Back to Journals » Drug Design, Development and Therapy » Volume 9
Isolation and evaluation of anticancer efficacy of stigmasterol in a mouse model of DMBA-induced skin carcinoma
Authors Ali H , Dixit S, Ali D , Alqahtani S, Alakahtani S, Alarifi S
Received 25 February 2015
Accepted for publication 16 April 2015
Published 28 May 2015 Volume 2015:9 Pages 2793—2800
DOI https://doi.org/10.2147/DDDT.S83514
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Professor Shu-Feng Zhou
Huma Ali,1 Savita Dixit,1 Daoud Ali,2 Saeed M Alqahtani,3 Saad Alkahtani,2 Saud Alarifi2
1Department of Chemistry, Maulana Azad National Institute of Technology, Bhopal, India; 2Department of Zoology, College of Science, King Saud University, Riyadh, Saudi Arabia; 3King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia
Abstract: Stigmasterol (99.9% pure) was isolated from Azadirachta indica and its chemopreventive effect on 7,12-dimethylbenz[a]anthracene (DMBA)-induced skin cancer was investigated in Swiss albino mice. Skin tumors were induced by topical application of DMBA and promoted by croton oil. To assess the chemopreventive potential of stigmasterol, it was orally administered at a concentration of 200 mg/kg and 400 mg/kg three times weekly for 16 weeks. Reduction in tumor size and cumulative number of papillomas were seen as a result of treatment with stigmasterol. The average latency period was significantly increased as compared with the carcinogen-treated control. Stigmasterol induced a significant decrease in the activity of serum enzymes, such as aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin as compared with the control. Stigmasterol significantly increased glutathione, superoxide dismutase, and catalase as compared with the control. Elevated levels of lipid peroxide and DNA damage in the control group were significantly inhibited by administration of stigmasterol. From the present study, it can be inferred that stigmasterol has chemopreventive activity in an experimental model of cancer. This chemopreventive activity may be linked to the oxidative stress of stigmasterol. The antigenotoxic properties of stigmasterol are also likely to contribute to its chemopreventive action.
Keywords: stigmasterol, papilloma, oxidative stress, skin carcinogenesis, chemoprevention, DNA damage
Introduction
A large number of plant species used in folk medicine have been used since the earliest days of humanity and have considerable importance in international trade.1 Azadirachta indica belongs to the Meliaceae family and has been extensively used in Ayurveda, Unani, and homeopathic medicine. More than 135 compounds have been isolated from different parts of A. indica.2 Plants produce different types of active compounds, including those that can cause DNA damage.3 In contrast, some plant metabolites have been shown to reduce the incidence of DNA damage.4 Therefore, isolation and characterization of plant products are important for the definition of strategies to reduce the risk of cancer in humans. In this respect, evaluation of the genotoxicity and antigenotoxicity of these plant products is extremely important.5
Phytosterols are triterpenes closely resembling cholesterol in both structure and function. They are insoluble in water, but soluble in organic solvents. Stigmasterol, also known as stigmasterin or wulzen anti-stiffness factor, is one of a group of plant sterols present in A. indica. The bark of A. indica contains tannins, which are phenolic compounds reported to have anti-inflammatory activity.6 Toxicology studies of A. indica leaf extract administered at a dose of 0.6–2.0 g/kg bodyweight did not have any lethal effects in terms of hematology, enzyme levels, or histopathological parameters in animals, whereas the leaf extract at 200 g/kg caused weight loss accompanied by weakness, anorexia, and histopathological defects.7 Stigmasterol is used in a number of chemical processes designed to yield synthetic and semisynthetic compounds in the pharmaceutical industry.8 Kaur et al6 reported that stigmasterol has various pharmacological properties, including antiosteoarthritic, hypoglycemic, antimutagenic, antioxidant, and anti-inflammatory activity. The anticarcinogenic activity of stigmasterol has not yet been fully explored. Some investigators have suggested that cancer could be prevented by avoidance of cancer-causing substances and by using chemopreventive agents,9 and evaluated the anticancer activity of stigmasterol using parameters such as oxidative stress, DNA damage, histopathological, and liver and kidney function indices.
The present study was designed to study the anticancer efficacy of stigmasterol in Swiss albino mice bearing 7,12-dimethylbenz[a]anthracene (DMBA) induced skin cancer.
Materials and methods
Plant material and extraction
The leaves of A. indica were collected from Maulana Azad Institute National Technology campus, Bhopal, India. The plant material was taxonomically identified by Zia-UL-Hasan, Department of Botany, Saifia College of Science, Bhopal. The voucher specimen (367/Bot/Saifia/12) was preserved in the above herbarium for future reference. Leaves from A. indica were dried in the shade for 7–10 days and pulverized using an electric grinder. The dried sample was extracted in a solvent of methanol and acetone at a ratio of 70:30 using Soxhlet apparatus. The residue was dried under reduced pressure using a rotary vacuum evaporator.
Isolation of stigmasterol
A column 40 cm in length and 3 cm in diameter was plugged with cotton and then packed with silica gel (70 g) up to a height of 23 cm under reduced pressure. The column was washed with n-hexane to facilitate compact packing. The sample was prepared by adsorbing 3.5 g of n-hexane soluble extract of A. indica onto silica gel (mesh size 60 mm×120 mm), then allowing it to dry, and subsequently applying it on top of the adsorbent layer. The column was then eluted with n-hexane followed by a mixture of n-hexane and dichloromethane, and then a mixture of dichloromethane and methanol. The polarity was gradually increased by adding increasing proportions of dichloromethane and methanol. Thirty fractions were collected, each in 100 mL beakers.
Characterization of stigmasterol
Ultraviolet spectral peaks of the compound isolated from A. indica showed maximum absorption at 244 nm, indicating the presence of the plant sterol. The Fourier transform infrared data indicated the presence of hydroxyl, alkenes methyl, and methylene groups in the spectrum (Figure 1).
  | Figure 1 Characterization of isolated compound (stigmasterol) of Azadirachta indica by Fourier transform infrared spectroscopy. |
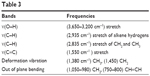
The 1H nuclear magnetic resonance spectrum of the isolated compound exhibited proton signals in μg/mL at δ5.37 (1H, H-6), δ5.14 (1H, H-22), δ5.04 (1H, H-23), δ2.29 (1H, H-3), δ1.09 (3H, CH3-10), δ0.95 (3H, CH3-20), δ0.846 (3H, CH3-27) δ0.81 (3H, CH3-26), and δ0.72 (3H, CH3-13).
The negative ion electrospray ionization mass spectrum of the isolated compound showed the loss of alkyl groups if the compound is stigmasterol the fragment resulted by loss of C4H2, loss of methyl and methylene at m/z 507. It showed loss of C3H2O2, loss of the CO2 fragment at m/z 453, and loss of C3H4 which is a resonance stabilized fragment at 435. The fragmentation pattern confirmed the presence of stigmasterol.
Chemicals and animals
The DMBA and croton oil were purchased from Sigma-Aldrich (St Louis, MO, USA). Other analytical grade chemicals were purchased from local sources. Swiss albino mice of either sex were selected randomly from the animal house at the Pinnacle Biomedical Research Institute, Bhopal. The animals were housed in polypropylene cages with sterile husk and had access to standard pellets and water ad libitum throughout the experiment. The animals were maintained on a 12-hour light/dark cycle at 22°C±2°C under controlled conditions. All animal experiments were performed with the prior permission of the institutional animal ethics committee at Pinnacle Biomedical Research Institute, Bhopal (1283/C/09/CPCSEA).
Exposure of stigmasterol on DMBA croton oil induced skin carcinoma
Four groups of Swiss albino mice (n=10 per group) were used in the study. The animals were dorsally shaved with a hair clipper. Group 1 animals were given Milli-Q water (10 mL/kg body weight), a normal diet, and tap water ad libitum daily. Group 2 animals received a single dose of DMBA (100 μg/100 μL of acetone) over the shaved area of dorsal skin after which 1% croton oil was applied to the skin three times a week for 16 weeks. After the single dose of DMBA, the group 3 and group 4 animals were treated with stigmasterol (200 and 400 mg/kg bodyweight) orally three times a week for 16 weeks, with application of 1% croton oil onto the skin 1 hour after exposure to stigmasterol. Two weeks after application of DMBA, the mice were monitored weekly for 16 weeks for the presence and size of skin tumors, body weight, and the average latency period. After 16 weeks, the mice were euthanized, and the dorsal skin was removed for histopathology and blood was taken for biochemical analysis.
Oxidative stress biomarkers
On the final day of the experiment, all the animals were euthanized by cervical dislocation. The dorsal skin was removed immediately and washed in ice-cold saline (0.9% NaCl), followed by removal of extraneous material. The skin was then weighed and blotted dry. A 10% tissue homogenate of skin was prepared in 0.15 M Tris-KCl (pH 7.4), and then centrifuged at 12,000 rpm for 15 minutes. For biochemical estimation, supernatant, were used on the same day. Protein content was measured by the Bradford method,10 using bovine serum albumin as the standard.
Lipid peroxidation assay
Lipid peroxidation (LPO) was estimated by measuring the formation of malondialdehyde.11 A mixture of 0.1 mL tissue lysate and 1.9 mL of 0.1 M sodium phosphate buffer (pH 7.4) was incubated at 37°C for 1 hour. After precipitation with 5% trichloroacetic acid, the incubation mixture was centrifuged (2,300× g for 15 minutes at room temperature) and the supernatant was collected. Next, 1.0 mL of 1% thiobarbituric acid was added to the supernatant and placed in boiling water for 15 minutes. After cooling to room temperature, the absorbance of the mixture was taken at 532 nm and expressed in nmol malondialdehyde per hour/mg protein using a molar extinction coefficient of 1.56×105/M/cm.
Estimation of glutathione
The glutathione (GSH) level was quantified using Ellman’s reagent.12 The assay mixture contained phosphate buffer, 5,5′-dithiobis-(2-nitrobenzoic acid), and tissue lysate. The reaction was monitored at 412 nm and the amount of GSH was expressed in terms of nmol of GSH/mg protein.
Measurement of superoxide dismutase
The activity of superoxide dismutase (SOD) was estimated using the method described by Kakkar et al.13 The assay mixture contained sodium pyrophosphate buffer, nitroblue tetrazolium, phenazine methosulfate, reduced nicotinamide adenine dinucleotide, and tissue lysate. One unit of SOD enzyme activity is defined as the amount of enzyme required to inhibit production of chromogen (560 nm) by 50% in 1 minute under assay conditions, and is expressed as specific activity in units/min/mg protein.
Measurement of catalase activity
Catalase activity was measured by following its ability to split hydrogen peroxide (H2O2) within 1 minute of incubation time. The reaction was then stopped by adding dichromate/acetic acid reagent, and the remaining H2O2 was determined by measuring at 570 nm the chromic acetate formed by reduction of dichromate/acetic acid in the presence of H2O2, as described earlier.14 Catalase activity was expressed as μmole H2O2 decomposed/min/mg protein.
Determination of serum enzymes in stigmasterol-treated mice
Blood was obtained from all animals by puncturing the retro-orbital plexus. The blood samples were allowed to clot for 45 minutes at room temperature. The serum was separated by centrifugation at 2,500 rpm and 30°C for 15 minutes and utilized for determination of various biochemical parameters. Aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin levels were estimated using the Span Diagnostics kit.
Histopathology
After treatment, the mice were sedated and euthanized by decapitation. Fresh portions of skin were rapidly dissected from each mouse, fixed in neutral buffered formalin (10%), and dehydrated with grades of ethanol (70%, 80%, 90%, 95%, and 100%). After dehydration, the samples were cleared in two changes of xylene. The samples were then impregnated with two changes of molten paraffin wax, embedded, and blocked out. Paraffin sections (4–5 μm) were stained with hematoxylin and eosin. Stained sections from the control and treated mice were observed and photographed using an optical microscope (model BX51 with a digital camera, Olympus, Tokyo, Japan) for alterations in architecture and for the presence of degeneration and necrosis.
Determination of DNA strand breakage
Alkaline single-cell gel electrophoresis was performed as a three-layer procedure with slight modification.15 The lymphocytes were separated from blood using Histopaque density gradient centrifugation and the cells were diluted 20-fold for the Comet assay. For the positive control, lymphocytes were treated with 100 μM H2O2 for 10 minutes at 4°C. Two slides per animal were prepared, and 25 cells per slide (250 cells per group) were scored randomly and analyzed using a Komet-5.5 image analysis system (Kinetic Imaging Ltd, Nottingham Business Park, UK) attached to a fluorescent microscope (Leica, Wetzlar, Germany) equipped with appropriate filters. The parameter selected for quantification of DNA damage was percent tail DNA as determined by the software.
Statistical analysis
The data obtained from the different groups were analyzed by analysis of variance. P<0.05 was considered to be statistically significant for all experiments.
Results
Chemopreventive effect of stigmasterol
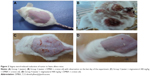
The results of the current study are presented in Table 1. A slow decrease in body weight was noticed in the different groups of animals. Group 3 and group 4 animals were continuously treated with different doses of stigmasterol and repeated application of croton oil, and showed significant in the cumulative number of papillomas and tumor size (Figure 2C and D; Table 1) as compared with the control group (group 2, Figure 2B). The latency period was found to be 10.10±5.17 weeks in the group treated with DMBA and croton oil, and was significantly higher in the stigmasterol-treated mice (Table 1).
Oxidative stress and biochemical enzymes
The activity of GSH, SOD, and catalase was increased in the skin of stigmasterol-treated mice (groups 3 and 4) when compared with the control mice (group 2, Figure 3). In contrast, LPO level were significantly decreased in stigmasterol-treated mice when compared with control mice (group 2, Figure 3). A significant decrease in blood aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, and bilirubin levels in group 3 and group 4 mice when compared with group 2 mice (Table 2).
Effect of stigmasterol on skin histopathology
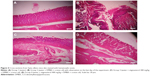
Generally, the dermal layer is composed of loose connective tissue and dense connective tissue known as the papillary and reticular layers, respectively. We observed no changes in the structure of the skin in group 1 (Figure 4A). In the animals treated with DMBA and croton oil (Figure 4B), these layers started to differentiate into a papillomatous form, with signs of an epidermal layer with an abnormal structure. Dysplastic changes in the squamous layer, stromal damage, hyperkeratosis, acanthosis, and cysts with horns were observed in the control group treated with DMBA and croton oil (Figure 4B). In the animals treated with stigmasterol, histological observation revealed signs of tumor, hyperkeratosis, and acanthosis, but to a lesser degree when compared with the control group (Figure 4C and D).
DNA damage
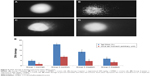
The DNA damage was measured as percent tail DNA and Olive tail moment in the control and treated groups. More DNA damage was observed in the lymphocytes of animals treated with DMBA and croton oil than in the stigmasterol-treated animals (group 2, Figure 5B and E); however, DNA damage was decreased when compared with the control (group 2, Figure 5C–E).
Discussion
Plant products are natural bioactive compounds that protect against stress and pathogenic attack. It has been reported that long-term use of certain medicinal plants overwhelms carcinogenesis in several human and animal organs.16 Thus, it is important to identify natural plant products that could suppress or reverse the process of cancer.1 In the present study, we observed several beneficial effects of stigmasterol in induced skin cancer. We used a subthreshold dose of DMBA as a carcinogen followed by regular treatment with croton oil as a promoter to induce skin tumors in experimental mice. With regard to the initiation and promotion stages, animal studies show that the promotion step takes more time to occur and is reversible initially, so prevention of cancer by inhibition of tumor promotion is expected to be an inventive approach. In the current study, stigmasterol could significantly inhibit formation DMBA-induced papilloma in terms of both incidence of tumors and the mean number of papillomas.
LPO is a free radical chain reaction and is well known to induce two main steps, ie, initiation and promotion of carcinogenesis. LPO level is increased and produced a complex reactive compounds, eg, malondialdehyde. The products of LPO have been reported to be mutagenic and carcinogenic. Roslida et al17 used chemopreventive agents to reduce production of free radicals in vivo. In our study, administration of stigmasterol significantly reduced the level of LPO in mice treated with DMBA and croton oil, and as a consequence, decreased the incidence of skin tumors.
GSH has an important role in normal cell metabolism, ie, inhibiting production of reactive oxygen species (ROS) and free radicals. We found that GSH activity was decreased in the control group (treated with DMBA + croton oil) but was increased in stigmasterol-treated mice, indicating its antioxidant activity. Klaunig et al18 reported that antioxidants have chemopreventive properties. Antioxidants are considered to be the first line of defense against oxidative stress, which suggests their usefulness in reducing the risk of oxidative damage during carcinogenesis. SOD and catalase are antioxidative enzymes that protect against ROS.19 We observed significant enrichment of GSH and SOD levels and catalase activity in the stigmasterol-treated group when compared with the control group. ROS play an important role in the process of apoptosis, and include the superoxide, hydrogen peroxide, and hydroxyl radicals, which damage cell components including DNA, ultimately leading to cell death.20
Exposure to DMBA induces LPO and ROS in the affected area of skin, leading to carcinogenesis. ROS cause permeabilization of the outer mitochondrial membrane, which releases soluble proteins from the inner membrane space into the cytosol, and promote caspase activation.21 Oxidative stress was observed in the control groups because LPO was high and GSH, SOD, and catalase levels were low. The beneficial effect of stigmasterol is probably due to its ability to stimulate antioxidant enzymes in cells. The increase in enzyme activity down the production of ROS and LPO in the skin, decreased the incidence of papilloma in the areas of treated skin.
Histopathological observation showed that DMBA caused severe skin damage. The histopathology study showed that stigmasterol repaired the degenerated epidermal and dermal layers of skin in the group 2 animals. However, significant histological changes were observed in the hypodermis, where there was degeneration of adipose tissue. Thus, the free hydroxyl group on the aromatic ring is responsible for the antioxidant properties. The Comet assay showed that DMBA induced significant DNA damage in lymphocytes. The protective effect of A. indica against DNA damage in lymphocytes might be due to the stigmasterol present in the ethanol leaf extract.
  | Table 3 |
The present results show the anticancer effect of stigmasterol at the higher concentrations tested (200 and 400 mg/kg body weight). Further, we found that stigmasterol had an antigenotoxic effect on DMBA-induced genotoxicity. These results suggest that the anticancer activity of stigmasterol may be due to its antioxidant and antigenotoxic properties.
Acknowledgment
The authors are grateful to the Deanship of Scientific Research at King Saud University for funding this research (RG-1435-076).
Disclosure
The authors report no conflicts of interest in this work.
References
Ebong PK, Atangwho IJ, Eyong EU, Egbung GE. The anti-diabetic efficacy of combined extracts from two continental plants: Azadirachta indica (A. juss) (Neem) and Vernonia amygdalina (Del.) (African bitter leaf). Am J Biochem Biotechnol. 2008;4:239–244. | ||
Chopra RN, Nayer SL, Chopra IC. Glossary of Indian Medicinal Plants. New Delhi, India: Council of Scientific and Industrial Research; 1956. | ||
Ansah C, Khan A, Gooderham NJ. In vitro genotoxicity of the West African antimalarial herbal Cryptolepis sanguinolenta and its major alkaloid cryptolepine. Toxicology. 2005;208:141–147. | ||
Vieira PM, Marinho LP, Ferri SC, Chen-Chen L. Protective effects of steroidal alkaloids isolated from Solanum paniculatum L. against mitomycin cytotoxic and genotoxic actions. An Acad Bras Cienc. 2013;85:553–560. | ||
Vieira PM, Santos SC, Chen-Chen L. Assessment of mutagenicity and cytotoxicity of Solanum paniculatum L. extracts using in vivo micronucleus test in mice. Braz J Biol. 2010;70:601–606. | ||
Kaur N, Chaudhary J, Jain A, Kishore L. Stigma sterol: a comprehensive review. Int J Pharm Sci Res. 2011;2:2259–2265. | ||
Ghimeray AK, Jin C, Ghimire BK, Cho DH. Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta indica (A. Juss) grown in foothills of Nepal. Afr J Biotechnol. 2009;8:3084–3091. | ||
Fernandes P, Cabral JM. Phytosterols: applications and recovery methods. Bioresour Technol. 2007;98:2335–2350. | ||
Sharma P, Parmar J, Verma P, Sharma P, Goyal PK. Anti-tumor activity of Phyllanthus niruri (a medicinal plant) on chemical-induced skin carcinogenesis in mice. Asian Pac J Cancer Prev. 2009;10:1089–1094. | ||
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. | ||
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. | ||
Ellman G. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. | ||
Kakkar PS, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. | ||
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–394. | ||
Ali D, Ray RS, Hans RK. UVA-induced cyototoxicity and DNA damaging potential of benz (e) acephenanthrylene. Toxicol Lett. 2010;199:193–200. | ||
Mouli KC, Vijaya T, Rao SD. Phyto-resources as potential therapeutic agents for cancer treatment and prevention. Journal of Global Pharma Technology. 2009;1:4–18. | ||
Roslida AH, Fezah O, Yeong LT. Suppression of DMBA/croton oil-induced mouse skin tumor promotion by Ardisia crispa root hexane extract. Asian Pac J Cancer Prev. 2011;12:665–669. | ||
Klaunig JE, Kamendulis LM, Hocevar BA. Oxidative stress and oxidative damage in carcinogenesis. Toxicol Pathol. 2010;38:96–109. | ||
Ekin S, Ozdemir H, Demir H, et al. Plantago major protective effects on antioxidant status after administration of 7, 12-dimethylbenz(a)anthracene in rats. Asian Pac J Cancer Prev. 2011;12:531–535. | ||
Parmar J, Sharma P, Verma P, Sharma P, Goyal PK. Anti-tumor and anti-oxidative activity of Rosmarinus officinalis in 7, 12 dimethyl benz(a) anthracene induced skin carcinogenesis in mice. Am J Biomed Sci. 2011;3:199–209. | ||
Blessy D, Suresh K, Manoharan S, Vijayaanand MA, Sugunadevi G. Evaluation of chemopreventive potential of Zingiber officinale roscoe ethanolic root extract on 7, 12-dimethyl benz[a]anthracene induced oral carcinogenesis. Res J Agric Biol Sci. 2009;5:775–781. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.