Back to Journals » Therapeutics and Clinical Risk Management » Volume 13
Isolated descemetorhexis for anterior synechiolysis prior to endothelial keratoplasty – case report and technique
Authors Droutsas K, Andreanos K, Lazaridis A, Georgalas I , Kymionis G, Papaconstantinou D
Received 18 June 2017
Accepted for publication 24 August 2017
Published 19 October 2017 Volume 2017:13 Pages 1443—1447
DOI https://doi.org/10.2147/TCRM.S144258
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Konstantinos Droutsas,1,2 Konstantinos Andreanos,1 Apostolos Lazaridis,2 Ilias Georgalas,1 George Kymionis,1,3 Dimitris Papaconstantinou1
1First Department of Ophthalmology, National and Kapodistrian University of Athens, Athens, Greece; 2Ophthalmology Department, Philipps University Marburg, Marburg, Germany; 3Ophthalmology Department, Jules Gonin Eye Hospital, University of Lausanne, Lausanne, Switzerland
Purpose: To describe the utilization of descemetorhexis for reformation of the anterior chamber in eyes with central iridocorneal synechiae before endothelial keratoplasty (EK).
Methods: A 71-year-old man with a history of trabeculectomy complicated by hypotony presented with bullous keratopathy in the presence of extensive iridocorneal synechiae and a flat anterior chamber. In order to proceed with EK, synechiolysis with the use of viscoelastic and scissors was attempted. Despite successful dissection of the peripheral strands, the pupillary margin of the iris remained attached to the endothelium. Therefore, descemetorhexis was performed to detach the Descemet membrane along with central synechiae and create sufficient space for safe EK at a later stage.
Results: Corneal clarity was restored by ultrathin Descemet stripping automated endothelial keratoplasty, leaving a fibrous membrane in the pupillary plane, which was excised 2 months later, allowing an improvement of best-corrected visual acuity to 0.5.
Conclusion: Isolated descemetorhexis was successfully employed to reform the anterior chamber and proceed with EK in a case of bullous keratopathy and resistant iridocorneal synechiae. This stepwise approach may be considered in similar cases in order to avoid a more invasive treatment, ie, penetrating keratoplasty and synechiolysis.
Keywords: endothelial keratoplasty, iridocorneal synechiae, bullous keratopathy, descemetorhexis
A Letter to the Editor has been received and published for this article.
Introduction
Endothelial keratoplasty (EK) has replaced penetrating keratoplasty (PK) in the treatment of endothelial dysfunctions to a great extent. Adequate anterior chamber depth is mandatory for successful positioning of the endothelial graft.1 Thus, in eyes with central iridocorneal synechiae, EK may prove difficult if not impossible. Several techniques for the safe and efficient dissection of anterior synechiae have been proposed, including the use of ophthalmic viscosurgical devices or scissors.2,3 Herein, we describe the utilization of descemetorhexis for removal of iridocorneal synechiae, aiming at restoring the anterior chamber volume prior to EK.
Case report
The patient involved provided a written informed consent in accordance with the tenets of the Declaration of Helsinki to having their medical data used for research purposes. Written informed consent was also obtained from the patient for publication of this case report and any accompanying images, according to Committee on Publication Ethics guidelines.
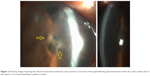
A 71-year-old male was referred for corneal transplantation due to severe bullous keratopathy in his right eye. He reported undergoing uneventful trabeculectomy 1 year before, followed by phacoemulsification due to significant cataract formation 6 months later. The postoperative course was complicated by prolonged athalamia (Figure 1) resulting in extensive central iridocorneal synechiae.
  | Figure 1 Slit-lamp images depicting the status after trabeculectomy and phacoemulsification before the onset of bullous keratopathy. Note the iridocorneal contact at the slit beam (yellow arrow). |
Best-corrected visual acuity (BCVA) was “counting fingers” with intact light projections in the right eye (OD) and 20/20 in the left eye (OS). Biomicroscopy OD showed extensive iridocorneal contact at the center and a very shallow peripheral anterior chamber. Intraocular pressure was 14 mmHg, while the vitreous space appeared normal in B-scan ultrasonography. Biomicroscopy and funduscopy OS were unremarkable, showing a clear crystalline lens and mild glaucomatous cupping of the optic nerve.
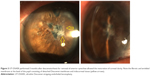
In order to allow reformation of the anterior chamber and proceed with EK at a later stage, synechiolysis was performed under subtenon’s block. However, despite repeated attempts, only part of the synechiae could be dissected, while a large portion of the pupillary margin of the iris remained attached to the corneal endothelium. Thus, in the presence of a deeper anterior chamber, we decided to perform descemetorhexis in order to detach the central portion of Descemet membrane (DM) along with the central synechiae and create sufficient space for EK. In brief, after removing the viscoelastic agent, the anterior chamber was completely filled with air to allow better visualization of the DM. With a reverse Sinskey hook (D.O.R.C., Zuidland, the Netherlands), DM was scored at a diameter of ~9.0 mm and stripped off from the posterior stroma along with the attached synechiae, causing it to retract and fall into the pupillary plane. Poor visibility, however, did not allow safe removal of the membrane. Indeed, 1 month later, no synechiae were visible on slit-lamp examination, the anterior chamber was deep, and the contracted, fibrotic DM was occupying the pupil (Figure 2).
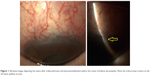
Two months later, ultrathin Descemet stripping endothelial keratoplasty was performed, allowing restoration of corneal clarity (Figure 3). One month later, a vitrectome-assisted membranectomy was performed, leading to a round and unobstructed pupil. During the postoperative period, the cornea remained clear and intraocular pressure within normal limits. One month later, BCVA increased to 0.5 (Figure 4) and remained at that level until the last follow-up 1 year later. Endothelial cell density decreased from 2,833 cells/mm2 (donor cornea) to 1,571 cells/mm2 (12 months after membranectomy).
Discussion
The present report describes the utilization of the descemetorhexis maneuver to remove central anterior synechiae and allow EK in a case with bullous keratopathy and athalamia.
Since its introduction a decade ago, EK has proven its superiority in terms of visual rehabilitation and safety compared to the older standard, ie, PK. Therefore, EK is currently the gold standard in the management of corneal endothelial dysfunction.4
During EK, the donor endothelium is inserted into the anterior chamber, centered, and attached by means of air bubble injection.1,5 Eyes with a shallow anterior chamber or with space occupied by a glaucoma tube or anterior synechiae are considered as challenging. In the present case, the extent and localization of the anterior synechiae rendered EK impossible, and therefore, synechiolysis was mandatory.
Several techniques for removal of anterior synechiae have been described, all of which are combined with PK. Jee et al2 described a technique for synechiolysis prior to PK using Healon needle and ophthalmic viscosurgical devices. The use of Castroviejo synechiae scissors and iris repository has also been suggested; however, there is a risk of iris bleeding and coloboma.3 In our case, viscoelatic-assisted synechiolysis could not release the extremely stiff synechiae and to avoid complications we did not use scissors. Instead, we chose to perform descemetorhexis and were therefore able to proceed successfully with EK.
Thus, to our knowledge, this is the first report of descemetorhexis as a technique performed specifically to release extremely adherent anterior synechiae.
Although PK could have restored corneal clarity and anterior chamber depth as a single procedure, we opted for the presented 3-step approach in order to proceed with EK for several reasons. Compared to PK, EK offers quicker rehabilitation, better and faster visual outcomes, and a lower risk for allograft rejection.6–9
Depending on anterior chamber visibility, the present approach could be applied in two steps, ie, descemetorhexis combined with removal of the iridocorneal membrane and EK surgery later. However, in the presented case, poor visibility of the anterior chamber did not allow visualization of the retracted DM to allow safe membranectomy, and therefore, ultrathin Descemet stripping endothelial keratoplasty was performed after descemetorhexis to allow corneal clearing and safe removal of the iridocorneal tissue.
To recapitulate, we propose isolated descemetorhexis to remove stiff anterior synechiae as a separate procedure prior to EK surgery. Performing a descemetorhexis maneuver and synechiotomy can help in releasing an adherent cornea from the underlying iris without risk of damaging the iris tissue. Once the anatomy of the anterior chamber depth has been restored, EK may be safely performed before or after removal of the retracted iridocorneal membrane lying on the pupillary plane, depending on the visibility of the anterior chamber. This technique can be helpful in cases with stiff anterior synechiae, such as in those with post-trabeculectomy athalamia and iridocorneal dystrophies.
Disclosure
The authors report no conflicts of interest in this work.
References
Dapena I, Moutsouris K, Droutsas K, Ham L, van Dijk K, Melles GR. Standardized “no-touch” technique for descemet membrane endothelial keratoplasty. Arch Ophthalmol. 2011;129:88–94. | ||
Jee D, Sung YM, Kim MS, Kim EC. Anterior synechiolysis with healon needle and ophthalmic viscosurgical devices after anterior lamellar dissection in penetrating keratoplasty. Semin Ophthalmol. 2016;31(6):554–558. | ||
Paton RT, McLean JM, et al. Corneal transplantation. Am J Ophthalmol. 1948;31(11):1365–1399. | ||
Kymionis GD, Mikropoulos DG, Portaliou DM, et al. New perspectives on lamellar keratoplasty. Adv Ther. 2014;31(5):494–511. | ||
Busin M, Bhatt PR, Scorcia V. A modified technique for descemet membranestripping automated endothelial keratoplasty to minimize endothelial cell loss. Arch Ophthalmol. 2008;126:1133–1137. | ||
Kim SE, Lim SA, Byun YS, Joo CK. Comparison of long-term clinical outcomes between descemet’s stripping automated endothelial keratoplasty and penetrating keratoplasty in patients with bullous keratopathy. Korean J Ophthalmol. 2016;30:443–450. | ||
Ishiyama S, Mori Y, Nejima R, et al. Comparison of long-term outcomes of visual function and endothelial cell survival after descemet stripping automated endothelial keratoplasty and penetrating keratoplasty using mixed-effects models. Cornea. 2016;35:1526–1532. | ||
Ang M, Soh Y, Htoon HM, Mehta JS, Tan D. Five-year graft survival comparing descemet stripping automated endothelial keratoplasty and penetrating keratoplasty. Ophthalmology. 2016;123:1646–1652. | ||
Heinzelmann S, Böhringer D, Eberwein P, Reinhard T, Maier P. Outcomes of descemet membrane endothelial keratoplasty, descemet stripping automated endothelial keratoplasty and penetrating keratoplasty from a single centre study. Graefes Arch Clin Exp Ophthalmol. 2016;254:515–522. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.