Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 13
Isomangiferin Attenuates Renal Injury in Diabetic Mice via Inhibiting Inflammation
Authors Yue S, Xue N, Li H, Chen Z, Huang B, Wang X
Received 23 August 2020
Accepted for publication 21 October 2020
Published 10 November 2020 Volume 2020:13 Pages 4273—4280
DOI https://doi.org/10.2147/DMSO.S276229
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Konstantinos Tziomalos
Shuwen Yue,1,* Ning Xue,2,* Honglei Li,3 Zhen Chen,1 Baosheng Huang,4 Xing Wang1
1Department of Pharmacology, School of Pharmacy, China Pharmaceutical University, Nanjing, People’s Republic of China; 2Department of Acupuncture, Jurong Hospital Affiliated to Jiangsu University, Zhenjiang, People’s Republic of China; 3Department of Pharmacy, Kangda College of Nanjing Medical University, Lianyungang, People’s Republic of China; 4Department of Neurosurgery, Sir Run Run Hospital, Nanjing Medical University, Nanjing, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xing Wang
China Pharmaceutical University, Nanjing, Jiangsu 211199, People’s Republic of China
Email [email protected]
Baosheng Huang
Sir Run Run Hospital, Nanjing Medical University, No. 101, Longmiandadao, Jiangning District, Nanjing, Jiangsu 211166, People’s Republic of China
Email [email protected]
Aim: Renal injury induced by diabetes is reported to be associated with inflammation. Isomangiferin (ISO), a xanthone C-glucoside from the Cyclopia subfamily, exhibits many pharmacological properties. This study aimed to evaluate the protection of ISO against renal damage in diabetic mice.
Methods: Serum glucose, insulin, uric acid, creatinine, total cholesterol (TC), triglyceride (TG), and inflammatory cytokines in serum and the kidney of db/db diabetes model mice were detected. The components of high mobility group protein B1 (HMGB1)/NACHT leucine-rich repeat- and PYD-containing 3 (NLRP3)/nuclear factor kappa-B (NF-κB) pathway in the kidney were detected by Western blot and immunohistochemical analysis.
Results: ISO improved lipid profile and glucose tolerance, and inhibited the production of inflammatory cytokines in a db/db model mice. Moreover, ISO decreased biochemical indexes in the serum and inhibited the activation of HMGB1/NLRP3/NF-κB signaling in the kidney of db/db model mice.
Conclusion: ISO provides protection against renal injury via inhibiting HMGB1/NLRP3/NF-κB signaling in a diabetic mouse model.
Keywords: isomangiferin, renal injury, inflammation, HMGB1/NLRP3/NF-κB, diabetics
Introduction
Diabetes mellitus (DM) is characterized by hyperglycemia because of abnormal insulin secretion, and causes a variety of complications including renal failure, atherosclerosis, liver injury, and blindness.1–4 Diabetic nephropathy (DN) is a common microvascular complication of diabetes, and is one main cause of death of diabetes patients.5,6 At present, the main treatment strategy for DN is to reduce renal injury by controlling blood glucose, blood fat, and anti-hypertension.7 Recent studies have revealed novel mechanisms underlying the pathogenesis of DN and suggested that targeting inflammation is a promising approach for DN treatment.8–10 The inflammatory pathways involved in DN mainly include mitogen-activated protein kinase (MAPK) and nuclear factor kappa-B (NF-κB).11–13 High mobility group protein B1 (HMGB1) can combine with receptor for advanced glycation end products (RAGE) to induce inflammation.14–16
Isomangiferin (ISO) is a xanthone C-glucoside found in plants of the Cyclopia family with anti-inflammation and anti-cancer activities.17 In particular, a recent study reported that ISO acted as a potent inhibitor of vascular endothelial growth factor receptor 2 kinase to suppress breast metastasis and angiogenesis.18 In addition, many Chinese herbal extracts, in particular those containing ISO, can improve diabetes and renal function.19–24 However, whether ISO is beneficial to DN remains unclear. Therefore, in this study we aimed to evaluate the efficacy of ISO on DN and explore possible mechanisms.
Methods
Reagents
ISO was purchased from YuanYe Biotech (Shanghai, China), glucose was from Sigma (St. Louis, USA), enzyme-linked immunosorbent assay (ELISA) kits for interleukin (IL)-6, IL-1β, and tumor necrosis factor (TNF)-α were from Elabscience (Wuhan, China), biochemical kits to measure triglyceride (TG), total cholesterol (TC), high-density lipoprotein (HDL); low-density lipoprotein (LDL); uric acid (UA), urea nitrogen, and creatinine (Cr) were from Jiancheng Biotech (Nanjing, China). Primary antibodies were purchased from Cell Signaling Technology.
Animals
Type 2 diabetes mellitus (T2DM) model mice (C57BL/KsJ db/db mice, male) and male db/m mice were provided by Animal Model Center of Nanjing University, and kept in specific pathogen-free (SPF) condition at room temperature of 21±1°C and humidity of 55±5%. The mice were divided randomly into six groups (n=10): db/m group, db/db group, db/db+ISO (ISO, 10 mg/kg) group, db/db+ISO (ISO, 20 mg/kg) group, db/db+metformin (met, 400 mg/kg) group, and db/db+saline group. ISO (10, 20 mg/kg) was administered orally every day for 12 weeks. The protocols were approved by Animal Care and Use Committee of China Pharmaceutical University and followed Chinese National Guidelines for Experimental Animal Welfare, and animals were euthanized by cervical dislocation.
Oral Glucose Tolerance Test (OGTT)
The mice were fasted for 18 hours and then received glucose (2 g/kg) orally. Blood samples collected at 0, 30, 60, 90, and 120 minutes were centrifuged at 4°C (4,000 g) for 10 minutes to separate serum. Glucose level was determined by automatic blood glucose instruments.
Elisa
The levels of IL-1β, IL-6, and TNF-α in the serum and renal tissues were measured using ELISA kits following the manufacturer’s protocols.
Immunohistochemistry
Renal tissues were fixed in 4% paraformaldehyde, embedded in paraffin, and cut into 5 μm sections. The sections were blocked by incubation with 5% sheep serum for 20 minutes, then incubated with antibodies to HMGB1 (1:1,000) and p-NF-κBp65 (1:200) at 4°C overnight. The sections were then washed and incubated with secondary antibody. The section were also stained with hematoxylin and eosin (H&E), and observed under an optical microscope (Nikon, Tokyo, Japan).
Western Blotting
Proteins were extracted from renal tissues using RIPA buffer (Beyonce, Nanjing, China), resolved on 10% polyacrylamide gel electrophoresis, and transferred to nitrocellulose membranes. The membranes were incubated with primary antibodies (Cell Signaling Technology) at 4°C overnight and then incubated with secondary antibody conjugated with horseradish peroxidase at room temperature for 1 hour. Protein bands were detected using an enhanced chemiluminescence kit (Tanon Tech., China).
Statistical Analysis
Data were presented as means±SD and analyzed by ANOVA. P<0.05 was considered significant.
Results
ISO Reduced UA and Cr Levels in Db/Db Mice
UA, Cr, urea nitrogen, and albuminuria levels were significantly higher in db/db mice than in db/m mice, while ISO (10, 20 mg/kg) significantly reduced high UA, Cr, urea nitrogen, and albuminuria levels in db/db mice (Figure 1). As the control, saline could not reduce high UA, Cr, urea nitrogen, and albuminuria levels in db/db mice.
ISO Reduced Serum Insulin Levels and Improved Lipid Profiles in Db/Db Mice
Blood glucose level was significantly higher in db/db mice than in db/m mice, while ISO (10, 20 mg/kg) significantly reduced blood glucose level (Figure 2A). In addition, ISO (10, 20 mg/kg) treatment reduced the high serum insulin level in db/db mice (Figure 2B).
Compared to db/m mice, plasma TC, TG, and LDL levels were significantly higher while serum HDL levels were significantly lower in db/db mice, and ISO (10, 20 mg/kg) significantly reduced serum TC, TG, and LDL levels while it increased serum HDL levels in db/db mice (Figure 2C–F).
ISO Attenuated Kidney Damage
Histopathological examination demonstrated significant lipid accumulation and infiltration of monocytes and neutrophils in db/db mice compared to db/m mice. Mice in the saline group showed significant tubular injuries, such as vacuolar degeneration. However, the animals treated with ISO (10, 20 mg/kg) showed less inflammation and tubular injuries (Figure 3).
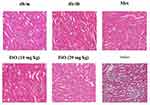 |
Figure 3 Effects of ISO on kidney pathology. Shown were H&E staining of the kidney tissues. Magnification: 20x. |
ISO Reduced Inflammatory Cytokine Levels in Serum and the Kidney
IL-1β, IL-6, and TNF-α levels were significantly higher in serum and kidney tissues of db/db mice compared to db/m mice, while ISO (10, 20 mg/kg) significantly decreased their levels in serum and kidney tissues (Figure 4A and B).
ISO Inhibited HMGB1/NACHT Leucine-Rich Repeat- and PYD-Containing 3 (NLRP3)/NF-κB Pathway in Db/Db Mice
Western blot analysis showed that protein levels of HMGB1, NLRP3, apoptosis-associated speck-like protein containing a caspase recruitment domain (ASC), interleukin-1β converting enzyme (Caspase-1), p-NF-κB, and IL-1β increased in db/db mice compared to db/m mice, while ISO (10, 20 mg/kg) significantly decreased their levels in db/db mice (Figure 5A and B). Furthermore, immunohistochemistry showed higher HMGB1 and p-NF-κBp65 levels in the kidney tissues of db/db mice compared to db/m mice, while ISO significantly reduced their levels in db/db mice (Figure 6).
 |
Figure 6 Effects of ISO on inflammatory factors in the kidney. Immunohistochemical staining of HMGB1 and p-NF-κB in the kidney tissues. Magnification: 20x. |
Discussion
Currently several methods have been developed for DN therapy, such as controlling blood pressure and blood sugar, but their efficacy is not satisfactory. In this study, we demonstrated that ISO had an anti-diabetes effect and attenuated kidney damage by targeting the HMGB1/NLRP3/NF-κB pathway.
DN is characterized by chronic hyperglycemia and proteinuria.25 It is important to diminish the risk of diabetes and diabetes complications to prevent DN caused by diabetes.26 In this study we showed that ISO significantly decreased serum TG and TC levels, reduced insulin resistance in db/db mice, reduced serum levels of uric acid and creatinine, and reduced kidney injury caused by hyperglycemia.
Kidney inflammation indicates the activation of immune response and the production of inflammatory cytokines such as IL-6 and TNF-α.27 The production of IL-1β inhibits insulin signaling, leading to insulin resistance.28 Additionally, increased serum IL-6 level indicates the progression of T2DM.29 In this study we found increased levels of IL-1β, TNF-α, and IL-6 in serum and kidney tissues of db/db mice, but ISO markedly reduced their levels in serum and kidney tissues.
It is acknowledged that HMGB1 plays a crucial role in inflammatory cascade. HMGB1 induces the upregulation of IL-6 to regulate inflammatory progression in macrophages.30–32 ISO reduced TC and TG levels and improved lipid metabolism by inhibiting HMGB1 in db/db mice. In particular, we found that ISO inhibited the production of pro-inflammatory factors IL-1β, IL-6, and TNF-α. Moreover, our results showed that ISO reduced the levels of HMGB1, ASC, NLRP3, Caspase-1, p-NF-κB, and IL-1β in db/db mice.
To our knowledge, this is the first study to demonstrate beneficial effects of ISO on DN. More importantly, our results provided a clue that beneficial effects of ISO on DN is related to anti-inflammation activity of ISO, consistent with the recent opinion that targeting inflammation is a promising therapeutic approach for DN.8 However, this study has some limitations. First, we could not perform quantitative analysis of the effects of ISO on pathological changes in the kidney of diabetic mice. Second, we could not determine the signaling pathway that mediates beneficial effects of ISO on DN by using in vitro cellular models.
In conclusion, ISO improved hyperglycemia, insulin resistance, and kidney inflammation in diabetes model mice, and these beneficial effects may be at least partially mediated by the inhibition of HMGB1/NLRP3/NF-κB signaling in the kidney. ISO may be a new agent to treat DN.
Acknowledgment
This study was supported by Natural Science Foundation of Jiangsu Province [BK20180574, BK20171064], which supported the animals, medicine, and page charges in the study design; the Technology Incubator Scheme (Social Programs) of Jiangning District [2018Ca12], the Research Foundation of Jiangsu Provincial Medical Youth Talent, the Project of Invigorating Health Care through Science, Technology and Education [QNRC2016858], the Nanjing Medical University Science Research and Development Foundation [2016NJMU013], the Natural Science Research Fund (General Program) for the Higher Education of Jiangsu Province of China [18KJB350005], and the Zhenjiang City 2017 Science and Technology Innovation Fund [SH2017043].
Disclosure
The authors declare no competing interests.
References
1. Makimattila S, Virkamaki A, Groop PH, Cockcroft J, Utriainen T, Fagerudd J. Chronic hyperglycemia impairs endothelial function and insulin sensitivity via different mechanisms in insulin-dependent diabetes mellitus. Circulation. 1996;94(6):1276–1282. doi:10.1161/01.CIR.94.6.1276
2. Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Res Clin Pract. 2014;103(2):137–149. doi:10.1016/j.diabres.2013.11.002
3. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi:10.2337/diabetes.54.6.1615
4. Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res. 2010;107(9):1058–1070. doi:10.1161/CIRCRESAHA.110.223545
5. Bentata Y, Haddiya I, Latrech H, Serraj K. Progression of diabetic nephropathy, risk of end-stage renal disease and mortality in patients with type-1 diabetes. Saudi J Kidney Dis Transpl. 2013;24(2):392–402. doi:10.4103/1319-2442.109617
6. Hernandez-Arteaga K, Soto-Abraham V, Perez-Navarro M, de Leon-garza B, Rodriguez-Matias A, Olvera-Soto MG. Thrombotic microangiopathy in patients with diabetic nephropathy is associated with low VEGF expression and end-stage renal disease. Clin Nephrol. 2018;89(6):429–437. doi:10.5414/CN109240
7. Ito D, Ikuma-Suwa E, Inoue K, et al. Effects of ipragliflozin on diabetic nephropathy and blood pressure in patients with type 2 diabetes: an open-label study. J Clin Med Res. 2017;9(2):154–162. doi:10.14740/jocmr2875w
8. Giraud Billoud M, Ezquer F, Bahamonde J, Ezquer M. Tubulointerstitial injury and proximal tubule albumin transport in early diabetic nephropathy induced by type 1 diabetes mellitus. Biocell. 2017;41:1–12. doi:10.32604/biocell.2017.41.001
9. Ram C, Jha AK, Ghosh A, et al. Targeting NLRP3 inflammasome as a promising approach for treatment of diabetic nephropathy: preclinical evidences with therapeutic approaches. Eur J Pharmacol. 2020;885:173503. doi:10.1016/j.ejphar.2020.173503
10. Giraud-Billoud M, Fader CM, Agüero R, Ezquer F, Ezquer M. Diabetic nephropathy, autophagy and proximal tubule protein endocytic transport: A potentially harmful relationship. Biocell. 2018;42:35–40. doi:10.32604/biocell.2018.07010
11. Watanabe N, Shikata K, Shikata Y, et al. Involvement of MAPKs in ICAM-1 expression in glomerular endothelial cells in diabetic nephropathy. Acta Med Okayama. 2011;65(4):247–257.
12. Ma J, Wu H, Zhao CY, Panchapakesan U, Pollock C. Requirement for TLR2 in the development of albuminuria, inflammation and fibrosis in experimental diabetic nephropathy. Int J Clin Exp Pathol. 2014;7(2):481–495.
13. Yang SM, Ka SM, Wu HL, et al. Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-kappaB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia. 2014;57(2):424–434. doi:10.1007/s00125-013-3115-6
14. Guzman-Ruiz R, Ortega F, Rodriguez A, et al. Alarmin high-mobility group B1 (HMGB1) is regulated in human adipocytes in insulin resistance and influences insulin secretion in beta-cells. Int J Obes. 2014;38(12):1545–1554. doi:10.1038/ijo.2014.36
15. Zhang C, Chen F, Feng L, et al. FBXW7 suppresses HMGB1-mediated innate immune signaling to attenuate hepatic inflammation and insulin resistance in a mouse model of nonalcoholic fatty liver disease. Mol Med. 2019;25(1):29. doi:10.1186/s10020-019-0099-9
16. Allette YM, Due MR, Wilson SM, Feldman P, Ripsch MS, Khanna R. Identification of a functional interaction of hmgb1 with receptor for advanced glycation end-products in a model of neuropathic pain. Brain Behav Immun. 2014;42:169–177. doi:10.1016/j.bbi.2014.06.199
17. Wu Z, Wei G, Lian G. Synthesis of mangiferin, isomangiferin, and homomangiferin. J Org Chem. 2010;75(16):5725–5728. doi:10.1021/jo100776q
18. Wang B, Shen J, Wang Z, Liu J, Ning Z, Hu M. A novel potent vascular endothelial growth factor receptor 2 kinase inhibitor, suppresses breast cancer growth, metastasis and angiogenesis. J Breast Cancer. 2018;21(1):11–20. doi:10.4048/jbc.2018.21.1.11
19. Montes FQ, Vázquez-Hernández A, Fenton-Navarro B. Active compounds of medicinal plants, mechanism for antioxidant and beneficial effects. Phyton, Int J Exp Botany. 2019;88:1–10.
20. Chellan N, Joubert E, Strijdom H, Roux C, Louw J. Muller CJ: aqueous extract of unfermented honeybush (Cyclopia maculata) attenuates STZ-induced diabetes and beta-cell cytotoxicity. Planta Med. 2014;80(8–9):622–629. doi:10.1055/s-0034-1368457
21. Seo CS, Ha H, Kim YJ, Jungb JY. HPLC-pDA simultaneous determination and protective effect of Anemarrhena asphodeloides against acute renal failure. Nat Prod Commun. 2014;9(6):829–832.
22. Zhang S, Yang J, Li H, et al. Skimmin, a coumarin, suppresses the streptozotocin-induced diabetic nephropathy in wistar rats. Eur J Pharmacol. 2012;692(1–3):78–83. doi:10.1016/j.ejphar.2012.05.017
23. Zhang S, Xin H, Li Y, et al. Skimmin, a coumarin from hydrangea paniculata, slows down the progression of membranous glomerulonephritis by anti-inflammatory effects and inhibiting immune complex deposition. Evid Based Complement Alternat Med. 2013;2013:819296.
24. Sen Z, Weida W, Jie M, Li S, Dongming Z, Xiaoguang C. Coumarin glycosides from Hydrangea paniculata slow down the progression of diabetic nephropathy by targeting Nrf2 anti-oxidation and smad2/3-mediated profibrosis. Phytomedicine. 2019;57(57):385–395. doi:10.1016/j.phymed.2018.12.045
25. Sun YM, Su Y, Li J, Wang LF. Recent advances in understanding the biochemical and molecular mechanism of diabetic nephropathy. Biochem Biophys Res Commun. 2013;433(4):359–361. doi:10.1016/j.bbrc.2013.02.120
26. Reddy GR, Kotlyarevska K, Ransom RF. The podocyte and diabetes mellitus: is the podocyte the key to the origins of diabetic nephropathy? Curr Opin Nephrol Hypertens. 2008;17(1):32–36. doi:10.1097/MNH.0b013e3282f2904d
27. Sasaki N, Itakura Y. Ganglioside GM1 contributes to extracellular/intracellular regulation of insulin resistance, impairment of insulin signaling and down-stream eNOS activation, in human aortic endothelial cells after short- or long-term exposure to TNFalpha. Oncotarget. 2018;9(5):5562–5577. doi:10.18632/oncotarget.23726
28. Aye IL, Jansson T. Interleukin-1beta inhibits insulin signaling and prevents insulin-stimulated system A amino acid transport in primary human trophoblasts. Mol Cell Endocrinol. 2013;381(1–2):46–55. doi:10.1016/j.mce.2013.07.013
29. Liu C, Feng X, Li Q, Wang Y, Li Q. Adiponectin, TNF-alpha and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine. 2016;86:100–109. doi:10.1016/j.cyto.2016.06.028
30. Nogueira-Machado JA, Volpe CM, Veloso CA. HMGB1, TLR and RAGE: a functional tripod that leads to diabetic inflammation. Expert Opin Ther Targets. 2011;15(8):1023–1035. doi:10.1517/14728222.2011.575360
31. Li L, Ling Y, Huang M, et al. Heparin inhibits the inflammatory response induced by LPS and HMGB1 by blocking the binding of HMGB1 to the surface of macrophages. Cytokine. 2015;72(1):36–42. doi:10.1016/j.cyto.2014.12.010
32. Tang D, Kang R, Livesey KM, et al. Endogenous HMGB1 regulates autophagy. J Cell Biol. 2010;190(5):881–892. doi:10.1083/jcb.200911078
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.