Back to Journals » Drug Design, Development and Therapy » Volume 14
Isoborneol Attenuates Low-Density Lipoprotein Accumulation and Foam Cell Formation in Macrophages
Authors Wang Y, Li Z, Liu B, Wu R, Gong H, Su Z, Zhang S
Received 30 September 2019
Accepted for publication 13 December 2019
Published 15 January 2020 Volume 2020:14 Pages 167—173
DOI https://doi.org/10.2147/DDDT.S233013
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qiongyu Guo
Yunfei Wang, 1, 2,* Zhengrong Li, 2,* Boxue Liu, 1, 2 Rumeng Wu, 1, 2 Haifeng Gong, 2 Zhanhai Su, 2 Shoude Zhang 1, 2
1State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University, Xining, Qinghai 810016, People’s Republic of China; 2Medical College of Qinghai University, Xining, Qinghai 810016, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Shoude Zhang
State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University, 251# Ningda Road, Xining, Qinghai 810016, People’s Republic of China
Tel +86-971-5366433
Fax +86-971-5201533
Email [email protected]
Purpose: Isoborneol has been used in the treatment of cardiovascular disease for several years in China. However, the mechanism is still unclear. The aim of this study was to identify the novel mechanism of isoborneol for its application in atherosclerotic disease.
Materials and Methods: The whole-genome gene expression profiles of MCF-7 cells treated with/or without isoborneol were detected by mRNA microarray analysis. The degree of similarity between the gene expression profiles was compared with the Connectivity Map (CMAP) database. An MTT assay was used to assess the toxicity of isoborneol on RAW 264.7 cells. Oil red O staining and a Dil-ox-LDL uptake assay in RAW 264.7 cells were also used to detect the accumulation of lipids in the macrophages and the uptake of oxidized low-density lipoprotein (ox-LDL).
Results: Isoborneol was proved to have mRNA expression profiles similar to that of ikarugamycin which can inhibit the uptake of ox-LDL. This process has proved to be an important cause of foam cell formation and early atherosclerotic lesions. It is speculated, therefore, that isoborneol may show similar activity to that shown by ikarugamycin. Subsequently, it was shown that RAW 264.7 cells reduced the absorption of ox-LDL and the accumulation of intracellular lipids after treatment with different concentrations of isoborneol.
Conclusion: The results indicate that isoborneol inhibits macrophage consumption of ox-LDL, thereby preventing the accumulation of lipids in the macrophages. These results provide evidence for the application of isoborneol in atherosclerotic disease.
Keywords: isoborneol, ikarugamycin, connectivity map, ox-LDL, atherosclerosis, macrophage
Introduction
Atherosclerosis-associated cardiovascular disease has one of the highest morbidity and mortality rates in Humans,1 and this condition is characterized by the slow formation of lesions and stenosis of blood vessel walls.2 Mono-nuclear cells migrate to the sub-endothelial to be activated and differentiated into macrophages, and macrophages can phagocytose modified low-density lipoprotein to form early foam cells when the scavenger receptor is activated.3,4 Foam cells will rupture and die with this constant accumulation of lipids.5 Foam cells, therefore, serve not only as marker cells for the early stage of arteriosclerosis6 but also participate in the entire atherosclerotic lesion process.7,8 These findings show that the inhibition of lipid accumulation in foam cells can effectively reduce the occurrence and development of atherosclerosis-related diseases.
The gene expression of cell lines can be impacted by small molecules and these changes can be captured by gene chips technology. The Connectivity Map (CMAP) is a collection of genome-wide transcriptional expression data from cultured human cells treated with a thousand bioactive small molecules. These data, used together with simple pattern-matching algorithms, can enable the discovery of functional connections between drugs, genes and diseases through the transitory feature of common gene expression changes. These algorithms can compare two sets of genes that are up-regulated and down-regulated in a specific condition with the whole CMAP database. CMAP can, therefore, be utilized to identify small molecules that create gene expression patterns similar to those being searched for.9
Isoborneol, an isomer of borneol, is a monoterpenoid alcohol present in the essential oils of numerous plants.10 Many Chinese medicines used for the treatment of cardiovascular disease contain borneol or isoborneol.11 Previous studies have shown that isoborneol has an anti-inflammatory effect12 and can protect the cardiovascular system.13 In addition, isoborneol has been proven to cross14 or assist other drugs in crossing the blood-brain barrier.15 However, there is little research on its action mechanism. The whole genome expression data of MCF-7 cells treated with/or without isoborneol were assessed using microarray technology (Table S1). To find the bio-activity of isoborneol and uncover its related mechanisms, the CMAP database was searched using the gene expression data of isoborneol-treated MCF-7 cells. Finally, this study identified that ikarugamycin created gene expression patterns similar to isoborneol. Ikarugamycin was originally identified as an antibiotic and has subsequently been found to inhibit the uptake of oxidized low-density lipoprotein (ox-LDL) in cells. It is proposed therefore that isoborneol may have similar activity with regard to inhibiting the uptake of ox-LDL and formation of foam cells. Currently, there are no reports in the literature concerning the effect of isoborneol on maintaining the lipid stability of macrophage foam cells. The aim of this study was to investigate whether isoborneol inhibits the uptake of low-density lipoprotein and the accumulation of intracellular lipids by RAW 264.7.
Materials and Methods
Reagents
Isoborneol was purchased from Chenguang Biotechnology (Baoji, Shanxi, China). The purity of isoborneol was greater than 97%. Dulbecco’s Modified Eagle’s Medium (DMEM) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Dil-labeled oxidized low-density lipoprotein (Dil-ox-LDL) was purchased from Solarbio (Beijing, China). Penicillin-streptomycin, RIPA lysis buffer, MTT kits and phosphate-buffered saline (PBS) were purchased from Beyotime Biotechnology (Shanghai, China). Human oxidized low-density lipoprotein (ox-LDL) was purchased from Yiyuan Biotechnologies (Guangzhou, China). Fetal bovine serum (FBS) was purchased from HyClone (GE Healthcare, Buckinghamshire, UK).
Microarray Analysis
After pre-treatment with 10 μM isoborneol, MCF7 cells were harvested, and total RNA was extracted using TRIzol reagent (Life Technologies, Carlsbad, CA, US) according to the manufacturer’s instructions. DMSO-treated cells were selected as a control. The gene expression profiles were assessed using microarray technology with Affymetrix Human Genome U133A 2.0 (Santa Clara, CA, US).
Comparison of Gene Expression Profiles
CMAP is a database with a collection of gene expression data sets from cultured human cell lines treated with various small molecules. The CMAP database was searched with two lists of genes from MCF-7 cells after treatment with isoborneol: one list comprised of the 20 most highly up-regulated genes and the other comprised of the 20 most heavily down-regulated genes. CMAP reported enrichment scores (which lie between −1 and 1) of all the drugs on the basis of the relative correlations between the signature of isoborneol and the reference gene expression profiles of individual drugs in the CMAP database. Higher absolute positive scores for a given signature indicate that the experimental results of the particular drug treatment in CMAP show a gene expression profile similar to the signature that is provided by the user.
Cell Culture
The mouse macrophage (RAW 264.7) was provided by Dr. Jigang Zhang from the laboratory of the Department of Clinical Pharmacy, Shanghai General Hospital. The use of this RAW 264.7 cell was approved by the institutional review board of Qinghai University. Cells were grown in DMEM supplemented with 10% FBS and 1% penicillin-streptomycin and were maintained in an incubator (Thermo Fisher Scientific, Marietta, USA) with humidified air containing 5% CO2 at 37°C. RAW 264.7 cells were grown to 70–80% fusion and were then used in the experiments. The MCF7 cells were purchased from the Chinese Academy of Sciences Cell Bank (Shanghai, China).
Oil Red O Staining
The lipid content was determined by using Oil Red O staining. Oil Red O staining was performed using an Oil Red O stain kit (Solarbio, Beijing, China). The RAW 264.7 cells were seeded in plates and pretreated with different doses of isoborneol for 24 hrs in DMEM with 1% FBS, then loaded with 50 μg/mL ox-LDL for 24 hrs and washed with PBS three times. Subsequently, the cells were fixed for 30 mins and stained for 15 mins at room temperature. After washing, an inverted microscope (Nikon Ti-S, Japan) was used to observe the accumulation of lipid in the macrophages. After oil red O staining, the lipid content was measured by isopropanol extraction. Absorbance was measured at 540 nm by using a microplate reader (Thermo Scientific, Varioskan, LUX, Finland).
Dil-ox-LDL Uptake Assay
The uptake of ox-LDL by the macrophages was detected by Dil-ox-LDL. RAW 264.7 cells were seeded in 12-well plates (1×105 cells/well). After 12 hrs, cells were separately treated with isoborneol (0 μM, 5 μM, and 20 μM) for 24 hrs followed by the addition of 20 μg/mL Dil-ox-LDL at 37°C for 4 hrs. After the cells were washed with PBS three times, they were viewed and photographed by fluorescence inverted microscope (Nikon Ti-S, Japan). The cells were lysed with RIPA lysis buffer to determine their fluorescence with 530-nm excitation and 590-nm emission filters using a microplate reader (Thermo Scientific, Varioskan, LUX, Finland).
Cell Viability Measurement
Cell viability was determined by MTT assay. RAW 264.7 cells were inoculated in a 96-well plate with a density of 1×104 cells/well, and the cell survival rate was determined by MTT assay. Briefly, the cells were treated with 50 μg/mL ox-LDL and 5 μM or 20 μM isoborneol for 24 hrs. MTT solution (5 mg/mL) was then added to the cells and left for 4 hrs. Following this, solution reagent was added to completely dissolve the formazan particles. The absorbance was determined with a microplate reader (Thermo Scientific, Varioskan, LUX, Finland) at a wavelength of 570 nm.
Statistical Analysis
Quantitative results are expressed as the mean ± SD. All the experimental results were confirmed by at least three independent experiments. P-values < 0.05 were deemed to be statistically significant.
Pathway Enrichment Analysis
Genes that had two-times fold changes were summitted to the STRING online sever and pathway enrichment analysis was performed by the sever. The enriched pathways were outputted by the score of false discovery rate.16
Results
To find the bioactivity of isoborneol and it’s related mechanism, the CMAP database was searched using datasets including the first 20 genes of MCF-7 cells that were up-regulated or down-regulated by isoborneol, as shown in Figure 1A and B (Table S1). The output from the CMAP search provided an enrichment score in the range from 1 to −1, and in this study, the absolute enrichment score was used as the metric according to previous research.17 Finally, ikarugamycin was identified as the top-ranking chemical compound (Figure 1C). To check whether gene expression profiles obtained for isoborneol and ikarugamycin also showed the same relationship in different settings, microarray results for ikarugamycin were obtained from the CMAP database and these again displayed a high level of similarity in a heat-map analysis between the first 20 genes that were up- or down-regulated (Figure 1D). Ikarugamycin was originally identified as an antibiotic and was subsequently found to inhibit the uptake of ox-LDL. From these results, it was assumed, therefore, that isoborneol may show similar activity. The following experiment was used to confirm whether isoborneol inhibited the uptake of ox-LDL.
It was necessary to determine the cytotoxicity of isoborneol, as this could affect subsequent experiments. Using the MTT assay, the cell viabilities of RAW 264.7 cells treated with 50 μg/mL ox-LDL alone or 50 μg/mL ox-LDL with 5 μM or 20 μM isoborneol were found to be 101.5% ± 9%, 99.8% ± 7%, and 96.5% ± 8%, respectively, when compared with untreated controls (100%). The results showed that 20 μM isoborneol in the presence of 50 μg/mL ox-LDL did not damage cell growth. The highest concentration of isoborneol used in this study was therefore 20 μM.
To investigate whether isoborneol influenced lipid accumulation in macrophages, the accumulation of intracellular lipids in mouse RAW 264.7 cells was examined after loading with 50 μg/mL oxidized LDL for 24 hrs with or without the presence of isoborneol. As shown in Figure 2, the occurrence of Oil Red O stained particles increased significantly in the macrophages. Compared with the ox-LDL group, the quantities of intracellular lipid accumulation were decreased in the group treated with isoborneol at different concentrations. The result suggests that isoborneol can decrease the quantities of intracellular lipid accumulation in ox-LDL-induced macrophages in a dose-dependent manner.
The formation of macrophage foam cells is an important part of atherosclerosis. Based on the experiments described above, it was shown that isoborneol has an inhibitory effect on the accumulation of lipids in macrophages. To further investigate whether isoborneol had an effect on the formation of foam cells, the uptake of Dil-ox-LDL in RAW 264.7 cells with or without the presence of isoborneol was examined. As shown in Figure 3, after the cells were loaded with Dil-ox-LDL for 4 hrs, the red Dil fluorescent-labeled particles in the macrophages increased significantly, and the content of the red fluorescent-labeled particles decreased depending on the dose of isoborneol (Figure 3A and B). These results indicate that isoborneol inhibits the formation of ox-LDL-induced macrophage foam cells.
To explore the mechanism of isoborneol, the isoborneol-regulated pathways were identified using pathway enrichment analysis. Nine enriched pathways were identified (Table 1). Among them, two pathways (hsa05200 and hsa05203) were related to cancer, five pathways (hsa04810, hsa04015, hsa04010, hsa04540, and hsa04072) were related to cell migration and cell polarity, one pathway (hsa05032) was related to addiction, and one pathway (hsa04750) related to inflammation.
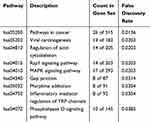 |
Table 1 Enriched KEGG Pathways |
Discussion
Atherosclerosis is a complex inflammatory vascular disease and persistent inflammation can promote the development of atherosclerosis.8 Inflammation, therefore, plays an important role in the formation of macrophage foam cells. Previous studies have suggested that isoborneol has anti-inflammatory activity and its derivatives can prevent the formation of macrophage foam cells and intracellular lipid accumulation.18 However, there are few studies investigating the role of isoborneol in the fight against atherosclerosis, such as the inhibition of the formation of macrophage-derived form cells. The present study found that isoborneol inhibited the accumulation of lipids and the uptake of ox-LDL in macrophages, which can prevent the formation of macrophage foam cells. Isoborneol may, therefore, be used independently or in combination with other drugs to combat atherosclerosis.
Previous research has proved that ox-LDL can inhibit the migration of aortic endothelial cells in vitro.19 In addition, ox-LDL was proved to induce loss of cell polarity and inhibit macrophage locomotion through its interaction with CD36, a transmembrane glycoprotein receptor expressed in a variety of cells including monocytes and macrophages.20 Here, five isoborneol-regulated pathways were identified that can control cell migration and polarity. It is suggested therefore that isoborneol plays the role of inhibiting the accumulation of lipids and the formation of macrophage foam cells in mouse RAW 264.7 cells, mainly through counteracting the adverse effect of ox-LDL on cell migration and polarity.
Recent decades have seen an increasing interest in the effect of traditional Chinese medicine and its active ingredients on atherosclerosis-related diseases.21 Isoborneol is a chemical component often found in traditional Chinese medicines, especially those used for the treatment of cardiovascular disease. This study not only provides a new molecular template for the treatment of atherosclerosis but also provides a basis for the treatment of cardiovascular disease using these traditional prescriptions.
Conclusion
In conclusion, isoborneol can inhibit the accumulation of lipids and the formation of macrophage foam cells in mouse RAW 264.7 cells. Isoborneol may, therefore, be a potential active ingredient from plants for use against atherosclerotic disease.
Abbreviations
LDL, low-density lipoprotein; ox-LDL, oxidized low-density lipoprotein; CMAP, Connectivity Map database.
Ethics Approval
All cell lines used in this work are non-primary cells and do not require ethics approval for their use.
Acknowledgments
We are very grateful to Dr. Jigang Zhang for providing us with macrophages. This work was supported by the Project of Qinghai Science & Technology Department (2018-ZJ-948Q).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation. 2019;139:10.
2. Heidi CW. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–1422. doi:10.1038/nm.2538
3. Tian K, Ogura S, Little PJ, Xu SW, Sawamura T. Targeting LOX-1 in atherosclerosis and vasculopathy: current knowledge and future perspectives. Ann N Y Acad Sci. 2019;1443(1):34–53. doi:10.1111/nyas.13984
4. Wang D, Yang Y, Lei Y, et al. Targeting foam cell formation in atherosclerosis: therapeutic potential of natural products. Pharmacol Rev. 2019;71(4):596–670.
5. Frank G, Alina VDG, Anton VDS, Jolanda W. Shear stress and advanced atherosclerosis in human coronary arteries. J Biomech. 2013;46(2):240–247. doi:10.1016/j.jbiomech.2012.11.006
6. Lusis AJ. Atherosclerosis. Nature. 2000;407(6801):233–241. doi:10.1038/35025203
7. Deguchi JO, Aikawa M, Tung CH, et al. Inflammation in atherosclerosis: visualizing matrix metalloproteinase action in macrophages in vivo. Circulation. 2006;114(1):55–62. doi:10.1161/CIRCULATIONAHA.106.619056
8. Moore K, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145(3):341–355. doi:10.1016/j.cell.2011.04.005
9. Lamb. The connectivity map: using gene-expression signatures to connect small molecules, genes, and disease. Science. 2006;313(5795):1929–1935. doi:10.1126/science.1132939
10. Tian LL, Zhou Z, Zhang Q, et al. Protective effect of (±) isoborneol against 6-OHDA-induced apoptosis in SH-SY5Y cells. Cell Physiol Biochem. 2007;20(6):1019–1032. doi:10.1159/000110682
11. Liu F, Du X, Liu PR, Sun YH, Zhang YM. Screening and analysis of key active constituents in Guanxinshutong capsule using mass spectrum and integrative network pharmacology. Chin J Nat Med. 2018;16(4):302–312. doi:10.1016/S1875-5364(18)30060-8
12. Zou L, Zhang Y, Li W, et al. Comparison of chemical profiles, anti-inflammatory activity, and UPLC-Q-TOF/MS-based metabolomics in endotoxic fever rats between synthetic borneol and natural borneol. Molecules. 2017;22(9):1446. doi:10.3390/molecules22091446
13. Jiang W, Xu D, Hu G, Lin B. Some Pharmacologic Effects Of The “Styrax pill for coronary disease” and the pharmacological basis of a simplified styrax-borneol preparation. Acta Pharmaceutica Sinica. 1979;14(11):655.
14. Lv Z, Yang Y, Wang J, Chen J, Li J, Di L. Optimization of the preparation conditions of borneol-modified ginkgolide liposomes by response surface methodology and study of their blood brain barrier permeability. Molecules. 2018;23(2):303. doi:10.3390/molecules23020303
15. Zheng C, Shixiang H, Yuanbo L, et al. Effect of borneol on the distribution of gastrodin to the brain in mice via oral administration. J Drug Target. 2008;16(2):178–184. doi:10.1080/10611860701794395
16. Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res. 2019;47(D1):D607–D613. doi:10.1093/nar/gky1131
17. Lee J, Liu J, Feng X, et al. Withaferin A is a leptin sensitizer with strong antidiabetic properties in mice. Nat Med. 2016;22(9):1023–1032. doi:10.1038/nm.4145
18. Wang J, Xu P, Xie X, et al. DBZ (Danshensu Bingpian Zhi), a novel natural compound derivative, attenuates atherosclerosis in apolipoprotein E-deficient mice. J Am Heart Assoc Cardiovasc. 2017;6(10):e006297.
19. Murugesan G, Chisolm GM, Fox PL. Oxidized low density lipoprotein inhibits the migration of aortic endothelial cells in vitro. J Cell Biol. 1993;120(4):1011–1019. doi:10.1083/jcb.120.4.1011
20. Park YM, Drazba JA, Vasanji A, Egelhoff T, Febbraio M, Silverstein RL. Oxidized LDL/CD36 interaction induces loss of cell polarity and inhibits macrophage locomotion. Mol Biol Cell. 2012;23(16):3057–3068. doi:10.1091/mbc.e11-12-1051
21. Sedighi M, Bahmani M, Asgary S, Beyranvand F, Rafieiankopaei M. A review of plant-based compounds and medicinal plants effective on atherosclerosis. J Res Med Sci off J Isfahan Univ Med Sci. 2017;22(1):30. doi:10.4103/jrms.JRMS_976_16
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



