Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 13
Isotretinoin and the Kidney: Opportunities and Threats
Authors Forouzani-Haghighi B , Karimzadeh I
Received 20 April 2020
Accepted for publication 8 July 2020
Published 28 July 2020 Volume 2020:13 Pages 485—494
DOI https://doi.org/10.2147/CCID.S259048
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jeffrey Weinberg
Bahareh Forouzani-Haghighi, Iman Karimzadeh
Department of Clinical Pharmacy, Faculty of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
Correspondence: Iman Karimzadeh
Department of Clinical Pharmacy, Faculty of Pharmacy, Shiraz University of Medical Sciences, Shiraz, Iran
Tel +98 (713) 2424128
Email [email protected]
Abstract: Retinoids are one of the most effective drugs in inducing complete or prolonged remission of severe acne vulgaris, but the adverse reactions associated with the use of them are raising a concern about the potential effect of these drugs on internal organs function such as the kidney. The aim of this review is to comprehensively gather data about isotretinoin, both potential adverse and beneficial effects on the kidney based on the current experimental and clinical findings. Very few studies, including five case reports, described that systemic oral isotretinoin within usual doses (40 mg/day or 0.5 mg/kg⁄day) within 1 to 4 months of treatment might be associated with different types of renal dysfunctions. These include acute interstitial nephritis, nephrotic syndrome, and hematuria with dysuria. The adverse reactions of systemic isotretinoin on the kidney and urinary system are unlikely and rare. In contrast, six experimental studies demonstrated the beneficial effects of either oral or parenteral low- (2 or 5 mg/kg/day) or high- (10, 20, 25, 40 mg/kg/day) dose isotretinoin on the kidney in the rat models of glomerulonephritis, obstructive nephropathy or allograft nephropathy. The nephroprotective functions of isotretinoin in these studies were attributed to its anti-proliferative, anti-fibrotic, and anti-inflammatory actions. However, clinical studies are warranted to elucidate the possible beneficial effects of isotretinoin in preventing or attenuating kidney injury in different settings.
Keywords: isotretinoin, kidney, adverse effects, beneficial effects
Introduction
Isotretinoin (13-cis RA), a prominent and commonly used member of the retinoid class, is one of the most prominent drugs for treating severe nodulocystic acne vulgaris. The recommended dose for treating severe acne vulgaris in a single course therapy is 0.5–1 mg/kg twice a day for 15–20 weeks, with the cumulative dose of 120–140 mg/kg. After this course of treatment, most patients usually experience complete or prolonged remission of the disease.1 The members of the retinoid class, including isotretinoin, act through influencing apoptosis, cellular morphogenesis, growth, and differentiation.2 Its mechanism of action has not been fully understood yet; however, recent investigations suggest that isotretinoin activates certain genes transcription by binding to a specific part of the gene promoter region named “retinoic acid response element” (RARE) and induces the differentiation of the cell. In contrast, the indirect negative regulatory mechanism is responsible for its anti-proliferative and anti-inflammatory activities. These are secondary to the downregulation of non-RARE promoter genes.3
Despite isotretinoin’s wide range of therapeutic effects, adverse reactions also have been reported with this agent. Some of its side effects are dose-related. So, specific monitoring must be considered.4 The most concerning side effects of isotretinoin relate to its potential teratogenicity and increased risk of spontaneous abortion. Therefore, at least two negative pregnancy tests (30 days apart) are necessary before starting its treatment. Effective contraception with two different methods must be considered until 1 month after treatment cessation.5 However, the most common side effects of isotretinoin are mucocutaneous reactions such as cheilitis, peeling, and skin, as well as mucosal dryness.6
Cholesterol and low-density lipoprotein levels are also affected by isotretinoin. The most significant elevating effect on serum lipids is seen in patients with elevated lipid levels at the baseline.7 Patients must undergo laboratory evaluations before the treatment, and those with a personal or family history of diabetes, cardiac disease, pancreatitis, hyperlipidemia, and liver disease should be monitored regularly during the treatment course of isotretinoin.8
Isotretinoin therapy can also associate with some ocular complications such as keratoconjunctivitis sicca (dry eyes), and even more concerning adverse reactions such as loss of dark adaptation and color vision which may be irreversible. Many possible ocular side effects of isotretinoin (eg, keratitis and photophobia) can be secondary to keratoconjunctivitis sicca.9
Neurological and psychiatric side effects of this drug are raising a concern about depression and suicidal thoughts in patients, as the acne patients have a higher rate of depression and anxiety on their own. However, there is no reliable evidence about this issue. Other neurologic side effects such as malaise, lethargy, and headache are usually transient and occur at the initiation of the treatment.10
Hematologic abnormalities such as neutropenia and thrombocytopenia are infrequent and regular monitoring is not recommended, unless in the cases with pre-existing hematologic disorders.11
However, what are isotretinoin plausible effects on the kidney? The purpose of this study is to critically review isotretinoin both potential adverse and beneficial effects on the kidney, based on the current experimental and clinical findings.
Methods
A systematic review was done according to the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guideline. Seven relevant databases, including Scopus, Medline, Embase, ISI Web of Knowledge, Cochrane central register of controlled trials, Cochrane database systematic reviews, and Google Scholar, were searched critically. The following search terms were exploited: “Isotretinoin", “13-cis retinoic acid”, "Accutane®", "Roaccutane®", "acute kidney injury (AKI)", "chronic kidney disease", "renal dysfunction", "renal impairment", "renal damage", "nephroprotection", "nephroprotective effects", "nephroprotective activity", and "renoprotective effects".
Titles, abstracts, and full text of studies were evaluated. Exclusion criteria were non-English language articles, congress abstracts, and newspaper articles. Also, studies on retinoids rather than isotretinoin such as tretinoin (all-trans-retinoic acid), etretinate, acitretin, and adapalene were not eligible for inclusion. Articles published up to December 2019 were taken into account. Finally, the required data were extracted from the selected and eligible articles. Eleven studies, including case reports (n=5) and experimental studies (n=6) related topics to isotretinoin potential negative and positive effects on the kidney, were finally considered for this review.
Results
Potential Renal and Urinary Adverse Reactions
There are few case reports of AKI and nephrotic syndrome following the use of isotretinoin in healthy individuals. The increased serum creatinine levels usually return to normal after the discontinuation of therapy.
Renal Colic and Moderate Renal Impairment
Pavese study group12 in 1997 reported a 34-year-old man with acne conglobata that had been treated with isotretinoin 40mg/day for the last 2 months. He admitted to the hospital due to severe bilateral lumbar pains suggesting renal colic. Blood pressure and body temperature were normal. His kidney size was also normal. He had an unremarkable nephrogram without any structural defect, lack of urinary concentration, and a normal bladder. Urine analysis revealed microscopic hematuria (500,000/mn) and proteinuria (0.8 g/l). His serum creatinine was 81 mmol/l. Non-specific markers of inflammation, including ESR and CRP, were moderately elevated. However, there was no clinical and laboratory clue in favor of either infectious or immunologic disease. Abdominal pain was attributed to chronic constipation. All signs and symptoms of renal impairment ultimately resolved 7 days after the discontinuation of isotretinoin along with intravenous hydration. The authors believed that renal impairment, in this case, is due to a drug toxicity of isotretinoin because the elimination half-life of isotretinoin as well as its major active metabolite is about 24 hrs and the time usually takes for them to be completely eliminated from the body is near 1 week.12
Minimal Change Disease
In another case report, Oers study group13 in 2000 described a 19-years-old previously healthy male patient with the chief complaint of the ankle, pre-tibial, and facial edema admitted to the outpatient department of nephrology. He had a weight gain of 12 kg during the past 2 months. His medical history only included severe acne vulgaris that had been treated with 40 mg/day oral isotretinoin for 4 months before the admission. Physical examinations and routine lab tests revealed blood pressure 130/80 mmHg, increased ESR (83 mm/hr), decreased hemoglobin (8.3 mmol/l) and decreased total serum protein and albumin (46 and 21 g/l, respectively), increased cholesterol level (8 mmol/l). Urine analysis revealed 10.3 g protein in the 24-hr urine. All other biochemical and serological tests were normal. A kidney ultrasound revealed no abnormality. Percutaneous renal biopsy showed no light microscopic aberrations. The immunofluorescence documented moderate sporadic mesangial deposition of IgM and C3. As these findings were suggestive for minimal change disease due to the anti-acne treatment, isotretinoin was discontinued, and 60 mg/day oral prednisolone was started. The nephrotic syndrome gradually disappeared after 4 weeks of treatment, and prednisolone was tapered to discontinue within 3 months. Interestingly, the authors noted that the manufacturer of isotretinoin (Roche pharmaceuticals) had been reported mild proteinuria 60 times in the recipients of this agent for 7 years. This case was one of the very few reports of full-blown nephrotic syndrome associated with the use of isotretinoin.13
Acute Interstitial Nephritis
Two case reports have described the possible association between isotretinoin and acute interstitial nephritis. Armaly study group14 in 2013 reported a case of previously healthy 17-year-old female, with a 5-day history of nausea, vomiting, and bilateral flank pain. Her past medical history only included the use of isotretinoin with a not-defined dose for acne treatment 2 months before admission. Initial lab tests revealed increased serum creatinine (2mg/dl) and blood urea nitrogen (20mg/dl). Additional blood tests such as complete blood count (CBC), full chemistry panel, rheumatoid factor (RF), anti-streptolysin O titer (ASOT), protein electrophoresis (PEP), antinuclear antibody (ANA) and complements levels were all normal. Urinalysis revealed pyuria (white blood cells [WBC] 25/μL), hematuria (red blood cells [RBC] 10/μL), and protein +1, without any evidence of WBC, RBC or granular casts. 24-hrs urine collection showed creatinine clearance of 33 mL/min and urine protein of 390 mg/day. Ultrasound sonography of both kidneys was normal. She was treated with IV fluids, but her kidney function dropped in the next days of her admission. Repeated urine analysis tests also revealed 5 WBC casts, 20–30 WBC/HPF, no RBCs or other casts, and Wright’s staining for eosinophils was positive. Eventually, on the base of these clinical and laboratory findings, the diagnosis of acute interstitial nephritis (AIN) was raised for this patient. However, a kidney biopsy was not performed. Rescue therapy with glucocorticoids was suggested. However, it was not initiated because her serum creatinine levels started to decrease in the fourth day of treatment, and fortunately, the serum creatinine and urine proteins returned to normal values. This study suggests that isotretinoin induced AKI can be due to autoimmune interstitial nephritis (AIN) in the absence of renal biopsy confirmatory findings. The authors proposed that kidney function tests must be considered in routine follow up tests during the treatment course of isotretinoin.14
Eosinophilic tubulointerstitial nephritis was also reported by Aksoy study group15 in 2016 in a 16-year-old male patient. He had received 40 mg/day isotretinoin in the last 3 months. The patient was admitted to the hospital with the diagnosis of AKI. Routine lab tests revealed an increase in blood urea nitrogen (21 mg/dl), serum creatinine (1.68 mg/dL) as well as cystatin C (1.15 mg/L), and a decrease in estimated glomerular filtration rate based on the serum cystatin C (56.5 mL/min/1.73 m2). Other biochemical and hematologic tests were normal and unremarkable. His renal biopsy showed interstitial mononuclear cell and eosinophilic infiltration. Eventually, regarding lab data and physical examination, he was diagnosed with eosinophilic, interstitial nephritis likely secondary to isotretinoin. Therefore, isotretinoin was discontinued, and 60mg/day prednisolone was given orally. After 6 days, his serum creatinine decreased to the normal value (79.7µmol/L). Prednisolone treatment was continued for and tapered within 3 months.15
Terminal Hematuria
Sarifakioglu16 study group described a case report of a 16-year-old boy who developed terminal hematuria along with dysuria after 1 month of treatment with isotretinoin (0.5 mg/kg⁄day) for treatment of scarring acne vulgaris. Kidney function tests, urine culture, ultrasonography, and computed tomography of the urinary system were all normal. Biochemical and immunologic tests were also unremarkable. He denied taking any food (eg, rhubarb, beets, blueberries) or medication (eg, rifampin, nitrofurantoin, phenazopyridine) that may cause red pigmenturia. De-challenging and re-challenging isotretinoin were associated with disappearing and reappearing hematuria, respectively. The authors attributed the gross hematuria of isotretinoin to its xerotic mucosal side effects, similar to the nasal mucosa, causing nasal bleeding.16 The summary of these case reports is listed in Table 1.
 |
Table 1 Summary of Studies About Adverse Effect of Isotretinoin on the Kidney |
Potential Beneficial Effects on the Kidney Function
Despite these few case reports of isotretinoin adverse reactions on renal performance, retinoids generally induce immune-regulatory, anti-inflammatory, and anti-proliferative effects in most cell types.
Acute Mesangioproliferative Glomerulonephritis
Wagner study group17 in 2000 provided an experimental study in a rat model of acute mesangioproliferative glomerulonephritis induced by anti-Thy1.1. Treatment protocol included either all-trans-retinoic acid (10 mg/kg/day) or isotretinoin (40 mg/kg/day) injected subcutaneously. Isotretinoin was administered solely as a post-treatment protocol (3 through 8 days after the induction of anti-Thy1.1 nephritis). Isotretinoin post-treatment resulted in a significant decrease in a 24-h albumin excretion rate in the urine. Also, glomerular cell number, PCNA-positive cells, and platelet-derived growth factor-B (as indexes of glomerular proliferation) declined significantly by the isotretinoin. Finally, isotretinoin significantly reduced the number of monocytes/macrophages along with glomerular fibrin deposition. According to this study, high-dose isotretinoin is highly effective in limiting glomerular proliferation as well as lesions, along with albuminuria in an established model of renal damage.17
In a similar study on the same model of renal damage in rats published in 2001, Morath study group18 demonstrated that cortical TGF-β1 gene expression, as well as TGF receptor II, were significantly attenuated with isotretinoin (40 mg/kg/day injected subcutaneously as a post-treatment). Glomerular collagen III and IV expressions were also decreased significantly after isotretinoin treatment. However, isotretinoin did not affect the content of fibronectin protein. The authors concluded that the beneficial effects of retinoids on glomerular damage in a rat model of acute mesangioproliferative glomerulonephritis are via mitigating fibrosis secondary to the reduction of renal transforming growth factor-β1 (TGF-β1) and TGF receptor II gene expression.18
Chronic Mesangioproliferative Glomerulonephritis
In another experimental study in 2001, Schaier study group19 investigated the dose-dependent effect of isotretinoin in the rat model of chronic mesangioproliferative glomerulonephritis induced by a monoclonal antibody. Rats were treated with either low (2 mg/kg/day orally) or high dose (10 mg/kg/day orally) of isotretinoin initiated 1 day after injection of the antibody. The study continued for 60 days. Isotretinoin treatment attenuated an elevation in blood pressure and also significantly decreased albuminuria. Interstitial cell counts, the number of glomerular and interstitial macrophages along with the area of the interstitial space significantly decreased in both low- and high-dose isotretinoin recipients. Renal gene expression of fibronectin-1, pro-collagen-1, and TGF-β1, as indexes of interstitial fibrosis and glomerulosclerosis, were also significantly reduced after isotretinoin treatment. Data of this study suggest that both low- and high-dose isotretinoin treatment can markedly reduce renal damage and normalize both glomerular and interstitial structure in a rat model of chronic mesangioproliferative glomerulonephritis via its anti-inflammatory, anti-proliferative, and anti-fibrotic actions.19
Unilateral Ureteral Obstruction
Two years later in 2003, Schaier research group20 reported the results of another study about isotretinoin potential effect on the rat model of unilateral ureteral obstruction (UUO). They compared control rats with UUO ones treated with vehicle, low-dose (5 mg/kg/day) or high-dose (25 mg/kg/day) isotretinoin by subcutaneous injection for 7 days. In comparison to the vehicle group, both low- and high-dose isotretinoin treatment significantly preserved tubulointerstitial structure. Isotretinoin treatment also inhibited the proliferation of tubular as well as interstitial cells determined by the number of Ki-67 positive cells. Isotretinoin also protected against interstitial infiltration by monocytes/macrophages. Finally, both low- and high-dose isotretinoin treatment abrogated the increased gene expression of TGF-β1 as well as its type II receptor, collagen I, and fibronectin in the interstitial space. In brief, isotretinoin, in a dose-dependent manner, had beneficial and protective effects on proliferation, inflammation, and fibrosis in the obstructive nephropathy model.20
Acute Renal Allograft Rejection
The protective effects of isotretinoin against cellular damage of kidneys have been demonstrated by Kiss study group21 in a rat model of acute renal allograft rejection. Low-dose (2 mg/kg/day) or high-dose (20 mg/kg/day) isotretinoin was given subcutaneously. It was initiated on the day of transplantation and continued for either 7 or 14 days. Both HD and LD isotretinoin treatment significantly reduced albuminuria (by about two-thirds). Serum creatinine, as a classic marker of renal function, was also lower in isotretinoin recipients than those given the vehicle. However, isotretinoin did not alter arterial blood pressure. On day 14, after transplantation, both LD and HD isotretinoin significantly decreased vascular injury. HD isotretinoin, but not LD, significantly reduced glomerular as well as tubulointerstitial damage and cell proliferation; the latter was indicated by the number of Ki67(+) cells. On day 14, after transplantation, indexes of both tubulointerstitial and glomerular inflammation, including the number of cytotoxic T cells, monocytes, and macrophages, also significantly decreased. The results of this study demonstrated the anti-rejection functions of isotretinoin via its anti-proliferative, immunosuppressive, and anti-inflammatory actions in the Fisher-to-Lewis model of acute renal graft rejection.21
Chronic Allograft Nephropathy
Adams study group22 conducted another experimental study about protective actions of isotretinoin on the Fisher-to-Lewis model of chronic allograft nephropathy (CAN). Two dosage regimen of isotretinoin was exploited, including low dose (2 mg/kg/day) and high dose (20 mg/kg/day), both were administered orally. Isotretinoin was given either for 8 weeks starting on the day of renal transplantation or for 6 weeks starting on day 14 after transplantation when chronic damage could already be seen. Mean serum creatinine levels were significantly lower in the isotretinoin recipients in comparison to untreated rats. Beside biochemical index, both low- and high-dose isotretinoin dramatically reduced the pathologic markers of chronic rejection, including sub-endothelial fibrosis of pre-glomerular vessels, glomerulosclerosis, tubular atrophy, and chronic tubulointerstitial damage. The expression of pro-inflammatory cytokines/chemokines (MCP-1/CCL2, MIP-1α/CCL3, IP-10/CXCL10, RANTES/CCL5, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, GM-CSF, TNF-α, and IFN-γ) and proteins associated with fibrosis (plasminogen activator inhibitor-1, transforming growth factor-1, and collagens I and III) were all significantly lowered by isotretinoin. Besides efficacy, both low and high doses of isotretinoin treatment were not associated with classic and prominent side effects of retinoid, including hair loss, cheilitis, conjunctivitis, liver damage, and bone loss. These data suggested that isotretinoin has anti-inflammatory and anti-fibrotic activities, can preserve renal function, and inhibit or attenuate the progress of CAN without any side effects.22 Table 2 shows a summary of experimental studies about the possible nephroprotective effects of isotretinoin.
 |
Table 2 Summary of Studies About the Possible Nephroprotective Effects of Isotretinoin |
Discussion
The potential adverse effects of isotretinoin on the kidney and urinary system were quite scarce and only limited to a few case reports. These include drug toxicity (n=1), nephrotic syndrome as minimal change disease type (n=1), acute interstitial nephritis (n=2), and terminal hematuria (n=1). Kidney biopsy was only done in 2 out of 5 case reports. Therefore, despite temporal relationship and relevant clinical as well as paraclinical findings, the diagnosis of kidney injury secondary to isotretinoin may not be definite.
From the aspect of pathophysiology, minimal change disease is one of the most common causes of nephrotic syndrome that is associated with significant proteinuria due to glomerular injury. It can lead to subsequent peripheral edema and loss of intravascular volume.23 Acute interstitial nephritis is characterized by immunologic reactions (usually T cell-mediated) against certain endogenous or exogenous antigens (such as specific medications) in kidney interstitium that causes renal function impairment.24 Terminal hematuria is a term referred to inflammatory reactions due to drugs or toxins in the urine stream that appears with hematuria and might mimic bladder cancer symptoms. The inflammation might either occur in glomeruli or post-renal structures such as ureters and bladder.25
The isotretinoin indication in all the above cases was the treatment of acne vulgaris. The doses taken by these patients were either 40 mg/day or 0.5 mg/kg⁄day that were within the recommended range. The duration of isotretinoin treatment before developing kidney injury varied from 1 to 4 months. Fortunately, all the above cases of kidney injury were recovered by only discontinuing the offending agent or along with initiating corticosteroid/hydration therapy. FDA (U.S. food and drug administration) also has reported proteinuria as one of the common side effects associated with the use of isotretinoin (Accutane®) as a result of clinical trials and post-marketing surveillance.26 It was noteworthy that acute rhabdomyolysis and myoglobinuria27,28 or isolated elevation of serum creatine kinase in patients under isotretinoin treatment29 had been reported. However, kidney function appears to be intact and it was not adversely affected by isotretinoin in these studies.26–28 Finally, microscopic haematuria, proteinuria, and other kidney abnormalities are sometimes detected as laboratory findings in the setting of acne fulminans.30 Acne fulminans can be either triggered or rarely precipitated during isotretinoin therapy. However, a literature review by Alakeel study group31 on the acne fulminans demonstrated no kidney involvement in isotretinoin recipients.
On the contrary side, the protective effects of isotretinoin against kidney damage have been reported in several settings, including acute (n=2) as well as chronic (n=1) mesangioproliferative glomerulonephritis, obstructive nephropathy (n=1), and acute (n=1) as well as chronic (n=1) allograft nephropathy. All these six studies were experimental and performed in the rat model. The isotretinoin dosage regimen exploited ranged from 2 to 40 mg/kg/day. This include either low dose (2 or 5 mg/kg/day) or high dose (10, 20, 25, 40 mg/kg/day) isotretinoin administered parenterally or orally. Although the nephroprotective activities of isotretinoin have been demonstrated to be dose-dependent, both low and high doses of isotretinoin are capable of improving renal function. Initiation of isotretinoin treatment was concurrent with or a short time after the kidney insult. Interestingly, both these approaches had significant nephroprotective effects. Classic biochemical indexes of kidney function, including serum creatinine and albuminuria, were improved by isotretinoin treatment. At both the molecular and cellular levels, the possible nephroprotective mechanisms of isotretinoin have also been investigated in the relevant experimental studies. Retinoic acid receptors (RAR) have different subtypes such as RAR α, β, γ, and retinoid X receptors (RXR). These receptors are responsible for specific gene modulation by these compounds.32 Activator protein-1 (AP-1) is one of the regulatory transcription factors that control specific cellular processes such as apoptosis, proliferation, and cytokine release. AP-1 consists of dimeric proteins such as Jun, Fos, ATF, and each member has a specific function in different cellular processes.33
Furthermore, subsequent activation of AP-1 due to angiotensin II release results in inflammatory cells infiltration and tubular damage in the kidney. So, AP-1 is significantly implicated in renal and vascular damage pathology.34 Members of the retinoid class generally have an antagonistic effect on AP-1 expression, and this is one of the most important mechanisms of their protective effect on the kidney in response to injuries.35 The antagonistic effect on AP-1 is also involved in the acne relieving and wound healing process as well.36
Isotretinoin can also attenuate renal injury by inhibiting pro-inflammatory pathways such as nuclear factor-κB (NF-κB), creb-binding protein/p300.37 In addition, both prominent mediators of Th1 (IFN-γ) and Th2 (IL-10) were significantly decreased by isotretinoin treatment. Besides these mediators, isotretinoin reduced other inflammatory cytokines (eg, IL-1α, IL-1β, IL-2, IL-4, IL-6, and GM-CSF) in the kidney tissue. The evidence of decreased infiltration of monocytes and macrophages in urine samples also supports the theory of retinoid anti-inflammatory properties.38
Further investigations suggest that anti-proliferative properties of isotretinoin are mediated through the endothelin (ET) system because of the mitogenic effect of this system on mesangial cells. Not only ET-1 expression but also its receptor expression has been decreased during isotretinoin treatment.39 It has been claimed that the retinoid anti-proliferative feature is not linked to specific receptor subtypes; both RAR and RXR may be involved. Anti-proliferative effects of isotretinoin involve both immunologic (eg, monocytes and macrophages) and non-immunologic cells (eg, mesangial, vascular smooth muscle, endothelial, and tubular epithelial).40
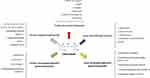
The anti-fibrotic activities of isotretinoin can be secondary to its anti-inflammatory and anti-proliferative properties. Also, isotretinoin has direct and independent anti-fibrotic functions. In this regard, the amount of expression or content of plasminogen activator inhibitor-1, transforming growth factor-1, collagens I as well as III, and fibronectin in different parts of the kidney was significantly reduced by isotretinoin treatment.41 Figure 1 shows a summary of key pro-inflammatory mediators suppressed by isotretinoin in different animal models of renal injury.
Conclusion
Retinoids are one of the most effective drugs in inducing complete or prolonged remission of severe acne vulgaris, but the adverse reactions associated with the use of them are raising a concern about the potential effect of these drugs on internal organs function such as the kidney. The most commonly prescribed and prominent agent of the retinoid class, isotretinoin, is considered in this systematic review. Very few studies including 5 case reports described that systemic oral isotretinoin within usual doses (40 mg/day or 0.5 mg/kg⁄day) within 1 to 4 months of treatment might be associated with different types of renal dysfunctions. These include acute interstitial nephritis, nephrotic syndrome, and hematuria with dysuria. The adverse reactions of systemic isotretinoin on the kidney and urinary system are unlikely and rare. Therefore, monitoring of renal performance at baseline and regularly during isotretinoin treatment appears not to be necessary currently. However, it seems that certain underlying conditions (eg, diabetes, hypertension), severe intravascular volume depletion, and concurrent use of nephrotoxic agents (eg, NSAIDs, aminoglycosides, vancomycin) might predispose patients to those mentioned renal adverse reactions of isotretinoin.42 Improvement of each of these conditions might be helpful in reducing isotretinoin nephrotoxicity risk.
In contrast, six experimental studies demonstrated the beneficial effect of either oral or parenteral low-dose (2 or 5 mg/kg/day) or high-dose (10, 20, 25, 40 mg/kg/day) isotretinoin on the kidney in the rat models of glomerulonephritis, obstructive nephropathy or allograft nephropathy. The nephroprotective functions of isotretinoin in these studies were attributed to its anti-proliferative, anti-fibrotic, and anti-inflammatory actions. However, performing clinical studies are essential to elucidate the possible beneficial effects of different isotretinoin doses for preventing or attenuating kidney injury in different settings (eg, acute as well as chronic glomerulonephritis or allograft rejection). Safety aspects and maximum cumulative dose of isotretinoin in these studies should also be considered.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rademaker M. Isotretinoin: dose, duration and relapse. What does 30 years of usage tell us? Australas J Dermatol. 2013;54(3):157–162. doi:10.1111/j.1440-0960.2012.00947.x
2. Rigopoulos D, Larios G, Katsambas AD. The role of isotretinoin in acne therapy: why not as first-line therapy? facts and controversies. Clin Dermatol. 2010;28(1):24–30. doi:10.1016/j.clindermatol.2009.03.005
3. Nelson AM, Zhao W, Gilliland KL, Zaenglein AL, Liu W, Thiboutot DM. Isotretinoin temporally regulates distinct sets of genes in patient skin. Invest Dermatol. 2009;129(4):1038. doi:10.1038/jid.2008.338
4. Charakida A, Mouser P, Chu A. Safety and side effects of the acne drug, oral isotretinoin. Expert Opin Drug Saf. 2004;3(2):119–129. doi:10.1517/14740338.3.2.119
5. Chan A, Hanna M, Abbott M, Keane RJ. Oral retinoids and pregnancy. Med J Aust. 1996;165(3):164–167. doi:10.5694/j.1326-5377.1996.tb124895.x
6. Kaymak Y, Ilter N. The results and side effects of systemic isotretinoin treatment in 100 patients with acne vulgaris. Dermatol Nurs. 2006;18(6):576–581.
7. McCarter TL, Chen YK. Marked hyperlipidemia and pancreatitis associated with isotretinoin therapy. Am J Gastroeneral. 1992;87:12.
8. Brecher AR, Orlow SJ. Oral retinoid therapy for dermatologic conditions in children and adolescents. J Am Acad Dermatol. 2003;49(2):171–182. doi:10.1067/S0190-9622(03)01564-0
9. Fraunfelder F, LaBraico JM, Meyer SM. Adverse ocular reactions possibly associated with isotretinoin. Am J Ophthalmol. 1985;100(4):534–537. doi:10.1016/0002-9394(85)90676-2
10. Marqueling AL, Zane LT, Marqueling AL, Zane LT. Depression and suicidal behavior in acne patients treated with isotretinoin: a systematic review. Semin Cutan Med Surg. 2005;24(2):92–102. doi:10.1016/j.sder.2005.04.003
11. Friedman SJ. Leukopenia and neutropenia associated with isotretinoin therapy. Arch Dermatol. 1987;123(3):293–295. doi:10.1001/archderm.1987.01660270025004
12. Pavese P, Kuentz F, Belleville C, Rouge P, Elsener M. Renal impairment induced by isotretinoin. Nephrol Dialysis Transplant. 1997;12(6):1299. doi:10.1093/ndt/12.6.1299a
13. van Oers JA, de Leeuw J, van Bommel EF. Nephrotic syndrome associated with isotretinoin. Nephrol Dial Transplant. 2000;15(6):923–924. doi:10.1093/ndt/15.6.923
14. Armaly Z, Haj S, Bowirrat A, et al. Acute kidney injury following isotretinoin treatment. Am J Case Rep. 2013;14:554.
15. Aksoy GK, Koyun M, Akkaya B, Comak E, Gemici A, Akman S. Eosinophilic tubulointerstitial nephritis on treatment with isotretinoin. Eur J Prdiatr. 2016;175(12):2005–2006. doi:10.1007/s00431-016-2778-7
16. Sarifakioglu E, Yilmaz AE, Erpolat S. Terminal hematuria associated with oral isotretinoin treatment in a patient with acne vulgaris. Pediatr Dermatol. 2012;29(5):668–669. doi:10.1111/j.1525-1470.2011.01552.x
17. Wagner J, Dechow C, Morath C, et al. Retinoic acid reduces glomerular injury in a rat model of glomerular damage. J Am Soc Nephrol. 2000;11(8):1479–1487.
18. Morath C, Dechow C, Lehrke I, et al. Effects of retinoids on the TGF-β system and extracellular matrix in experimental glomerulonephritis. J Am Soc Nephrol. 2001;12(11):2300–2309.
19. Schaier M, Jocks T, Grone H-J, Ritz E, Wagner J. Retinoid agonist isotretinoin ameliorates obstructive renal injury. J Urol. 2003;170(4):1398–1402. doi:10.1097/01.ju.0000084620.64255.b3
20. Schaier M, Lehrke I, Schade K, et al. Isotretinoin alleviates renal damage in rat chronic glomerulonephritis. Kidney Int. 2001;60(6):2222–2234. doi:10.1046/j.1523-1755.2001.00056.x
21. Kiss E, Adams J, Gröne H-J, Wagner J. Isotretinoin ameliorates renal damage in experimental acute renal allograft rejection1. Transplantation. 2003;76(3):480–489. doi:10.1097/01.TP.0000066354.31050.5A
22. Adams J, Kiss E, Arroyo AB, et al. 13-cis retinoic acid inhibits development and progression of chronic allograft nephropathy. Am J Path. 2005;167(1):285–298. doi:10.1016/S0002-9440(10)62973-2
23. Waldman M, Crew RJ, Valeri A, et al. Adult minimal-change disease: clinical characteristics, treatment, and outcomes. Clin J Am Soc Nephro. 2007;2(3):445–453. doi:10.2215/CJN.03531006
24. Praga M, González E. Acute interstitial nephritis. Kidney Int. 2010;77(11):956–961. doi:10.1038/ki.2010.89
25. Fairley KF, Birch DF. Hematuria: a simple method for identifying glomerular bleeding. Kidney Int. 1982;21(1):105–108. doi:10.1038/ki.1982.16
26. On SCJ, Zeichner J. Isotretinoin updates. Ther. 2013;26(5):377–389.
27. Guttman-Yassky E, Hayek T, Muchnik L, Bergman R. Acute rhabdomyolysis and myoglobinuria associated with isotretinoin treatment. Int J Dermatol. 2003;42(6):499.
28. Paulsrud C, Stender I, Schmidt L. Rhabdomyolysis after isotretinoin treatment in a 17-year-old male. Ugeskr Laeger. 2017;179:40.
29. Landau M, Mesterman R, Ophir J, et al. Clinical significance of markedly elevated serum creatine kinase levels in patients with acne on isotretinoin. Acta Derm Venerol. 2001;81:5.
30. Zaba R, Schwartz R, Jarmuda S, Czarnecka–Operacz M, Silny W. Acne fulminans: explosive systemic form of acne. J Eur Acad Dermatol Venerol. 2011;25(5):501–507. doi:10.1111/j.1468-3083.2010.03855.x
31. Alakeel A, Ferneiny M, Auffret N, Bodemer C. Acne fulminans: case series and review of the literature. Pediatr Dermatol. 2016;33(6):e388–e92. doi:10.1111/pde.12983
32. Schüle R, Rangarajan P, Yang N, et al. Retinoic acid is a negative regulator of AP-1-responsive genes. Proc Natl Acad Sci. 1991;88(14):6092–6096. doi:10.1073/pnas.88.14.6092
33. Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3(11):859. doi:10.1038/nrc1209
34. Ruiz-Ortega M, Lorenzo O, Rupérez M, Blanco J, Egido J. Systemic infusion of angiotensin II into normal rats activates nuclear factor-κB and AP-1 in the kidney: role of AT1 and AT2 receptors. Am J Pathol. 2001;158(5):1743–1756. doi:10.1016/S0002-9440(10)64130-2
35. Simonson MS. Anti-AP-1 activity of all-trans retinoic acid in glomerular mesangial cells. Am J Physiol Renal. 1994;267(5):F805–F15. doi:10.1152/ajprenal.1994.267.5.F805
36. Kang S, Cho S, Chung JH, Hammerberg C, Fisher GJ, Voorhees JJ. Inflammation and extracellular matrix degradation mediated by activated transcription factors nuclear factor-κB and activator protein-1 in inflammatory acne lesions in vivo. Am J Pathol. 2005;166(6):1691–1699. doi:10.1016/S0002-9440(10)62479-0
37. Chakravarti D, LaMorte VJ, Nelson MC, et al. Role of CBP/P300 in nuclear receptor signalling. Nature. 1996;383(6595):99. doi:10.1038/383099a0
38. Karadag A, Ertugrul D, Bilgili S, Takci Z, Akin K, Calka O. Immunoregulatory effects of isotretinoin in patients with acne. Brit J Dermatol. 2012;167(2):433–435. doi:10.1111/j.1365-2133.2012.10949.x
39. Lehrke I, Schaier M, Schade K, et al. Retinoid receptor-specific agonists alleviate experimental glomerulonephritis. Am J Physiol Renal. 2002;282(4):F741–F51. doi:10.1152/ajprenal.00026.2001
40. Meister B, Fink F, Hittmair A, Marth C, Widschwendter M. Antiproliferative activity and apoptosis induced by retinoic acid receptor-gamma selectively binding retinoids in neuroblastoma. Anticancer Res. 1998;18(3A):1777–1786.
41. Kiss E, Popovic ZV, Bedke J, et al. Peroxisome proliferator-activated receptor (PPAR) γ can inhibit chronic renal allograft damage. Am J Pathol. 2010;176(5):2150–2162. doi:10.2353/ajpath.2010.090370
42. Haysom L, Ziegler DS, Cohn RJ, Rosenberg AR, Carroll SL, Kainer G. Retinoic acid may increase the risk of bone marrow transplant nephropathy. Pediatr Nephrol. 2005;20(4):534–538. doi:10.1007/s00467-004-1775-7
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.