Back to Journals » OncoTargets and Therapy » Volume 7
Isolation, detection, and immunomorphological characterization of circulating tumor cells (CTCs) from patients with different types of sarcoma using isolation by size of tumor cells: a window on sarcoma-cell invasion
Authors Chinen L, Melo C, Ali Abdallah E, Ocea L, Buim M, Breve N, Gasparini Junior J, Fanelli M, Paterlini-Bréchot P
Received 13 February 2014
Accepted for publication 13 May 2014
Published 16 September 2014 Volume 2014:7 Pages 1609—1617
DOI https://doi.org/10.2147/OTT.S62349
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Ludmilla T Domingos Chinen,1 Celso A Lopes Mello,2 Emne Ali Abdallah,1 Luciana MM Ocea,1 Marcilei E Buim,1 Natália M Breve,1 José Luiz Gasparini Junior,1 Marcello F Fanelli,2 Patrizia Paterlini-Bréchot3
1International Research Center, 2Department of Clinical Oncology, AC Camargo Cancer Center, São Paulo, Brazil; 3Unité INSERM U807, Université Paris Descartes, Paris, France
Background: Sarcomas are rare and heterogeneous neoplasms with poor prognosis that are thought to spread to distant organs mainly by hematogenous dissemination. However, circulating tumor cells (CTCs) have never been visualized in sarcomas.
Objectives: To investigate the feasibility of using isolation by size of tumor cells (ISET) for isolation, identification, and characterization of CTCs derived from patients with high-grade and metastatic sarcomas.
Patients and methods: We studied eleven patients with metastatic/recurrent or locally advanced soft-tissue sarcomas (STSs), six of whom had synovial sarcomas. Blood samples (8 mL) were collected from patients with advanced STS and treated by ISET, a marker- independent approach that isolates intact CTCs from blood, based on their larger size compared with leukocytes. CTCs were identified by cytomorphology and characterized by dual-color immunocytochemistry using antivimentin or anti-Pan CK, and anti-CD45.
Results: All patients with STS included in this study showed CTCs, with numbers ranging from two to 48 per 8 mL of blood.
Conclusion: This study shows the feasibility of isolating, identifying, and characterizing CTCs from patients with different types of sarcomas and the presence of circulating sarcoma cells in all the tested patients. Our results set the basis for further studies aimed at exploring the presence, number, and immunomolecular characteristics of CTCs in different types of sarcoma, and bring more light to the mechanisms of tumor invasion for these tumors.
Keywords: sarcoma, circulating tumor cells, ISET
Introduction
Sarcomas are relatively rare neoplasms with poor prognosis. They are broadly classified as either soft-tissue sarcomas (STSs), including more than 50 histologic subtypes, or bone neoplasms. Sarcomas represent 1% of all cancers in adults, 10% in children, and 8% in adolescents and young adults.1 Despite their rarity, sarcomas contribute to a substantial loss of years of life compared to other cancers, because of the many children, adolescents, and young adults diagnosed. Furthermore, their rarity and diversity across ages render diagnosis and treatment difficult.
Sarcomas most typically present spontaneously as a mass without a demonstrable cause. However, they have been associated with exposure to radiation, chemotherapeutic agents, viral infections, occupational factors, and hereditary syndromes.1 Their prognosis is variable according to the histologic type, but often poor and presumably related to hematogenous spread. For instance, only 16% of patients surgically treated for osteosarcoma have long-term survival, suggesting that micrometastasis is present in an overwhelming majority of newly diagnosed patients.1 Many STSs display a remarkable predilection for metastasizing through hematogenous circulation to the lungs and/or other sites, such as the liver, bones, and subcutaneous tissue. A minority of subtypes (synovial sarcoma, rhabdomyosarcoma, epithelioid sarcoma, clear-cell sarcoma, and angiosarcoma) may metastasize to lymph nodes and other sites of the body. However, lymph-node metastasis is rarely found in STS.2,3
Surgery and adjuvant radiation therapy result in high rates of control of localized diseases, but reliable staging of localized tumors is very challenging. On average, more than 50% of patients with high-grade STS develop tumor relapse and die due to tumor progression.4 Patients with metastatic disease are treated with palliative chemotherapy, with modest impact on survival and median survival of 12 months on most trials.5,6
As a result, there is an urgent need for new markers helping to identify patients without metastasis but with “early stage” invasive sarcoma, and to follow the tumor response to treatment in order to optimize the timing, dose, and type of anticancer therapy. The ideal marker in this domain is represented by circulating tumor cells (CTCs). CTCs are the earliest hallmark of tumor invasion. They are known to circulate in the blood for months or years before metastases develop,7–9 and can thus reliably help to identify patients in the intermediate stage between localized and metastatic, who are presently not identified and thus either untreated or treated with nonpersonalized protocols.
CTCs are also the natural target of antitumor treatments, and thus their persistence beyond anticancer treatments can provide the important information that the treatment has been inefficient before metastases develop. Finally, the CTC compartment is meant to contain the most malignant tumor cells,9 which can be characterized by immunological and molecular studies to help unravel the invasion mechanisms and find new and effective targeted treatment.
Despite the clinical evidence that CTCs should circulate in the blood of patients with sarcomas, few studies have dealt with this subject, as CTCs are rare and their isolation is a technical challenge. Furthermore, the majority of approaches developed to isolate CTCs have targeted CTCs derived from carcinomas and used (for the cell-capture step) antibodies directed against epithelial antigens, which are certainly not suitable to isolate CTCs from sarcoma.
Isolation by size of tumor cells (ISET) was initially developed to isolate CTCs from carcinomas. However, since the approach is independent of any cellular marker and relies on the tumor cellular size larger than the size of leukocytes, which is a physical property common to the vast majority of cancer cells from solid tumors, we thought that the ISET system could also be able to efficiently isolate CTCs from patients with sarcoma. We show here that by using ISET, CTCs were found in the blood of all the tested patients with advanced STS. This result should open new pathways for the follow-up and treatment of patients with sarcomas.
Patients and methods
Patients
Blood samples (8 mL) were collected from patients and healthy subjects who gave written informed consent to this study, which obtained approval by the local research ethics committee (protocol 1367-10). Eleven patients with metastatic/recurrent or locally advanced disease STS treated at the AC Camargo Cancer Center in São Paulo, Brazil were included in this study between July and October 2013. The majority of patients (six of eleven) presented synovial sarcoma. The main site of metastasis was lung (five of eleven), and age varied between 26 and 62 years. Patients’ clinicopathological parameters were obtained from medical records. Patients were evaluated individually, according to the tumor’s histological subtype (Table 1). Thirty healthy subjects (blood donors) were also studied as controls.
  | Table 1 Clinical characteristics of included patients |
In vitro sensitivity of ISET for isolation of mesenchymal tumor cells
The in vitro sensitivity of ISET for isolation of sarcoma cells was tested by spiking 25, 50, 100, and 150 HT1080 cells, derived from a human fibrosarcoma, in triplicate in 1 mL of blood obtained from healthy blood donors. We counted the cells by making dilutions in a counting chamber before spiking the cells in 1 mL of blood. Then, we filtered the blood using the ISET system, as described by Rarecells Diagnostics (Paris, France). The filters were stained with antibody against CD45 to identify leukocytes, counterstained with hematoxylin, and read on a light microscope.
Isolation of circulating tumor cells by the ISET approach
Blood samples were collected in ethylenediaminetetraacetic acid tubes, stored at room temperature for up to 4 hours under homogenization, and treated by ISET according to the manufacturer’s instructions. ISET methodology isolates intact CTCs from blood through direct filtration without using antibodies, thus exploiting the larger size of tumor cells compared with leukocytes, and uses polycarbonate membrane with 8 μm-diameter cylindrical pores. We used procedures of this platform and CTC definition criteria according to a previous detailed report.10 Images of results obtained with this technique were taken using a light microscope (Axioskop 40; Carl Zeiss Meditec, Jena, Germany) coupled to a digital camera (Cyber-Shot DSC-S75; Sony, Tokyo, Japan) at 40× and 100× magnification.
Although ISET is used for research only, it has undergone technical11,12 and clinical validation13 showing the prognostic value of the system in patients with early and late-stage non-small-cell lung cancer. We have also reported its advantage compared with a “marker-related” approach in showing progression of metastatic lung cancer before the progression became evident by imaging.14
Immunocytochemistry of cells isolated by ISET
ISET membrane spots were cut out and used for single- or dual-color immunocytochemistry for CTC identification and characterization according to the manufacturer’s instructions. To evaluate and distinguish CTCs from white blood cell (WBC) contaminants, in addition to cytomorphological analysis, dual-color immunocytochemistry (DAB+/Permanent Red; Dako, Glostrup, Denmark) was carried out using the following antibodies: antivimentin (1:1,500, clone V9; Dako), a mesenchymal-related marker, and anti-CD45 (1:100, clone 2B11+ PD7/26; Dako), a WBC surface marker. We also performed dual-color immunocytochemistry replacing vimentin by anti-Pan CK (1:1,000, clone AE1/AE3; Dako), an epithelial-related marker.
Single immunocytochemistry (DAB+) was performed to certify that there were no endothelial cells in our sample (anti-CD34, 1:100, clone QBEnd 10; Dako). For CTC counting, eight spots on the ISET filter were used (corresponding to 8 mL of blood), including four stained with hematoxylin and eosin and the others stained by immunocytochemistry as described in previous studies.12,15 Negative and positive controls were performed for each immunohistochemical staining. For controls, we used blood from healthy individuals spiked with cells from the cell lines A549, HCT 116, or HT1080. WBCs from healthy individuals were used as positive control for CD45. For a negative reaction, a spot was obtained without antibody. Circulating tumor microemboli were defined as groups or clusters of tumor cells containing three or more distinct nuclei and previously identified in metastatic cancer patients.13 The STS CTCs were defined based on size and morphology, after excluding WBCs (anti-CD45) and endothelial cells (anti-CD34). All the slides were reviewed by a pathologist specialized in the diagnosis of sarcoma (IWC).
Results
ISET sensitivity test
We spiked, in triplicate, 25, 50, 100, and 150 cells from HT1080 cell line in 1 mL of blood from healthy donors and treated the blood by the ISET system. The filters were stained with antibody against CD45 to identify leukocytes, counterstained with hematoxylin, and read with a light microscope. The triplicate tests were read by three different operators, and the mean values were calculated. For the spiking of 25, 50, 100, and 150 HT1080 cells, we found mean numbers of 25, 54, 100, and 155 cells, respectively. This result shows the excellent sensitivity of ISET for the isolation from blood of sarcoma cells.
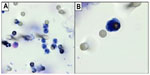
The numbers we found were not identical to the numbers of cells we spiked. However, this is normal, as cells were counted in a counting chamber by dilution and not one by one. Counting cells one by one for spiking tests is technically too cumbersome and not feasible for more than 20 cells. A picture of a sarcoma cell recovered by ISET in shown in Figure 1B.
Controls
We tested with ISET the blood (8 mL) of 30 subjects without cancer (healthy blood donors). None of them were found to have CTC. We detected only some leukocytes, as shown in Figure 1A.
Patients
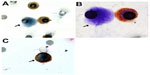
All patients showed CTCs in their blood samples, and their counts ranged from two to 48 per 8 mL of blood (Table 1). CTCs were identified by their differential morphology, as described in the Patients and methods section, and by their negative expression of CD45 (specific marker for WBCs) (Figure 2) and for CD34 (specific marker for endothelial cells) by single immunocytochemistry.
We also performed double staining with CD45 and vimentin, a mesenchymal marker that is also found in leukocytes. Our aim was to assess if CTCs from STS express vimentin. CTCs from three patients expressed vimentin in their cytoplasm (Figure 2). The CTC spread from STS present the typical morphology of a neoplastic cell, as described by Krebs et al,12 with high nuclear-to-cytoplasmic ratio (>0.8), irregular shape, and hyperchromatic nuclei with a diameter >10 μm. The identification of all CTCs was performed by a pathologist from our center specialized in the pathological diagnosis of sarcomas. Interestingly, in four patients (three with synovial sarcoma and one with liposarcoma), ISET allowed the detection of circulating tumor microemboli, which are known in the literature as a marker of poor prognosis.16,17
Discussion
This is the first report showing that isolation, detection, and characterization of CTCs from the blood of patients with sarcoma is feasible without using polymerase chain reaction (PCR)-based molecular tests and through a cytopathological approach that is valid for all types of sarcoma.
Few pioneer attempts of CTC detection in patients with sarcoma have been published (Table 2), including three targeting five or fewer patients,18–20 and seven targeting more than five patients.21–27 These reports used reverse-transcription (RT)-PCR-based methods to detect tumor cells in peripheral blood and/or bone marrow, taking advantage of specific gene translocations detected in Ewing’s sarcoma20–22,24,25,27 and alveolar sarcoma,19,23,24 or from gene expressions associated with sarcoma.18,23,26
However, these approaches have several limitations. In fact, they are specific to a type of sarcoma and cannot be extended to other types; their “sensitivity” depends on the level of expression of the targeted transcript in the tumor cells, which can be variable, and their specificity depends on the absence of similar transcripts: in the case of nonmutated transcripts, in peripheral blood cells. Finally, they do not allow a reliable counting of CTCs nor to visualize CTC morphology to study their protein expression and invasive potential.
Dubois et al20 were the first to study the blood of a patient with Ewing’s sarcoma in an attempt to provide CTC counting. They isolated by Ficoll (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA) 1–5 million peripheral blood mononuclear cells (PBMCs) from 10 mL of blood and analyzed them by flow cytometry for the presence of the CD99 sarcoma marker and the absence of the CD45 leukocyte marker. The sensitivity of this test was shown to be one tumor cell per 500,000 PBMCs (approximately 20 tumor cells per milliliter of blood). However, the authors also reported a background rate of 0.00019% in PBMCs from healthy donors, thus just below one in 500,000 cells, which was their sensitivity rate.
Therefore, while studies on CTCs from patients with carcinoma have evolved from RT-PCR-based methods to magnetic cell-sorter methods that allows CTC counting (CellSearch®; Johnson & Johnson, New Brunswick, NJ, USA) and to cytopathological methods (ISET, Rarecells Diagnostics, Paris, France) which allow CTC diagnosis, counting and immunomolecular characterization; the domain of CTCs in patients with sarcoma did not show the same evolution, missing important information (CTC number, phenotypic and genotypic characterization) about the invasion process. However, preliminary studies focused on CTCs in patients with sarcoma showed a trend26 or clear evidence25,27 of the prognostic value of CTC detection.
This setting prompted us to realize this study and test the feasibility of immunocytopathological detection of CTCs in patients with sarcoma using ISET, a “universal” and diagnostic approach potentially valid for all solid tumors, including carcinomas and sarcomas.
The CTC domain is technically challenging, as CTCs are very rare, just a few per milliliter of blood. They have a highly heterogeneous phenotype, and are not the only rare cells in blood, implying that they have to be distinguished from epithelial and nonepithelial nontumor cells, atypical nontumor cells, endothelial cells, and other rare circulating cells, such as stem cells. Many authors have studied CTCs from carcinomas, mostly after their isolation using epithelial markers, and have shown their prognostic value in metastatic breast, colon, and prostate cancers.28–32 However, the majority of CTC methods use epithelial antigen-targeted antibodies for CTC isolation, and thus they cannot isolate the most malignant CTCs undergoing epithelial–mesenchymal transition, nor can they be applied to the detection of CTCs from sarcomas.
ISET is a marker-independent approach to isolate from blood intact rare cells in order to diagnose and characterize them through further morphological and immunomolecular analyses. It is based on the observation that WBCs are the smallest cells in the body, and thus smaller than tumor cells from organs. ISET was initially developed to sensitively isolate rare epithelial tumor cells.11 However, since the approach does not use epithelial markers, but only a physical cell property, we and others have shown that it isolates CTCs that have undergone epithelial–mesenchymal transition and do not express epithelial antigens.14,33,34 It was thus clear to us, after these reports, that ISET could also isolate CTCs from the blood of patients with sarcomas. Furthermore, Hofman et al15 reported CTCs from patients with sarcoma (five patients with leiomyosarcoma, liposarcoma, and synovial sarcoma; P Hofman, University of Nice Sophia Antipolis [Nice, France], personal communication, February, 2014) using ISET, but the number of CTCs per patient was not known. Our results show that CTCs were able to be isolated and reliably identified and counted in all the patients with sarcoma we studied.
The sensitivity and specificity of ISET in detecting CTCs from patients with carcinoma has been shown. There is a total of 607 controls without cancer, including 327 healthy subjects without cancer, studied by ISET published by different independent teams,11,13,33–40 without any detection of CTCs. However, we also tested by ISET in this study 30 additional subjects without cancer (healthy subjects), and found them without any CTCs, as reported in the Results section.
In this study, we assessed the ability and sensitivity of the ISET method to isolate sarcoma cells (from HT 1080 cell line derived from a human fibrosarcoma) from blood, by performing spiking analyses. We spiked in triplicate 25, 50, 100, and 150 HT1080 cells in 1 mL of blood, which was then filtered and analyzed by CTC counting. We found mean numbers of 25, 54, 100, and 155 cells, respectively. The slight difference in numbers we found between spiked cells and detected cells is normal, as cells were counted in a counting chamber by dilution. Our results show that the ISET method practically does not lose tumor cells from sarcoma. This result is consistent with previous data showing the very high sensitivity of ISET11,12 and its ability to isolate large cells based only on their size, since sarcoma cells are known to be larger than lymphocytes. However, we set up the spiking test with sarcoma cells to precisely assess this issue, as it is key for the clinical relevance of CTC detection by ISET in patients with sarcoma.
A burning issue in the field of CTCs is circulating stem tumor cells and the possibility that these are lost by ISET. Interesting studies have been published41–43 showing that during chemically induced osteoblast differentiation, flexibility, cell size, and circularity decrease. Therefore, stem mesenchymal tumor cells are expected to have increased flexibility, circularity, and size. However, increased flexibility cannot lead to stem cell loss by ISET, as the ISET methodology fixates all the blood cells (through blood dilution with the ISET buffer) prior to filtration.11 The cells thus become rigid, and their deformability and flexibility is lost. The CTCs we found in patients with sarcoma were generally round cells. They had a high nucleus:cytoplasm ratio, and their membrane was very irregular. This observation and the reported decrease in cell size with cell differentiation in the osteoblast-differentiation model42 suggest that ISET is able to isolate the most aggressive and stem tumor cells. Further studies are needed in this field, in particular we have no reliable markers of circulating sarcoma-derived stem cells. When these markers become available, it will be possible to use them to characterize tumor cells isolated by ISET in patients with sarcoma.
We were able to include in the study different histological subtypes of sarcoma to test if CTCs are spread via the bloodstream in all cases. We studied six cases of synovial sarcoma, a tumor that is thought to spread through the lymphatic vessels. However, we also found hematogenous spreading of synovial sarcoma, as we found CTCs in all six cases. Three of our patients (of eleven) were found to have small numbers of CTCs (less than on CTC per milliliter). These numbers of CTCs in STS patients could be related to the treatment that patients received prior to CTC analysis. However, our data show that CTC identification and counting could be used in patient follow-up, for personalized monitoring of the anticancer treatment. It is also noteworthy that we included different types of sarcomas in our study, the majority of which are not characterized by genetic mutations or fusion transcripts, and thus could never benefit from a CTC test based on PCR-based analyses.
Our study characterized CTCs from STS patients and distinguished them from endothelial cells, leukocytes, and epithelial cells by dual staining using immunolabeling. We think that cell-morphology visualization is important in the field of CTCs, and has potential advantages over RT-PCR or circulating cell-free deoxyribonucleic acid (cfDNA) techniques. These methods may give false-positive results. For instance, RT-PCR methods are exposed to the risk of false-positive results due to nonspecific markers, nonspecific primers, and illegitimate transcription. cfDNA-marker detection is bound to the presence of mutations in the cfDNA, is spread from the tumor even when the tumor mass is not invasive, may derive from necrotic or apoptotic cells (thus not be involved in the invasion process and more sensitive to antitumor treatments), and may contain “early” mutations that may not be the end point of cancer transformation or a hallmark of cancer, but only one of the first steps of carcinogenesis. Circulating CTC visualization and morphological analysis allows the evaluation of the specialist cytopathologist to be obtained and the study of the cellular immunomolecular profile. As shown in Figure 1, ISET allows the study of cell morphology and cell immunomolecular characterization to be performed.
By using immunocytochemistry, we detected vimentin in CTCs from three STSs. Vimentin is an interesting marker, as it has been associated with AKT1 activation in the process of sarcoma tumor-cell motility and invasiveness.44 Although not specific for STS, vimentin expression in CTCs from sarcomas could be the sign of a more aggressive and invasive cell phenotype. Several authors have found that the expression of vimentin in circulating tumor cells from carcinomas is associated with their epithelial–mesenchymal transition, “stemness”, and more malignant characteristics.9,12,45–47 With this in mind, future studies should be planned to better understand the biological significance of vimentin expression in CTCs from sarcomas, which are mesenchymal tumors, and to correlate the expression of this marker in CTCs with the clinical outcome. Specifically, it is important to understand if the expression of vimentin in CTCs from sarcomas is to be considered, as for CTCs from carcinomas, a sign of stemness and if it is potentially related to a more invasive cellular behavior.
Sarcomas are currently diagnosed based on histological evaluation combined with immunohistochemistry and molecular techniques.2 Despite modest clinical benefits obtained using chemotherapy, the side effects of the most aggressive anticancer-drug combinations are high. As a result, it is important to develop new diagnostic tools for the early identification of nonresponders, thus avoiding useless drug-related morbidity and suffering. The availability of a noninvasive method allowing sensitive detection, reliable identification, and molecular characterization of CTC in sarcoma patients, independently of the need for gene-mutation or fusion-transcript detection, as in our study, is expected to allow for further larger investigations and potentially has great clinical value.
In conclusion, by using ISET, we were able to identify CTCs in all patients with high-grade sarcomas we studied. Detection of CTCs in the peripheral blood of STS patients is expected to have an important role as a biomarker of invasion and to provide an opportunity to improve staging and prognosis in patients with STS. In fact, most STS histotypes spread mainly through the hematogenous route.
Although the number of patients we studied was limited, as sarcomas are rare tumors, our results bring a proof of concept that should stimulate further studies aimed at detecting and characterizing CTCs in larger numbers of patients with different sarcoma subtypes. Multicenter studies should target CTC counting and protein expression, focus on the correlation between quantitative cellular data and patient clinical data, and follow up in order to assess the prognostic value of the CTC analyses.
We think that our results, obtained with a highly sensitive cytopathological and immunocytopathological approach and with the relative controls, are reliable and can be the basis to foster studies aimed at improving the follow-up and treatment of patients with sarcoma.
Acknowledgments
We thank Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) for financial support (project number 2012/01273-8). We would like to make a special acknowledgment to Dr Adriane Siqueira, Dr Silvia Regina Rogatto, and his student Juan Augusto Moyano Munõz for help with the culture of the sarcoma-cell lineage HT1080.
Disclosure
The authors report no conflicts of interest in this work.
References
Amankwah EK, Conley AP, Reed DR. Epidemiology and therapies for metastatic sarcoma. Clin Epidemiol. 2013;5:147–162. | |
Pennacchioli E, Tosti G, Barberis M, et al. Sarcoma spreads primarily through the vascular system: are there biomarkers associated with vascular spread? Clin Exp Metastasis. 2012;29(7):757–773. | |
Gronchi A, Lo Vullo S, Colombo C, et al. Extremity soft tissue sarcoma in a series of patients treated at a single institution: local control directly impacts survival. Ann Surg. 2010;251(3):506–511. | |
[No authors listed]. Adjuvant chemotherapy for localised resectable soft-tissue sarcoma of adults: meta-analysis of individual data. Sarcoma Meta-analysis Collaboration. Lancet. 1997;350(9092):1647–1654. | |
Santoro A, Tursz T, Mouridsen H, et al. Doxorubicin versus CYVADIC versus doxorubicin plus ifosfamide in first-line treatment of advanced soft tissue sarcomas: a randomized study of the European Organization for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group. J Clin Oncol. 1995;13(7):1537–1545. | |
Casali PG. Histology- and non-histology-driven therapy for treatment of soft tissue sarcomas. Ann Oncol. 2012;23 Suppl 10:x167–xS169. | |
Paterlini-Brechot P, Benali NL. Circulating tumor cells (CTC) detection: clinical impact and future directions. Cancer Lett. 2007;253(2):180–204. | |
Klein CA. Parallel progression of primary tumours and metastases. Nat Rev Cancer. 2009;9(4):302–312. | |
Rhim AD, Mirek ET, Aiello NM, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;(148):349–361. | |
Khoja L, Backen A, Sloane R, et al. A pilot study to explore circulating tumour cells in pancreatic cancer as a novel biomarker. Br J Cancer. 2012;106(3):508–516. | |
Vona G, Sabile A, Louha M, et al. Isolation by size of epithelial tumor cells. A new method for the immunomorphological and molecular characterization of circulating tumor cells. Am J Pathol. 2000;156(1):57–63. | |
Krebs MG, Hou JM, Sloane R, et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J Thorac Oncol. 2012;7(2):306–315. | |
Hofman V, Long E, Ilie M, et al. Morphological analysis of circulating tumour cells in patients undergoing surgery for non-small cell lung carcinoma using the isolation by size of epithelial tumour cell (ISET) method. Cytopathology. 2012;23(1):30–38. | |
Chinen LT, Carvalho FM, Rocha BM, et al. Cytokeratin-based CTC counting unrelated to clinical follow up. J Thoracic Dis. 2013;5(5):593–599. | |
Hofman V, Ilie MI, Bonnetaud C, et al. Cytopathologic detection of circulating tumor cells using the isolation by size of epithelial tumor cell method: promises and pitfalls. Am J Clin Pathol. 2011;135(1):146–156. | |
Friedl P, Gilmour D. Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol. 2009;10(7):445–457. | |
Ilina O, Friedl P. Mechanisms of collective cell migration at a glance. J Cell Sci. 2009;122(18):3203–3208. | |
Gattenloehner S, Dockhorn-Dworniczak B, Leuschner I, Vincent A, Müller-Hermelink HK, Marx A. A comparison of MyoD1 and fetal acetylcholine receptor expression in childhood tumors and normal tissues:implications for the molecular diagnosis of minimal disease in rhabdomyosarcomas. J Mol Diagn. 1999;1(1):23–31. | |
Hoshino M, Ogose A, Kawashima H, et al. Molecular analyses of cell origin and detection of circulating tumor cells in the peripheral blood in alveolar soft part sarcoma. Cancer Genet Cytogenet. 2009;190(2):75–80. | |
Dubois SG, Epling CL, Teague J, Matthay KK, Sinclair E. Flow cytometric detection of Ewing sarcoma cells in peripheral blood and bone marrow. Pediatr Blood Cancer. 2010;54(1):13–18. | |
Pfleiderer C, Zoubek A, Gruber B, et al. Detection of tumour cells in peripheral blood and bone marrow from Ewing tumour patients by RT-PCR. Int J Cancer. 1995;64(2):135–139. | |
Peter M, Magdelenat H, Michon J, et al. Sensitive detection of occult Ewing’s cells by the reverse transcriptase-polymerase chain reaction. Br J Cancer. 1995;72(1):96–100. | |
Kelly KM, Womer RB, Barr FG. Minimal disease detection in patients with alveolar rhabdomyosarcoma using a reverse transcriptase-polymerase chain reaction method. Cancer. 1996;78(6):1320–1327. | |
Thomson B, Hawkins D, Felgenhauer J, Radich J. RT-PCR evaluation of peripheral blood, bone marrow and peripheral blood stem cells in children and adolescents undergoing VACIME chemotherapy for Ewing’s sarcoma and alveolar rhabdomyosarcoma. Bone Marrow Transplant. 1999;24(5):527–533. | |
Avigad S, Cohen IJ, Zilberstein J, et al. The predictive potential of molecular detection in the nonmetastatic Ewing family of tumors. Cancer. 2004;100(5):1053–1058. | |
Gallego S, Llort A, Roma J, Sabado C, Gros L, de Toledo JS. Detection of bone marrow micrometastasis and microcirculating disease in rhabdomyosarcoma by a real-time RT-PCR assay. J Cancer Res Clin Oncol. 2006;132(6):356–362. | |
Schleiermacher G, Peter M, Oberlin O, et al. Increased risk of systemic relapses associated with bone marrow micrometastasis and circulating tumor cells in localized Ewing tumor. J Clin Oncol. 2003;21(1):85–91. | |
Cristofanilli M, Budd GT, Mathew JE, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–791. | |
Maheswaran S, Haber DA. Circulating tumor cells: a window into cancer biology and metastasis. Current Opin Gen Dev. 2010;20(1):96–99. | |
O’Flaherty JD, Gray S, Richard D, et al. Circulating tumor cells, their role in metastasis and their clinical utility in lung cancer. Lung Cancer. 2012;76(1):19–25. | |
Gorges TM, Pantel K. Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunol Immunother. 2013; 62(5):931–939. | |
Ligthart ST, Couman FA, Bidard FC, et al. Circulating tumor cells count and morphological features in breast, colorectal and prostate cancer. PLoS One. 2013;8(6):e67148. | |
Hofman VJ, Bonnetaud C, Ilie MI, et al. Preoperative circulating tumor cells detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin Cancer Res. 2011;(17):827–835. | |
Hofman V, Ilie M, Long E, et al. Detection of circulating tumor cells as a prognostic factor in patients undergoing radical surgery for non-small-cell lung carcinoma: comparison of the efficacy of the Cell Search Assay™ and the isolation by size of epithelial tumor cell method. Int J Cancer. 2011;(129):1651–1660. | |
Vona G, Estepa L, Béroud C, et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology. 2004;39(3):792–797. | |
Pinzani P, Salvadori B, Simi L, et al. Isolation by size of epithelial tumor cells in peripheral blood of patients with breast cancer: correlation with real-time reverse transcriptase-polymerase chain reaction results and feasibility of molecular analysis by laser microdissection. Hum Pathol. 2006;37(6):711–718. | |
Pinzani P, Mazzini C, Salvianti F, et al. Tyrosinase mRNA levels in the blood of uveal melanoma patients: correlation with the number of circulating tumor cells and tumor progression. Melanoma Res. 2010;20(4):303–310. | |
De Giorgi V, Pinzani P, Salvianti F, et al. Application of a filtration- and isolation-by-size technique for the detection of circulating tumor cells in cutaneous melanoma. J Invest Dermatol. 2010;130(10):2440–2447. | |
De Giorgi V, Pinzani P, Salvianti F, et al. Circulating benign nevus cells detected by ISET technique: warning for melanoma molecular diagnosis. Arch Dermatol. 2010;146(10):1120–1124. | |
Mazzini C, Salvianti F, Scatena C, et al. Circulating tumor cells detection and counting in uveal melanomas by a filtration-based method. Cancers (Basel). 2014;6(1):323–332. | |
Xu W, Mezencev R, Kim B, Wang L, McDonald J, Sulchek T. Cell stiffness is a biomarker of the metastatic potential of ovarian cancer cells. PLoS One. 2012;7(10):e46609. | |
Wang G, Mao W, Byler R, et al. Stiffness dependent separation of cells in a microfluidic device. PLoS One 2013;8(10):e75901. | |
Bongiorno T, Kazlow J, Mezencev R, et al. Mechanical stiffness as an improved single-cell indicator of osteoblastic human mesenchymal stem cell differentiation. J Biomech. 2014;47(9):2197–2204. | |
Zhu QS, Rosenblatt K, Huang KL, et al. Vimentin is a novel AKT1 target mediating motility and invasion. Oncogene. 2011;30(4):457–470. | |
Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331(6024):1559–1564. | |
Hou JM, Krebs M, Ward T, et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am J Pathol. 2011;178(3):989–996. | |
Krebs MG, Metcalf RL, Carter L, Brady G, Blackhall FH, Dive C. Molecular analysis of circulating tumour cells – biology and biomarkers. Nat Rev Clin Oncol. 2014;11(3):129–144. | |
Burchill SA, Lewis IJ, Abrams KR, et al. Circulating neuroblastoma cells detected by reverse transcriptase polymerase chain reaction for tyrosine hydroxylase mRNA are an independent poor prognostic indicator in stage 4 neuroblastoma in children over 1 year. J Clin Oncol. 2001;19(6):1795–1801. | |
West DC, Grier HE, Swallow MM, Demetri GD, Granowetter L, Sklar J. Detection of circulating tumor cells in patients with Ewing’s sarcoma and peripheral primitive neuroectodermal tumor. J Clin Oncol. 1997;15(2):583–588. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.