiveness as well as efficacy, in individual patients through mechanisms such as N – of – 1 trials. Such mechanisms, when properly conducted, promise to actually enhance the evidence base of CFC therapies, relative to current requirements for patient numbers, which are arbitrarily specified and have caused mounting treater concern.94 We support the use of patient-centered outcomes by HTA agencies when they are synthesizing recommendations regarding the added benefit coming from the plethora of new products. Such outcomes should include measures of health-related quality of life, which may need to be more nuanced than the instruments used currently by these agencies. All bodies involved in the delivery of CFC treatments should embed ethical principles in their assessments, which negate harm to patients, and these should be transparent to manufacturers seeking a therapeutic claim and to investigators responsible for clinical trials. These concepts, synthesized in Figure 1, should optimize patient and societal outcomes in a compassionate and efficient health care system.
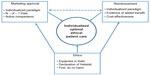  | Figure 1 Patient-centric delivery of hemophilia treatment products. |
Disclosure
Albert Farrugia provides contractual services to the manufacturers of therapies mentioned in this work. The authors report no other conflicts of interest in this work.
References
Olivieri M, Kurnik K, Pfluger T, Bidlingmaier C. Identification and long-term observation of early joint damage by magnetic resonance imaging in clinically asymptomatic joints in patients with haemophilia A or B despite prophylaxis. Haemophilia. 2012;18(3):369–374. | |
Den Uijl I, Fischer K, Van Der Bom JG, Grobbee DE, Rosendaal FR, Plug I. Analysis of low frequency bleeding data: the association of joint bleeds according to baseline FVIII activity levels. Haemophilia. 2011;17(1):41–44. | |
Rogers GL, Herzog RW. Gene therapy for hemophilia. Front Biosci (Landmark Ed). 2015;20:556–603. | |
Spencer HT, Denning G, Gautney RE, et al. Lentiviral vector platform for production of bioengineered recombinant coagulation factor VIII. Mol Ther. 2011;19(2):302–309. | |
Elvidge S. EMA’s parallel advice workshop bridges regulatory and reimbursement divide. Nat Rev Drug Discov. 2013;13(1):8–8. | |
Baghaipour MR, Steen Carlsson K. Strategies for inhibitor treatment and costs in the short and long term: a critical evaluation of recent clinical studies. Eur J Haematol. 2015;94(Suppl 77):30–37. | |
Cochrane AL. Effectiveness and Efficiency: Random Reflections on Health Services [Internet]. Nuffield Provincial Hospitals Trust; 1972 [cited April 16, 2015]. Available from: http://www.nuffieldtrust.org.uk/sites/files/nuffield/publication/Effectiveness_and_Efficiency.pdf. Accessed May 25, 2015. | |
Hill AB. The environment and disease: association or causation? Proc R Soc Med. 1965;58(5):295–300. | |
Food and Drug Administration. Summary Basis for Regulatory Action – Treatment of Acute Bleeding in Patients with Congenital Fibrinogen Deficiency, Afibrinogenemia and Hypofibrinogenemia [Internet]; 2009 [cited February 23, 2015]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM244507.pdf. Accessed May 25, 2015. | |
European Medicines Agency. Scientific Discussion – Novoseven European Public Assessment Report [Internet]; 2005 [cited February 4, 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000074/WC500030713.pdf. Accessed May 25, 2015. | |
Aledort L, Ljung R, Blanchette V. On Behalf of the International Prophylaxis Study Group (IPSG). Are randomized clinical trials the only truth? Not always. J Thromb Haemost. 2006;4(3):503–504. | |
Manco-Johnson MJ, Abshire TC, Shapiro AD, et al. Prophylaxis versus episodic treatment to prevent joint disease in boys with severe hemophilia. N Engl J Med. 2007;357(6):535–544. | |
Manco-Johnson ML, Blades TA, Funk S, et al. Long-term orthopedic effects of delaying prophylaxis in severe hemophilia a until age 6 years: results of the joint outcome study continuation (JOSc). Blood. 2013;122(21):210–210. | |
US FDA. Statistical Review and Evaluation – Recombinant Factor IX [Internet]; 2012 [cited February 6, 2014]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM363188.pdf. Accessed May 25, 2015. | |
Solomon M. Just a Paradigm: Evidence-Based Medicine in Epistemological Context; 2009 [cited November 18, 2013]. Available from: http://philsci-archive.pitt.edu/4793/. Accessed May 25, 2015. | |
McMullan V, Alston RP. III. Aprotinin and cardiac surgery: a sorry tale of evidence misused. Br J Anaesth. 2013;110(5):675–678. | |
Hébert PC, Fergusson DA, Hutton B, et al. Regulatory decisions pertaining to aprotinin may be putting patients at risk. Can Med Assoc J. 2014;186(18):1379–1386. | |
Wise J. Boldt: the great pretender. BMJ. 2013;346(mar19 1):f1738–f1738. | |
European Medicines Agency Committee for Proprietary Medicinal Products. Points to Consider upon Application with (1) Meta-analysis (2) One Pivotal Study [Internet]; 2011 [cited February 6, 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003657.pdf. Accessed May 25, 2015. | |
Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J Clin Epidemiol. 2008;61(8):763–769. | |
Brougher JT. The Biosimilars Act: promoting or discouraging the development of generic biologics? Biotechnol Healthc. 2010;7(4):22–23. | |
Franchini M, Lippi G. Immune tolerance induction for patients with severe hemophilia A: a critical literature review. J Thromb Thrombolysis. 2011;32(4):439–447. | |
Collins PW, Fischer K, Morfini M, Blanchette VS, Björkman S; On Behalf of International Prophylaxis Study Group (IPSG) Pharmacokinetics Expert Working Group. Implications of coagulation factor VIII and IX pharmacokinetics in the prophylactic treatment of haemophilia. Haemophilia. 2011;17(1):2–10. | |
Collins PW, Björkman S, Fischer K, et al. Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe hemophilia A: influences of variance in pharmacokinetics and treatment regimens. J Thromb Haemost. 2010;8(2):269–275. | |
Collins PW. Personalized prophylaxis. Haemophilia. 2012;18: 131–135. | |
Schlenkrich S, Schubert C. Secondary prophylaxis in an adult patient with severe haemophilia A. Factor VIII consumption and effectiveness. Hamostaseologie. 2013;33(3):241–244. | |
Guyatt GH, Heyting A, Jaeschke R, Keller J, Adachi JD, Roberts RS. N of 1 randomized trials for investigating new drugs. Control Clin Trials. 1990;11(2):88–100. | |
Kravitz RL, Duan N, Niedzinski EJ, Hay MC, Subramanian SK, Weisner TS. Whatever happened to N-of-1 trials? Insiders’ perspectives and a look to the future. Milbank Q. 2008;86(4):533–555. | |
Scuffham PA, Nikles J, Mitchell GK, et al. Using N-of-1 trials to improve patient management and save costs. J Gen Intern Med. 2010; 25(9):906–913. | |
Kravitz RL, Duan N, White RH. N-of-1 trials of expensive biological therapies. Arch Intern Med. 2008;168(10):1030–1033. | |
Björkman S, Folkesson A, Berntorp E. In vivo recovery of factor VIII and factor IX: intra- and interindividual variance in a clinical setting. Haemophilia. 2007;13(1):2–8. | |
Den Uijl IE, Mauser Bunschoten EP, Roosendaal G, et al. Clinical severity of haemophilia A: does the classification of the 1950s still stand? Haemophilia. 2011;17(6):849–853. | |
Kravitz RL, Duan N. Design and Implementation of N-of-1 Trials: A User’s Guide [Internet]. Agency for Healthcare Research and Quality; 2014. Available from: http://effectivehealthcare.ahrq.gov/ehc/products/534/1844/n-1-trials-report-130213.pdf. Accessed May 25, 2015. | |
Lindvall K, Astermark J, Björkman S, et al. Daily dosing prophylaxis for haemophilia: a randomized crossover pilot study evaluating feasibility and efficacy. Haemophilia. 2012;18(6):855–859. | |
Alamelu J, Bevan D, Sorensen B, Rangarajan S. Pharmacokinetic and pharmacodynamic properties of plasma-derived vs recombinant factor IX in patients with hemophilia B: a prospective crossover study. J Thromb Haemost. 2014;12(12):2044–2048. | |
Collins P, Faradji A, Morfini M, Enriquez MM, Schwartz L. Efficacy and safety of secondary prophylactic vs on-demand sucrose-formulated recombinant factor VIII treatment in adults with severe hemophilia A: results from a 13-month crossover study. J Thromb Haemost. 2010;8(1):83–89. | |
Zucker DR, Schmid CH, McIntosh MW, D’Agostino RB, Selker HP, Lau J. Combining single patient (N-of-1) trials to estimate population treatment effects and to evaluate individual patient responses to treatment. J Clin Epidemiol. 1997;50(4):401–410. | |
Duan N, Kravitz RL, Schmid CH. Single-patient (n-of-1) trials: a pragmatic clinical decision methodology for patient-centered comparative effectiveness research. J Clin Epidemiol. 2013;66(8 0):S21–S28. | |
Kumar D. The personalised medicine. A paradigm of evidence-based medicine. Ann Ist Super Sanita. 2011;47(1):31–40. | |
De Leon J. Evidence-based medicine versus personalized medicine: are they enemies? J Clin Psychopharmacol. 2012;32(2):153–164. | |
Food and Drug Administration. Guidance for Industry Adaptive Design Clinical Trials for Drugs and Biologics [Internet]; 2010 [cited February 24, 2015]. Available from: http://www.fda.gov/downloads/Drugs/Guidances/ucm201790.pdf. Accessed May 25, 2015. | |
European Medicines Agency. Guideline on Clinical Trials in Small Populations [Internet]; 2005 [cited February 24, 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2009/09/WC500003615.pdf. Accessed May 25, 2015. | |
Facey K, Granados A, Guyatt G, et al. Generating health technology assessment evidence for rare diseases. Int J Technol Assess Health Care. 2014;30(4):416–422. | |
Institute for Quality and Efficiency in Health Care. General Methods 4.1 [Internet]; 2013 [cited February 24, 2015]. Available from: https://www.iqwig.de/download/IQWiG_General_Methods_Version_%204-1.pdf. Accessed May 25, 2015. | |
Sackett DL, Rosenberg WMC, Gray JAM, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996; 312(7023):71–72. | |
Cochrane Collaboration. Our Strategy [Internet]; 2015 [cited February 11, 2015]. Available from: http://www.cochrane.org/about-us/our-strategy. Accessed May 25, 2015. | |
Coppola A, Windyga J, Tufano A, Yeung C, Di Minno MND. Treatment for preventing bleeding in people with haemophilia or other congenital bleeding disorders undergoing surgery. In: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley and Sons, Ltd; 2015 [cited February 10, 2015]. Available from: http://www.cochrane.org/CD009961/CF_preventing-bleeding-in-people-with-congenital-bleeding-disorders-during-and-after-surgery. Accessed May 25, 2015. | |
Iorio A, Marchesini E, Marcucci M, Stobart K, Chan AK. Clotting factor concentrates given to prevent bleeding and bleeding-related complications in people with hemophilia A or B. In: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley and Sons, Ltd; 2011 [cited February 10, 2015]. Available from: http://www.cochrane.org/CD003429/CF_regular-clotting-factor-replacement-therapy-to-prevent-joint-disease-in-people-with-severe-hemophilia-a-or-b. Accessed May 25, 2015. | |
Gøtzsche PC, Johansen HK. Intravenous alpha-1 antitrypsin augmentation therapy for treating patients with alpha-1 antitrypsin deficiency and lung disease. In: The Cochrane Collaboration, editor. Cochrane Database of Systematic Reviews [Internet]. Chichester, UK: John Wiley and Sons, Ltd; 2010 [cited February 10, 2015]. Available from: http://www.cochrane.org/CD007851/AIRWAYS_intravenous-alpha-1-antitrypsin-augmentation-therapy-for-treating-patients-with-alpha-1-antitrypsin-deficiency-and-lung-disease. Accessed May 25, 2015. | |
Shen L-J, Chou H, Huang C-F, Chou G-M, Chan WK, Wu F-LL. Economic benefits of sponsored clinical trials on pharmaceutical expenditures at a medical center in Taiwan. Contemp Clin Trials. 2011;32(4):485–491. | |
Grossi F, Genova C, Gaitan ND, et al. Free drugs in clinical trials and their potential cost saving impact on the National Health Service: a retrospective cost analysis in Italy. Lung Cancer. 2013;81(2):236–240. | |
US National Institutes of Health. Search for Factor VIII AND Hemophilia [Internet]; 2015 [cited February 25, 2015]. Available from: https://clinicaltrials.gov/ct2/results?term=Factor+VIII+AND+hemophilia&Search=Search. Accessed May 25, 2015. | |
European Medicines Agency. EMA-HTA Workshop Bringing Together Stakeholders for Early Dialogue in Medicines Development – Report from the Public Workshop Hosted by the European Medicines Agency (EMA) in London on 26 November 2013 [Internet]; 2014 [cited February 13, 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Report/2014/05/WC500166228.pdf. Accessed May 25, 2015. | |
Singal AG, Higgins PDR, Waljee AK. A primer on effectiveness and efficacy trials. Clin Transl Gastroenterol. 2014;5(1):e45. | |
Whitehead SJ, Ali S. Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. 2010;96(1):5–21. | |
Caro JJ, Nord E, Siebert U, et al. The efficiency frontier approach to economic evaluation of health-care interventions. Health Econ. 2010;19(10):1117–1127. | |
European Network of HTA Agencies. HTA Core Model [Internet]; 2014 [cited February 13, 2015]. Available from: http://meka.thl.fi/htacore/model/AE-tables-pharma-2.0.pdf. Accessed May 25, 2015. | |
Finnish Office for Health Technology Assessment. Assessment Element Tables for HTA Core Model Application for Diagnostic Technologies [Internet]; 2014 [cited February 25, 2015]. Available from: http://meka.thl.fi/htacore/model/AE-tables-diagnostics-2.0.pdf. Accessed May 25, 2015. | |
Barnett D. The HTA View [Internet]. EMA-HTA Workshop Parallel Advice in Scientific Drug Development, London UK; 2013 [cited February 25, 2015]. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2013/11/WC500155669.pdf. Accessed May 25, 2015. | |
European Medicines Agency. Reflection Paper on the Regulatory Guidance For the Use of Health related Quality of Life (HRQL) Measures in the Evaluation of Medicinal Products [Internet]; 2005 [cited February 25, 2015]. Available from: http://www.ispor.org/workpaper/emea-hrql-guidance.pdf. Accessed May 25, 2015. | |
Centre for Innovation in Regulatory Science, Ltd. New Drug Approvals in ICH Countries 2003–20 [Internet]; 2013 [cited February 25, 2015]. Available from: http://www.cirsci.org/sites/default/files/CIRS_R&D_Briefing_52_May_2013.pdf. Accessed May 25, 2015. | |
Berntorp E, Astermark J, Baghaei F, et al. Treatment of haemophilia A and B and von Willebrand’s disease: summary and conclusions of a systematic review as part of a Swedish health-technology assessment. Haemophilia. 2012;18(2):158–165. | |
O’Mahony B, Noone D, Giangrande PLF, Prihodova L. Haemophilia care in Europe: a survey of 19 countries. Haemophilia. 2011;17(1):35–40. | |
Farrugia A, Hermans C, Franchini M. Assessing options for treating haemophilia with inhibitors. Haemophilia. 2015. | |
IQWIG. [A13-07] Treatment of Haemophilia Patients – Rapid Report [Internet]; 2012 [cited February 25, 2015]. Available from: https://www.iqwig.de/en/projects-results/projects/drug-assessment/a13-07-treatment-of-haemophilia-patients-rapid-report.3253.html#overview. Accessed May 25, 2015. | |
Gemeinsamer Bundesausschuss. Beschluss des Gemeinsamen Bundesausschusses über eine Änderung der Arzneimittel-Richtlinie (AM-RL): Anlage XII – Beschlüsse über die Nutzenbewertung von Arzneimitteln mit neuen Wirkstoffen nach § 35a SGB V – Turoctocog alfa (Decision [Federal Joint Committee] of the Federal Joint Committee amending the Prescription Drug Guidelines (AM-RL): Appendix XII – Decisions on the benefit assessment of drugs with new active substances in accordance with § 35a SGB V [Social Security Code V] – Turoctocog Alfa) [Internet]; 2014 [cited February 25, 2015]. Available from: https://www.g-ba.de/downloads/39-261-2026/2014-07-03_AM-RL-XII_Turoctocog%20alfa_2014-01-15-D-092_BAnz.pdf. Accessed May 25, 2015. | |
Gemeinsamer Bundesausschuss. Tragende Gründe 1 zum Beschlussentwurf des Gemeinsamen Bundesausschusses über eine änderung der Arzneimittel-Richtlinie (AM-RL): Anlage XII – Beschlüsse über die Nutzenbewertung von Arzneimitteln mit neuen Wirkstoffen nach § 35a SGB V – Turoctocog alfa (Justification [Federal Joint Committee] of the Decision of the Federal Joint Committee amending the Prescription Drug Guidelines (AM-RL): Appendix XII – Decision on the Benefit Assessment of Drugs with New Active Substances in accordance with § 35a SGB V [Social Security Code V] – Turoctocog Alfa) [Internet]; 2014 [cited February 25, 2015]. Available from: https://www.g-ba.de/downloads/40-268-2871/2014-07-03_AM-RL-XII_Turoctocog%20alfa_2014-01-15-D-092_TrG.pdf. Accessed May 25, 2015. | |
IQWIG. Simoctocog Alfa for Haemophilia A: No Suitable Data [Internet]; 2015 [cited March 6, 2015]. Available from: https://www.iqwig.de/en/press/press-releases/press-releases/simoctocog-alfa-for-haemophilia-a-no-suitable-data.6593.html. Accessed May 25, 2015. | |
Ruggeri K, Nolte E. Pharmaceutical Pricing – The Use of External Reference Pricing [Internet]; 2013. Available from: http://www.rand.org/content/dam/rand/pubs/research_reports/RR200/RR240/RAND_RR240.pdf. Accessed May 25, 2015. | |
Petkantchin V. Economic Effects of Germany’s Reference Pricing Policy for Drugs [Internet]; 2006 [cited February 26, 2015]. Available from: http://www.institutmolinari.org/economic-effects-of-germany-s,491.html. Accessed May 25, 2015. | |
Powell JS, Pasi KJ, Ragni MV, et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N Engl J Med. 2013;369(24):2313–2323. | |
Mahlangu J, Powell JS, Ragni MV, et al. Phase 3 study of recombinant factor VIII Fc fusion protein in severe hemophilia A. Blood. 2014; 123(3):317–325. | |
Food and Drug Administration. Rixubis – Statistical Review and Evaluation [Internet]; 2012 [cited February 26, 2015]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM363882.pdf. Accessed May 25, 2015. | |
Manco-Johnson MJ, Kempton CL, Reding MT, et al. Randomized, controlled, parallel-group trial of routine prophylaxis vs on-demand treatment with sucrose-formulated recombinant factor VIII in adults with severe hemophilia A (SPINART). J Thromb Haemost. 2013;11(6):1119–1127. | |
Food and Drug Administration. Summary Basis for Regulatory Action – Novoeight/Antihemophilic Factor (Recombinant) [Internet]; 2013 [cited February 26, 2015]. Available from: http://www.fda.gov/downloads/BiologicsBloodVaccines/BloodBloodProducts/ApprovedProducts/LicensedProductsBLAs/FractionatedPlasmaProducts/UCM373769.pdf. Accessed May 25, 2015. | |
CSL Behring. A Safety and Efficacy Study of a Recombinant Fusion Protein Linking Coagulation Factor IX with Albumin (rIXFP) in Patients with Hemophilia B [Internet]; 2015 [cited February 26, 2015]. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01496274?term=Factor+IX+CSL&rank=3.. Accessed May 25, 2015. | |
Bayer. A Trial to Compare Prophylaxis Therapy to On-demand Therapy with a New Full Length Recombinant FVIII in Patients with Severe Hemophilia A (Leopold II) [Internet]; 2014 [cited April 12, 2015]. Available from: https://clinicaltrials.gov/ct2/show/results/NCT01233258?term=Bayer+Factor+VIII&rank=22. Accessed May 25, 2015. | |
Bayer. A Trial Investigating Safety and Efficacy of Treatment with BAY94-9027 in Severe Hemophilia A (PROTECT-VIII) [Internet]; 2015 [cited April 12, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01580293?term=BAY+94-9027&rank=3. Accessed May 25, 2015. | |
Baxter. Study Investigating a PEGylated Recombinant Factor VIII (BAX 855) for Hemophilia A (PROLONG-ATE Study) [Internet]; 2014 [cited April 12, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01736475?term=BAX+855&rank=2. Accessed May 25, 2015. | |
Octapharma. Efficacy and Safety Study of Human-cl rhFVIII in PTPs With Severe Hemophilia A [Internet]; 2013 [cited April 12, 2015]. Available from: https://clinicaltrials.gov/ct2/show/study/NCT01125813?term=GENA-08&rank=1§=X01256. Accessed May 25, 2015. | |
Novonordisk. Evaluation of Safety and Efficacy, Including Pharmacokinetics, of NNC 0129-0000-1003 When Administered for Treatment and Prophylaxis of Bleeding in Subjects with Haemophilia A (pathfinderTM2) [Internet]; 2015 [cited April 12, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT01480180?term=N8-GP&rank=3. Accessed May 25, 2015. | |
Novonordisk. Safety and Efficacy of Nonacog Beta Pegol (N9-GP) in Previously Untreated Patients with Haemophilia B (paradigmTM6) [Internet]; 2015 [cited April 12, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT02141074?term=N9-GP&rank=1. Accessed May 25, 2015. | |
CSL Behring. An Open-label Safety and Efficacy Study of Recombinant FVIII in Patients with Severe Hemophilia A [Internet]; 2015 [cited April 12, 2015]. Available from: https://clinicaltrials.gov/ct2/show/NCT02172950?term=rVIII-SingleChain&rank=2. Accessed May 25, 2015. | |
Klein H. Optimal Clinical Use of Blood and Plasma Derivatives Background and Perspectives [Internet]. Optimal Use of Coagulation Factors and Immunoglobulins Meeting, Wildbad Kreuth, Bavaria, Germany; 2013 [cited February 26, 2015]. Available from: https://www.edqm.eu/medias/fichiers/session_1.pdf. Accessed May 25, 2015. | |
Manco-Johnson MJ, Kempton CL, Reding MT, et al. Randomized, controlled, parallel-group trial of routine prophylaxis vs on-demand treatment with sucrose-formulated recombinant factor VIII in adults with severe hemophilia A (SPINART). J Thromb Haemost. 2013;11(6):1119–1127. | |
Freedman B. Equipoise and the ethics of clinical research. N Engl J Med. 1987;317(3):141–145. | |
Gringeri A, Lundin B, von Mackensen S, Mantovani L, Mannucci PM; ESPRIT Study Group. A randomized clinical trial of prophylaxis in children with hemophilia A (the ESPRIT Study). J Thromb Haemost. 2011;9(4):700–710. | |
Mann H, London AJ, Mann J. Equipoise in the enhanced suppression of the platelet IIb/IIIa receptor with integrilin trial (ESPRIT): a critical appraisal. Clin Trials. 2005;2(3):233–243. | |
Tcheng JE, Madan M, O’Shea JC, et al. Ethics and equipoise: rationale for a placebo-controlled study design of platelet glycoprotein IIb/IIIa inhibition in coronary intervention. J Interv Cardiol. 2003;16(2):97–105. | |
Freedman B. Placebo-controlled trials and the logic of clinical purpose. IRB. 1990;12(6):1–6. | |
World Medical Association. World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects [Internet]; 2008 [cited February 26, 2015]. Available from: http://www.wma.net/en/30publications/10policies/b3/17c.pdf. Accessed May 25, 2015. | |
Goodyear MDE, Lemmens T, Sprumont D, Tangwa G. Does the FDA have the authority to trump the Declaration of Helsinki? BMJ. 2009;338:b1559. | |
International Conference on Harmonization. ICH Harmonised Tripartite Guideline for Good Clinical Practice [Internet]; 1996 [cited February 26, 2015]. Available from: http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R1_Guideline.pdf. Accessed May 25, 2015. | |
Mannucci PM. Evolution of the European guidelines for the clinical development of factor VIII products: little progress towards improved patient management. Haemophilia. 2013;19(3):344–348. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
