Back to Journals » Drug Design, Development and Therapy » Volume 13
Isolation, pharmacological evaluation and molecular docking studies of bioactive compounds from Grewia optiva
Authors Ul Bari W, Zahoor M , Zeb A , Sahibzada MUK , Ullah R, Shahat AA , Mahmood HM, Khan I
Received 23 June 2019
Accepted for publication 2 August 2019
Published 26 August 2019 Volume 2019:13 Pages 3029—3036
DOI https://doi.org/10.2147/DDDT.S220510
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Prof. Dr. Yan Zhu
Wasim Ul Bari,1 Mohammad Zahoor,1 Alam Zeb,2 Muhammad Umar Khayam Sahibzada,3 Riaz Ullah,4 Abdelaaty A Shahat,4,5 Hafiz Majid Mahmood,6 Irfan Khan1
1Department of Chemistry, University of Malakand, Dir Lower, Chakdara, KPK 18800, Pakistan; 2Department of Biotechnology, University of Malakand, Dir Lower, Chakdara, KPK 18800, Pakistan; 3Department of Pharmacy, Sarhad University of Science and Information Technology, Peshawar, KPK 25000, Pakistan; 4Medicinal, Aromatic and Poisonous Plants Research Center (MAPRC), College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia; 5Phytochemistry Department, National Research Centre, Giza, Egypt; 6Department of Pharmacology, College of Pharmacy, King Saud University, Riyadh 11451, Saudi Arabia
Correspondence: Mohammad Zahoor
Department of Chemistry, University of Malakand Chakdara, Dir Lower, KPK 18800, Pakistan
Email [email protected]
Muhammad Umar Khayam Sahibzada
Department of Pharmacy, Sarhad University of Science and Information Technology, Peshawar, KPK 25000, Pakistan
Email [email protected]
Background: Traditionally, Grewia optiva is widely used for the treatment of many diseases like dysentery, fever, typhoid, diarrhea, eczema, smallpox, malaria and cough.
Methods: Shade-dried roots of G. optiva were extracted with methanol. Based on HPLC results, chloroform and ethyl acetate fractions were subjected to silica column isolation and four compounds: glutaric acid (V), 3,5 dihydroxy phenyl acrylic acid (VI), (2,5 dihydroxy phenyl) 3’,6’,8’-trihydroxyl-4H chromen-4’-one (VII) and hexanedioic acid (VIII) were isolated in pure form. Ellman’s assay was used to determine the anticholinesterase potential of isolated compounds while their antioxidant potential was estimated by DPPH and ABTS scavenging assays.
Results: Amongst the isolated compounds, VI and VII exhibited excellent percent inhibition of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) (83.23±1.11, 82.72±2.20 and 82.11±2.11, 82.23±1.21, respectively, at 1000 μg/mL) with IC50 of 76, 90, 78 and 92 μg/mL, respectively. Highest percent radicals scavenging against DPPH and ABTS (87.41±1.20 and 86.13±2.31) with IC50 of 64 and 65 μg/mL, respectively, were observed for compound VII. Molecular docking studies also supported the binding of compound VI and VII with the target enzyme. The para-hydroxyl group of the phenolic moiety is formed hydrogen bonds with the active site water molecule and the side chain carbonyl and hydroxyl residues of enzyme.
Conclusion: The isolated compounds inhibited the DPPH and ABTS-free radicals, and AChE and BChE enzymes. It was concluded that these compounds could be used in relieving the oxidative stress and pathological symptoms associated with excessive hydrolysis of acetyl and butyryl choline. The results of the study were supported by docking studies for compounds VI and VII.
Keywords: G. optiva, antioxidant, acetylcholinesterase, butyrylcholinesterase, molecular docking
Introduction
Grewia optiva belongs to the family Tiliaceae, having almost 150 species dispersed widely in various parts of the world, mainly in tropical and subtropical regions. Most of the species belonging to genus Grewia are reported from India, northern territories of Pakistan and Bangladesh, South Africa, Northern Australia, China, Thailand, Madagascar and Malaysia. Ten species have been reported up till now from Pakistan and are used to cure fever, dysentery, cough, malaria, typhoid, smallpox, eczema and diarrhea by local communities. Previously, antibacterial, antioxidant and antimalarial activities have been reported for many species of this genus.1,2
In human body, a number of reactive/hazardous substances are continuously produced which are detoxified by the intricate body machinery. However, human is prone to a number of threats not posed by nature but created by human itself like industrial contaminants and excessive use of seasoning agent in food which leads to the formation of excessive amounts of free radicals than the body detoxifying capacity. Plants, like human, have intricate systems and being self-nourished they can produce secondary metabolites if they face threats from surrounding environment. Therefore, plants all over the world have been explored by scientists to isolate compounds for curing other diseases and oxidative stress.3–7 The compounds that help in relieving oxidative stress are called antioxidants. Although in food industries the preservatives in use are synthetic antioxidants, they have shown toxic and carcinogens properties.3,8–12 As plant products are associated with low side effects, isolation of antioxidants from plant origin is the cry of the day.
A very common neurodegenerative syndrome caused by behavioral turbulence, limitation in the routine activities and cognitive dysfunction is called Alzheimer disease (AD), which is reported to have its maximum incidence amongst the old age peoples.13 Acetylcholine (ACh) and butyrylcholine (BCh), the important neurotransmitters and dynamic in the procurement and storage of transmitted memory, have an important role in the impulse transmission through the synapse. It is found that the levels of ACh and BCh are insufficient in AD.14 To restore and control the activities of ACh and BCh at the synapse, inhibitors of acetylcholinesterase (AChE) and butyrylcholinesterase (BChE) are used. Two natural occurring compounds including rivastigmine and galanthamine are clinically approved inhibitors of cholinesterase.15,16 However, they are not 100% efficient and new plants are under investigation to isolate efficient inhibitors of the mentioned enzymes.
Compounds like nitidanin, grewin, Harman, contanoic acid, vitexin and gulonic acid have been reported from different species of genus Grewia.17,18 Heneicosanoic acid, β-sitosterol, propyl palmitate and catechin have been isolated from G. Biloba by Liu.19 Ahamed reported γ-lactone and Gulconic acid from G. tiliafolia in 2009. Later on, he also isolated lactone, gluonic acid and D-erythro-2-hexenoic acid from the same plant.17,20 Compounds with anti-diabetic potential, viz., cyclopentadeca-4, 12-dienone from Grewia hirsute have been reported by Abirami.21 Tridecanoic acid, Octanoic acid, Eicosanoic acid and Octadecatrienoic acid from Grewia tenax have been reported by Mital.22
After extensive review of literature, we found that roots of G. optiva have not been investigated for phytochemicals so far. So this study was designed to isolate compounds from the roots of G. optiva, which resulted in the isolation of four active chemical constituents (from chloroform and ethyl acetate fractions): glutaric acid (V), 3,5 dihydroxy phenyl acrylic acid (VI), (2,5 dihydroxyl phenyl) 3',6',8' trihydroxyl-4H chromen −4'- one (VII) and Hexanedioic acid (VIII). The structures of new compounds have been confirmed through 1D, 2D NMR and LC-MS spectroscopic analysis. The in-silico molecular docking of the synthesized compounds was performed using AChE and BChE crystal structure (PDB ID: 1ACL, 4BOP) to ascertain the role of different functionalities in the formation of ligand protein complex.
Materials and methods
For isolation of active compounds, silica gel column (mesh size 70–230, E. Merck, 0.063–0.200 Mm) was used. Purification of compounds was carried by Biotage Grace and HPLC. Preparative TLC was done with the help of silica gel 60 PF254 (E. Merck). The compounds were visualized with the help of solid iodine and CeSO4 spray and detected at 254 and 266 nm.1 H and13 C NMR, COSY, DEPT, HMBC and HSQC spectra were drawn through Bruker Spectrometers (Avance Av 500, 600/150 MHz) and chemical shifts (δ) in ppm/coupling constants (J) in Hz were measured.
Plant materials
The roots of the selected plant were collected from village Sia Haidar, Lower Dir, KPK, Pakistan, in March 2016. The plant was authenticated by an expert from the Department of Botany, University of Malakand and voucher specimen (1022HU) was deposited in Herbarium of University.
Extraction and fractionation
About 5 kg root samples were cleaned to remove dust using tap water which were then dried in shade for a few weeks. The dried roots were then mechanically ground to fine powder and macerated in methanol:water (95:5) mixture for 3 days. At the end of this period, the solvent was allowed to settle down, decant (without disturbing the sediment) and filtered using Whattman filter paper. The solvent was removed using a rotary evaporator under vacuum at 45ºC and crude extract of about 400 g in semi-solid state was obtained. The crude extract was fractionated into aqueous, chloroform, ethyl acetate, butanol and n-hexane fractions. Almost all crude extract (400 g) was added into 1800 mL of distilled water and fractionated through solvent/solvent extraction. Based on HPLC results, chloroform and ethyl acetate fractions were found rich in bioactive compounds and were subjected to column isolation.
The ethyl acetate and chloroform soluble fractions were subjected to evaporation under vacuum to get gummy residue of ethyl acetate (23 g) and chloroform (31 g) which were separately subjected to isolation through column chromatography. The process of elution was performed with n-hexane–ethyl acetate in an increasing order of polarity. After elution, few of the fractions were recombined on the basis of TLC profiling to afford twenty fractions.
The fraction (A-16) was eluted from the chloroform main fraction that yielded C-4 and C-5 sub-fractions. These were recombined on the basis of TLC analysis and further eluted using pencil column which leads to the formation of impure crystals. These crystals were collected and washed with non-polar organic solvents to yield 22 mg of compound (V).
The fraction (A-12) was subjected to Biotage (normal phase chromatography) and obtained Z-1 to Z-4 fractions. The Z-2 fraction was further analyzed using Grace Column (Reverse phase chromatography) and obtained D-2 fraction which was subjected to LC-MS but only a few impurities were detected. To remove the impurities, it was further subjected to HPLC and the compound (VI) weighing about 21 mg was purified, later confirmed by LC-MS.
The fraction (A-1) was subjected to Biotage and obtained Z-10 to Z-17 fractions. TLC analysis revealed that some of these fractions have same RF values, so they were combined and subjected to Grace Column. After treating with Grace Column, compound (VII) was obtained along with impurities, which were removed using HPLC. At last, 18 mg of this compound was obtained.
The fraction (A-11) was subjected to Biotage and obtained Z-22 to Z-32 fractions. Z-24 was further run on Grace Column and V-11 fraction was obtained, which was later on subjected to LC-MS analysis for the detection of impurities. The impurities were removed by HPLC and 24 mg of compound (VIII) was obtained which was purified and confirmed by LC-MS.
DPPH antioxidant assay
The values of antioxidant potential of the isolated compounds were calculated using diphenyl picrylhydrazyl (DPPH) assay.23 About 10 mg of DPPH was dissolved in 50 mL of methanol which was further diluted to adjust its absorbance to 0.70 at 515 nm. For the generation of free radicals, the stock solution was put in dark for 24 hrs. Sample solutions were prepared by dissolving 5 mg of the isolated compounds in 5 mL methanol. Different diluted solutions having concentrations 62.5, 125, 250, 500, 1000 μg/mL were prepared and 1.5 mL from each sample solutions were mixed with 1.5 mL DPPH and incubated for 15 mins. Percent DPPH scavenging by the isolated compounds was determined using the following relation:
(i)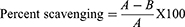
A = Control absorbance, B = Sample absorbance.
ABTS antioxidant assay
The isolated compounds were also tested by another antioxidant assay (ABTS) using already reported procedure in literature.24,25 Different dilutions of samples prepared in the above step were added to ABTS solution and incubated for 15 mins. After incubation, the absorbance was measured at 745 nm. Scavenging activities of isolated were calculated by equation (i).
Anticholinesterase assay
To evaluate the isolated compounds for their AChE and BChE inhibitory potential Ellmen’s assay was used with slight modifications.14,15,23,24 Standard solutions of compounds were prepared by dissolving 5 mg of isolated compounds in 5 mL of methanol, each of which was further diluted to prepare working standards (125, 250, 500, 1000 μg/mL). Enzyme and substrate solutions were prepared in 6.8 PH phosphate buffer solutions. Following standard procedure of Ellmen’s assay, the samples after 15 mins incubation were subjected to UV/Visible analysis. The absorbance values were inserted in following relations to calculate reaction rate, percent inhibition and activities of the enzymes.
(ii) (iii) (iv)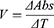
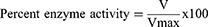
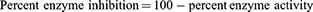
Molecular docking simulations
In the present study, all the isolated compounds (V–VIII) were subjected to molecular docking simulation studies for the possible binding orientations with the crystal structure of the AChE and BChE. For this purpose, we used the crystal structure of the AChE and BChE (PDB: 1ACL, 4BOP) from the RCSB Protein Data Bank. All ligands were prepared by (Schrödinger) Lig: prepared in their neutral form and optimizing their conformation in the OPLS-3 force field. The protein structure was prepared by using the Protein Preparation (Schrödinger) for adding hydrogen and setting protonation states appropriate at pH 7. The receptor grid box was defined as 20 Å box with the active site water molecule in its center. Molecular Docking was performed with Glide (Schrödinger) using XP extra precision with evasion settings and glide scoring function, reporting the 15 top-ranked poses for each ligand. Visual review of the binding pose and generation of figure was done with (Schrödinger) Maestro.26
Results and discussion
Our exploration led to the isolation of four compounds (V–VIII) from chloroform and ethyl acetate fractions. The chemical structures of these compounds have been presented in Figure 1.
Glutaric acid (V)
Glutaric acid is an amorphous solid having melting point 95–99°C and is slightly soluble in water. LR-EI-MS confirm its molecular formulae C5H8O4 and molecular mass giving molecular ion peak [M+] at 131.12 g/mol.
1H NMR (600 MHz, Methanol-d4): δ 2.38 (t, J =7.4 Hz, 4H, H-2, 4), 1.89 (p, J =7.4 Hz, 2H, H-3), 12.01 (s, 2H, H-1, 5).
13C NMR (151 MHz, Methanol-d4): δ 19.95 (C-3), 32.52 (C-2, 4), 175.50 (C-1, 5). HMBC, HSQC and COSY also confirm the structure.
3,5 dihydroxy phenyl acrylic acid (VI)
The 3,5 dihydroxy phenyl acrylic acid is an amorphous solid, slightly soluble in water and have melting point 222–228°C. LR-EI-MS confirm its molecular formulae C9H8O4 giving molecular ion peak [M+] at 180.16 g/mol.
1H NMR (600 MHz, DMSO-d6): δ 7.41 (d, J =15.8 Hz, 1H, H-6), 7.02 (d, J =2.1 Hz, 1H, H-2), 6.96 (dd, J =8.2, 2.1 Hz, 1H, H-4), 6.76 (d, J =8.1 Hz, 1H, H-7), 6.17 (d, J =15.8 Hz, 1H, H-8), 9.13 (s, 1H, H-3), 9.52 (s, 1H, H-5).
13C NMR (151 MHz, DMSO-d6): δ40.09 (C-9), 115.09 (C-4), 116.19 (C-6), 121.60 (C-7), 126.15 (C-8), 145.04 (C-1), 146.01 (C-2), 148. 59 (C-5), 168.37 (C-3). HMBC, HSQC and COSY also confirm the structure.
(2,5dihydroxyl phenyl) 3',6',8' trihydroxyl-4H chromen-4'- one (VII)
The (2,5dihydroxyl phenyl) 3',6',8' trihydroxyl-4H chromen −4'- one is an amorphous solid, melting point is 312–316°C and is soluble in water. LR-EI-MS confirm its molecular formulae C15H10O7 giving molecular ion peak [M+] at 302.23 g/mol.
1H NMR (600 MHz, DMSO-d6): δ10.78 (s, 1OH, H-6'),9.59 (s, 1OH, H-8'),9.36 (s, 1OH, H-3'),9.30 (s, 1OH, H-2), 7.68 (d, J =2.2 Hz, 1H, H-3), 7.54 (dd, J =8.5, 2.2 Hz, 1H, H-4), 6.89 (d, J =8.4 Hz, 1H, H-6), 6.41 (d, J =2.1 Hz, 1H, H-9'), 6.17 (d, J =6 Hz, 1H, H-7').
13C NMR (151 MHz, DMSO-d6): δ93.80 (C-9'), 98.64 (C-7'), 103.46 (C-5'), 115.52 (C-1,6), 116.06 (C-4), 120.43 (C-3), 136.19 (C3'), 145.52 (C-2',8), 147.27 (C-5,6'), 148.16 (C-2,10), 164.38 (C-4'). HMBC, HSQC and COSY also confirm the structure.
Hexanedioic acid (VIII)
Hexanedioic acid is an amorphous solid; slightly soluble in water and having melting point 152.1°C. LC-MS confirm its molecular formulae C6H10O4, giving molecular ion peak [M+] at 146.14 g/mol.
1H NMR (600 MHz, DMSO-d6): δ 2.20 (ddt, J =7.4 Hz, 4H, 2CH2), 1.50 (h, J =3.3 Hz, 4H, 2CH2), 10.32 (s, 2OH).
13C NMR (151 MHz, DMSO-d6): δ24.47 (C-3,4), 33.82 (C-2,5), 174.81 (C-1,6).
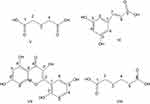 |
Figure 1 Structures of bioactive compounds (V–VIII) isolated from roots of G. optiva. |
Antioxidant potentials of isolated compounds (V–VIII)
Table 1 shows the antioxidant ability of the isolated bioactive compounds determined through DPPH and ABTS assays. Compounds VI and VII showed highest scavenging capabilities (85.21±1.10 and 84.13±2.1 at 1000 μg/mL) of DPPH radical with IC50 values of 65 and 64 μg/mL, respectively, followed by compound V (IC50=72 μg/mL, percent inhibition=82.13±1.21). The compounds that most potently scavenged ABTS-free radical were VI and VII again showing excellent percent inhibition of 84.13±2.11 and 86.13±2.31 at 1000 μg/mL, respectively, with IC50 of 67 and 65 μg/mL. Positive control ascorbic acid was used and its IC 50 value was about 35 μg/mL. Other two compounds also showed good inhibitory potential against ABTS-free radical.
 |
Table 1 Antioxidant potential of compounds V–VIII isolated from Grewia optiva |
Anticholinesterase potentials of isolated compounds
A number of pathological conditions relating to nervous coordination of human bodies are associated with the reduction of ACh and BCh levels. Their constant level can be restored if AChE and BChE are inhibited. The AChE and BChE percent inhibition by isolated compounds and their 1C50values are presented in Table 2. Compounds VI and VII showed excellent inhibition with the IC50 values of 76 and 90 μg/mL (87.66±1.93 and 82.72±2.20% inhibition) respectively, against AChE. Compounds V and VIII also showed good percent inhibition (79.13±1.42 and 79.61±2.10) with IC50 of 118 and 122 μg/mL, respectively.
 |
Table 2 Anticholinesterase activities of the bioactive compounds (V–VIII) isolated from Grewia optiva |
Compounds VI and VII were again good inhibitors of BChE (IC50 value of 78 and 92 μg/mL, and percent inhibition 82.11±2.11 and 82.23±1.21). Compounds V and VIII moderately inhibited BChE with IC50 of 123 and 124 μg/mL, respectively. As a positive control, galanthamine was used. Compounds VI and VII exhibited highest potentials against the studied enzymes; therefore, molecular docking studies were performed for these two to support the finding of the study.
Molecular docking studies
The computational docking studies were performed to predict the most favorable binding mode inside the binding pockets of the two proteins with suitable orientation in terms of docking score. The findings were counter varified from IC50 values in the anticholinesterase assay compounds VI and VII as both of them have shown excellent potential against AChE and BChE. The molecular structures of both of them showed excellent interaction with the enzyme crystal structures with the docking score of −9.64 and −8.31 Kcal/mol against AChE and −7.22 and −6.12 Kcal/mol against BChE, respectively. Such lower values indicate very decent fitness of the compound in the binding pocket of the protein showing that compound has established good interaction with protein. The selected pose of compound VII was visualized in order to determine the amino acids involved in binding with the ligand to the crystal structure of the protein. Here, the phenolic moiety of the compound interacts with the enzyme through strong H-bond (2.33A°) between the hydroxyl and the NH2 group of the active site (Gly119) of AChE. The PHE 331 (2.11A°) and SER 200 (2.33A°) of the active site were also interacted with OH of the ligand through H-bond. On the other hand, ILE-315 and LYS-327 amino acids of the active sites of BChE interact with ligand molecules by H-bond (2.05 A°, 1.44 A°); HOH-2016 of the protein molecule also interact with the said ligand (1.92 A°). Surface and ball and stick representation of the docking pose in the active site of AChE and BChE (1 ACL, 4BOP), showing the highly ranked pose for active compound VII (green). It was predicted that inhibitor bind with the active site in the space close to the water molecule.
The selected pose of compound VI was also visualized in order to find out binding of the amino acids of AChE in the crystal structure of the protein, involved with the ligand. Here), PHE-331 interact (2.30 A°) with phenolic moiety of the ligand. SER-200 also form H-bonding (1.80 A°) with the hydroxyl group of the ligand. In case of BChE, ATG-33 interact with water, ASP-332 and ARG-323 form strong H-bond interaction (2.06A°, 1.58 A°) with the hydroxyl group of ligand
Conclusion
In this study, we isolated four bioactive compounds, namely, glutaric acid (V), 3,5-dihydroxy phenyl acrylic acid (VI), (2,5 dihydroxyl phenyl) 3',6',8' trihydroxyl-4H chromen −4'- one (VII) and Hexanedioic acid (VIII). Although they have been reported previously from genus Grewia, for the first time they are reported from the roots of G. optiva. Compounds VI and VII showed strong anticholinesterase and antioxidant potentials. Though the potency of these compounds was less compared to the standard drugs, so far its dual efficiency (enzymes inhibition and free radicals scavenging capacity) may be of probable benefit. Docking studies of compounds VI and VII (be used as anticholinesterase inhibitors) support the finding of the study.
Availability of data
The data will be provided in the form of a thesis, and images for the molecular docking studies are also available, by request to the corresponding author.
Acknowledgments
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research grant number RG-1440-100. The authors are grateful for the financial support (Project No: 20-2515/R&D/HEC) from the Higher Education commission of Pakistan.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Uddin G, Ullah W, Siddiqui BS, Shah SQ. Grewialin and optivanin new constituents from the stem bark of grewia optiva drummond ex burret (Tiliaceae). Nat Prod Res. 2013;27(3):215–220. doi:10.1080/14786419.2012.666749
2. Anwar J, Shah HU, Ali R, Iqbal Z, Khan SM. Antioxidant activity and phytochemical screening of stem bark extracts of grewia optiva drummond ex burret. J Pharmacogn Phytochemi. 2015;3(6):179–182.
3. Zahoor M, Shafiq S, Ullah H, Sadiq A, Ullah F. Isolation of quercetin and mandelic acid from aesculus indica fruit and their biological activities. BMC Biochem. 2018;19(1):5. doi:10.1186/s12858-018-0095-7
4. Nazir N, Zahoor M, Nisar M, et al. Phytochemical analysis and antidiabetic potential of elaeagnus umbellata (Thunb.) in streptozotocin-induced diabetic rats: pharmacological and computational approach. BMC Complement Altern Med. 2018;18(1):332. doi:10.1186/s12906-018-2317-3
5. Zahoor M, Zafar R, Rahman NU. Isolation and identification of phenolic antioxidants from pistacia integerrima gall and their anticholine esterase activities. Heliyon. 2018;4(12):e01007. doi:10.1016/j.heliyon.2018.e01007
6. Zohra T, Ovais M, Khalil AT, et al. Bio-guided profiling and HPLC-DAD finger printing of atriplex lasiantha boiss. BMC Complement Altern Med. 2019;19(1):4. doi:10.1186/s12906-018-2416-1
7. Khayam SM, Zahoor M, Shah AB. Biological and phytochemical evaluation of cotoneaster microphyllus, ficus auriculata and calotropis procera. Latin Am J Pharm. 2019;38(5):945–953.
8. Sawadogo W, Maciuk A, Banzouzi J, et al. Mutagenic effect, antioxidant and anticancer activities of six medicinal plants from burkina faso. Nat Prod Res. 2012;26(6):575–579. doi:10.1080/14786419.2010.534737
9. Pieme C, Penlap V, Ngogang J, Costache M. In vitro cytotoxicity and antioxidant activities of five medicinal plants of malvaceae family from Cameroon. Environ Toxicol Pharmacol. 2010;29(3):223–228. doi:10.1016/j.etap.2010.01.003
10. Zhang L, Ravipati AS, Koyyalamudi SR, et al. Antioxidant and anti-inflammatory activities of selected medicinal plants containing phenolic and flavonoid compounds. J Agric Food Chem. 2011;59(23):12361–12367. doi:10.1021/jf203146e
11. Suryanti V, Marliyana S, Putri H. Effect of germination on antioxidant activity, total phenolics,[Beta]-carotene, ascorbic acid and [alpha]-tocopherol contents of lead tree sprouts (Leucaena leucocephala (lmk.) de Wit). Int Food Res J. 2016;23(1):167.
12. Hamid A, Aiyelaagbe O, Usman L, Ameen O, Lawal A. Antioxidants: its medicinal and pharmacological applications. Afr J Pure Appl Chem. 2010;4(8):142–151.
13. Ayaz M, Junaid M, Ullah F, et al. Anti-Alzheimer’s studies on β-sitosterol isolated from polygonum hydropiper L. Front Pharmacol. 2017;8:697. doi:10.3389/fphar.2017.00697
14. Ahmad S, Ullah F, Sadiq A, et al. Chemical composition, antioxidant and anticholinesterase potentials of essential oil of rumex hastatus D. Don collected from the North West of Pakistan. BMC Complement Altern Med. 2016;16(1):29. doi:10.1186/s12906-016-0998-z
15. Hasnat M, Pervin M, Lim B. Acetylcholinesterase inhibition and in vitro and in vivo antioxidant activities of ganoderma lucidum grown on germinated brown rice. Molecules. 2013;18(6):6663–6678. doi:10.3390/molecules18066663
16. Ullah F, Ayaz M, Sadiq A, et al. Phenolic, flavonoid contents, anticholinesterase and antioxidant evaluation of Iris germanica var; florentina. Nat Prod Res. 2016;30(12):1440–1444. doi:10.1080/14786419.2015.1057585
17. Ahamed MBK, Krishna V, Dandin CJ. In vitro antioxidant and in vivo prophylactic effects of two γ-lactones isolated from grewia tiliaefolia against hepatotoxicity in carbon tetrachloride intoxicated rats. Eur J Pharmacol. 2010;631(1–3):42–52. doi:10.1016/j.ejphar.2009.12.034
18. Waliullah GU, Rauf A, Siddiqui BS, Rehman T, Azam S, Qaisar M. Chemical constituents and biological screening of grewia optiva drummond ex burret whole plant. Am Eurasian J Agric Environ Sci. 2011;11(4):542–546.
19. Liu J, Wu J, Kou X, Hong Q. Studies on chemical constituents from grewia biloba. Zhong Yao Cai. 2008;31(10):1505–1507.
20. Ullah W, Uddin G, Siddiqui BS. Ethnic uses, pharmacological and phytochemical profile of genus grewia. J Asian Nat Prod Res. 2012;14(2):186–195. doi:10.1080/10286020.2011.639764
21. Natarajan A, Sugumar S, Bitragunta S, Balasubramanyan N. Molecular docking studies of (4 Z, 12 Z)-cyclopentadeca-4, 12-dienone from grewia hirsuta with some targets related to type 2 diabetes. BMC Complement Altern Med. 2015;15(1):73. doi:10.1186/s12906-015-0588-5
22. Aadesariya MK, Ram VR, Dave PN. Extraction, Isolation and Identification of Useful Phyto Constituents from Dichloromethane Leave Extract of Abutilon Pannosum and Grewia Tenax Using Q-TOF LC/MS. IJARCS. 2017;4(10):1-14. doi: 10.20431/2349-0403.0410001
23. Ovais M, Ayaz M, Khalil AT, et al. HPLC-DAD finger printing, antioxidant, cholinesterase, and α-glucosidase inhibitory potentials of a novel plant olax nana. BMC Complement Altern Med. 2018;18(1):1. doi:10.1186/s12906-018-2317-3
24. Ayaz M, Junaid M, Ahmed J, et al. Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from polygonum hydropiper L. BMC Complement Altern Med. 2014;14(1):145. doi:10.1186/1472-6882-14-145
25. Zahoor M, Shah AB, Gul S, Amin S. HPLC-UV analysis of antioxidants in citrus sinensis stem and root extracts. J Chem Soc Pak. 2018;40(3):595.
26. Pintus F, Matos MJ, Vilar S, et al. New insights into highly potent tyrosinase inhibitors based on 3-heteroarylcoumarins: anti-melanogenesis and antioxidant activities, and computational molecular modeling studies. Bioorg Med Chem. 2017;25(5):1687–1695. doi:10.1016/j.bmc.2017.01.037
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
